Abstract
On-pump techniques are the methods of choice for organ protection and heart support during thoracoabdominal aortic aneurysm surgical repair. We present a case of a patient with a symptomatic saccular aneurysm of the visceral segment of a severe atheromatous aorta requiring a surgical type IV aortic repair during which we adopted a temporary passive arterial shunt from the left axillary artery to ensure visceral and distal aortic perfusion. The old-fashioned technique of passive shunting to ensure visceral and distal aortic perfusion seems to offer an appealing alternative to on-pump techniques in selected patients.
keywords: Aortic aneurysm, Perfusion, Organ protection, Aortic operation, Off pump, TAAA surgical repair
Distal aortic perfusion strategies such as cardiopulmonary bypass and left heart bypass are the methods of references for organ protection and heart support during thoracoabdominal aortic aneurysm (TAAA) surgical repair.1,2 Even if the on-pump techniques have allowed standardization and a significant improvement in terms of morbidity and mortality, they are burdened by an increased risk of complications such as coagulation disorders and systemic inflammatory response syndrome.3 The old-fashioned technique of passive shunting seems to offer an appealing alternative in selected patients.4,5
We report a case of a saccular aneurysm of the visceral segment of a severe atheromatous aorta requiring a type IV aortic repair during which we adopted a temporary passive arterial shunt to ensure visceral and distal aortic perfusion.
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Case report
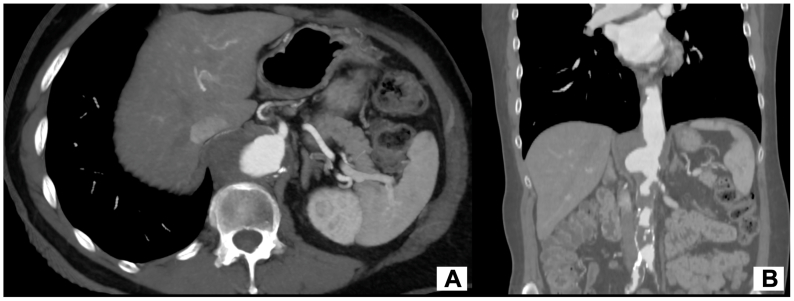
A 68-year-old man was admitted with nonspecific lumbar pain. Computed tomography angiography (CTA) incidentally revealed a 57 mm saccular aneurysm of the visceral segment of a severe atheromatous aorta (likely resulting from a penetrating atherosclerotic ulcer). CTA also demonstrated abundant multifocal thrombus apposition at the level of the thoracic and abdominal aorta extending to the common iliac arteries, as well as a focal dissection flap at the level of the abdominal aorta (Fig 1). The patient presented common cardiovascular risks factors such as active smoking, poorly managed hypertension, and dyslipidemia. No prior history of cardiovascular disease was reported. As a preoperative assessment, the patient had only undergone a transthoracic echocardiogram, which yielded normal results. We did not delve into a second-level examination because the patient was symptomatic, and we opted to expedite the preoperative process.
Fig 1.
Preoperative computed tomography angiography (CTA) showing a saccular aneurysm of the visceral segment of a severe atheromatous aorta with abundant multifocal thrombus apposition.
Treatment options were discussed in a multidisciplinary meeting. The amount of thrombus spreading along the whole aorta and the lack of adequate accesses and sealing zone were considered a contraindication to endovascular treatment which would have been high risk for potential embolization requiring to extend upward the proximal sealing zone to the lever of the mid descending thoracic aorta, potentially increasing the risk of spinal cord ischemia. Another technical challenge was the iliac outflow. In fact, the iliac arteries were heavily diseased and preservation of the internal iliac perfusion during and after surgery was a matter of concern. Therefore, the decision was made to proceed with open type IV aortic repair with visceral protection thought a passive shunt from the left axillary artery (Fig 2).
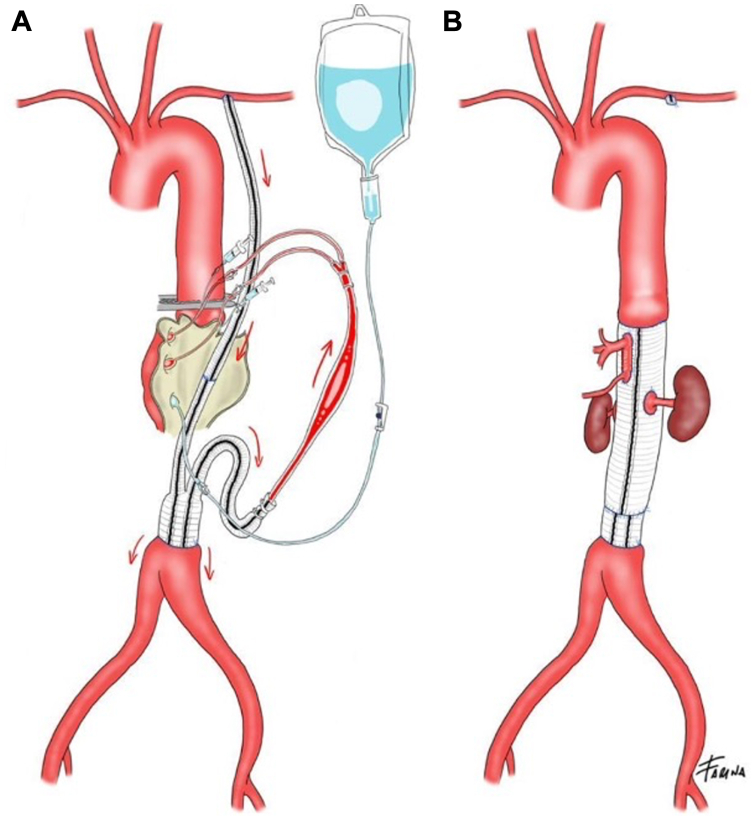
Fig 2.
Schematic representation of visceral, renal and lower limb perfusion strategy with a passive shunt for celiac trunk and superior mesenteric artery and Custodiol solution for renal arteries (A) and of the aorto-aortic bypass with reimplantation on a single aortic patch of the visceral vessels and direct reimplantation of the two renal arteries on the prosthetic body (B).
Aortic surgical exposure was performed through a thoracotomy at the VII intercostal space. First, we created the passive shunt suturing an 8-mm Dacron graft to the left axillary artery. The second step was the reconstruction of the distal aorta with infrarenal and iliac clamping, suturing a reverse bifurcated Dacron graft to the aortic bifurcation and connecting the passive shunt to one of the two limbs of the graft to immediately establish distal perfusion. Next, an intraoperative color Doppler ultrasound examination was performed to identify a favorable aortic site for clamping that was then marked with a dermographic pencil.
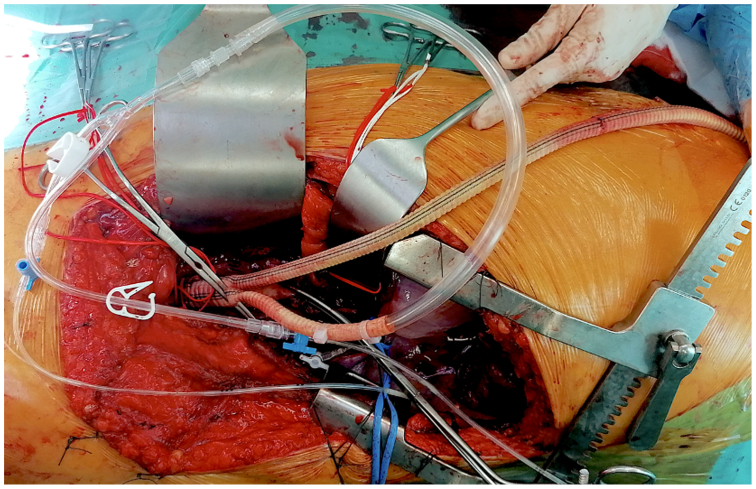
The superior mesenteric artery and the celiac trunk were perfused through a 9F Pruitt balloon-tipped perfusion catheters connected to one of the limbs of the reverse bifurcated graft. The renal arteries were perfused with cold Custodiol solution6,7 (Fig 3). Then, the thoracic aorta was clamped at the previously identified site, and we performed an aorto-aortic bypass with reimplantation on a single patch of aortic wall of the visceral vessels and direct reimplantation of the two renal arteries on the prosthetic body. The decision to perform direct reimplantation of the renal arteries and a small single aortic patch incorporating the ostia of the celiac trunk and superior mesenteric artery was made to minimize the amount of remaining native aorta decreasing the risk of developing a visceral patch aneurysm8 and because the origins of the renal arteries were widely apart from the visceral vessels as the result of the severe dilatation of the corresponding aortic segment.
Fig 3.
The reverse bifurcated Dacron graft sutured to the aortic bifurcation and connected to the passive shunt through one of the two limbs, whereas the visceral vessels are perfused through a 9F Pruitt balloon-tipped perfusion catheters connected to the other limb of the bifurcated graft.
The postoperative course was uneventful. The patient was discharged on postoperative day 14. CTA was performed at 3 months after surgery, showing satisfactory repair of the aorta with patent and adequately perfused visceral branches (Fig 4).
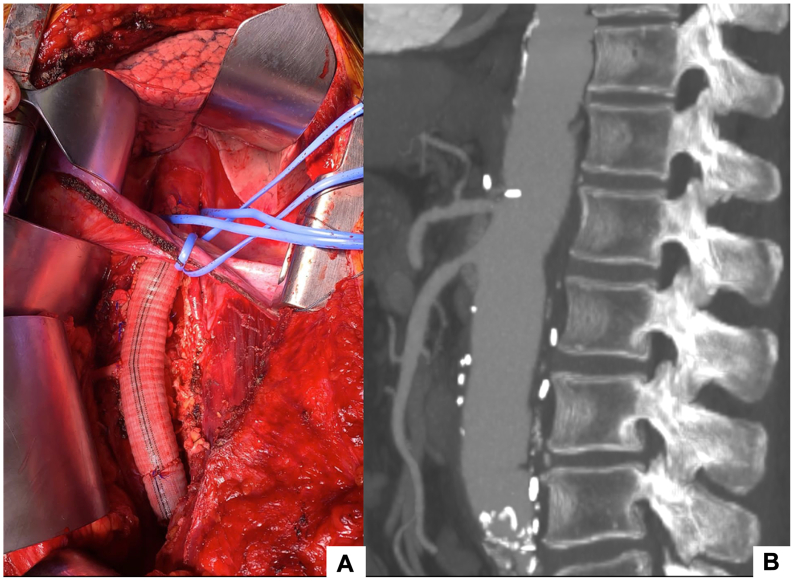
Fig 4.
Intraoperative image of the reconstructed aorta (A), postoperative computed tomography angiography (CTA) showing aortic replacement with reimplantation of celiac trunk, superior mesenteric artery and renal arteries. (B).
Discussion
The use of a passive shunt during TAAA surgery has been described since the 1970s, and it has been reported in several studies as an alternative to the use of an assisted extracorporeal circulation (cardiopulmonary bypass and left heart bypass) in the setting of TAAA surgery.9, 10, 11 The rationale for passive shunting is to divert blood from proximal to distal aortic sites, largely decreasing left ventricular afterload and thereby controlling proximal hypertension and, above all, ensuring a reliable way to maintain visceral and distal perfusion.12 Lower heparin administration and hemodynamic control are among the strengths that passive shunts offer4,13 potentially decreasing intraoperative coagulopathy and minimizing systemic inflammatory response.
The incidence of renal and visceral complications related to open TAAA repair remains substantial.14 We adopt both on-pump and passive shunt strategies to ensure renal and distal perfusion during TAAA open repair, depending on individual patient's characteristics. We consider passive shunting when we do not expect substantial intraoperative blood loss and when proximal clamping at the level of the aortic arch is not required.
Regardless of the visceral perfusion technique, we provide renal protection thought a cold histidine-tryptophan-ketoglutarate solution (Custodiol; Dr Franz-Kohler Chemie GmbH, Bensheim, Germany) that has proven to reduce postoperative acute kidney injury rates compared to Ringer's solution in TAAA open repair.7
In this case, visceral and distal aortic perfusion is provided by a passive shunt, wherein the inflow is taken from the left axillary artery and distal perfusion is provided to the aorta below the aneurysm. Moreover, the sequential aortic clamping and the almost constant lower limb and pelvic perfusion (briefly interrupted during the realization of the anastomosis on the aortic bifurcation and the side branches) provided great hemodynamic stability with minimal variation of the systemic pressure event at the time of decamping the visceral aorta. A single dose of heparin (5000 units) was administered reducing the risk of intraoperative and perioperative coagulopathy. Iliac arteries, which were a source of concern, were clamped for 10 minutes in total, decreasing the risk of clamp injury and thrombus formation at the level of heavily diseases vessels. In addition, during aortic cross-clamping, maintenance of antegrade blood flow into the hypogastric arteries may provide some degree of protection against spinal cord ischemia.15
For what concern the aortic repair we opted for an open type IV aortic repair with an aortoaortic bypass with reimplantation on a single patch of aortic wall of the visceral vessels and direct reimplantation of the two renal arteries on the prosthetic body.
Regarding the aortic repair, we opted for an open type IV aortic repair involving an aortoaortic bypass. The procedure included the reimplantation of the visceral vessels onto a single patch of aortic wall and the direct reimplantation of the two renal arteries onto the prosthetic graft.
Ballard's technique was not considered for three main reasons: the lack of access to a trifurcated graft, the limited expertise in this surgical technique, and the need for an additional anastomosis for the revascularization of the visceral vessels.16 Furthermore, both visceral and renal bypasses necessitate supplementary prosthetic material, potentially increasing the risk of infection. Additionally, their extra-anatomical pathways render them susceptible to kinking and tension once the retroperitoneum has been returned to its anatomical position after detorsion at the end of the procedure.
In such a complex clinical scenario, every detail counts and we believe the strategy with a passive shunt has allowed a very controlled surgery dividing a complex problem in a more accessible equation.
Conclusions
We are convinced that the use of passive shunt remains a very helpful if sometimes neglected tool when approaching TAAA surgery. This experience suggests that, in complex TAAA surgical repair, the old-fashioned technique of passive shunting to ensure visceral and distal aortic perfusion seems to offer an appealing alternative to on-pump techniques if performed in experienced centers.
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Riambau V., Böckler D., Brunkwall J., et al. Editor's choice - management of descending thoracic aorta diseases: clinical practice guidelines of the European society for vascular surgery (ESVS) Eur J Vasc Endovasc Surg. 2017;53:4–52. doi: 10.1016/j.ejvs.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Aftab M., Coselli J.S. Renal and visceral protection in thoracoabdominal aortic surgery. J Thorac Cardiovasc Surg. 2014;148:2963–2966. doi: 10.1016/j.jtcvs.2014.06.072. [DOI] [PubMed] [Google Scholar]
- 3.Hanssen S.J., Derikx J.P., Vermeulen Windsant I.C., et al. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann Surg. 2008;248:117–125. doi: 10.1097/SLA.0b013e3181784cc5. [DOI] [PubMed] [Google Scholar]
- 4.Comerota A.J., White J.V. Reducing morbidity of thoracoabdominal aneurysm repair by preliminary axillofemoral bypass. Am J Surg. 1995;170:218–222. doi: 10.1016/s0002-9610(99)80290-6. [DOI] [PubMed] [Google Scholar]
- 5.Ouriel K. The use of an aortoiliac side-arm conduit to maintain distal perfusion during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2003;37:214–218. doi: 10.1067/mva.2003.72. [DOI] [PubMed] [Google Scholar]
- 6.Tshomba Y., Kahlberg A., Melissano G., et al. Comparison of renal perfusion solutions during thoracoabdominal aortic aneurysm repair. J Vasc Surg. 2014;59:623–633. doi: 10.1016/j.jvs.2013.09.055. [DOI] [PubMed] [Google Scholar]
- 7.Kahlberg A., Tshomba Y., Baccellieri D., et al. Renal perfusion with histidine-tryptophan-ketoglutarate compared with Ringer's solution in patients undergoing thoracoabdominal aortic open repair. J Thorac Cardiovasc Surg. 2023;165:569–579.e5. doi: 10.1016/j.jtcvs.2021.02.090. [DOI] [PubMed] [Google Scholar]
- 8.Tshomba Y., Bertoglio L., Marone E.M., Melissano G., Chiesa R. Visceral aortic patch aneurysm after thoracoabdominal aortic repair: conventional vs hybrid treatment. J Vasc Surg. 2008;48:1083–1091. doi: 10.1016/j.jvs.2008.05.079. [DOI] [PubMed] [Google Scholar]
- 9.Lawrence G.H., Hessel E.A., Sauvage L.R., Krause A.H. Results of the use of the TDMAC-heparin shunt in the surgery of aneurysms of the descending thoracic aorta. J Thorac Cardiovasc Surg. 1977;73:393–398. [PubMed] [Google Scholar]
- 10.Verdant A., Pagé A., Cossette R., Dontigny L., Pagé P., Baillot R. Surgery of the descending thoracic aorta: spinal cord protection with the Gott shunt. Ann Thorac Surg. 1988;46:147–154. doi: 10.1016/s0003-4975(10)65887-0. [DOI] [PubMed] [Google Scholar]
- 11.Verdant A., Cossette R., Pagé A., Baillot R., Dontigny L., Pagé P. Aneurysms of the descending thoracic aorta: three hundred sixty-six consecutive cases resected without paraplegia. J Vasc Surg. 1995;21:385–390. doi: 10.1016/s0741-5214(95)70280-6. discussion 390-391. [DOI] [PubMed] [Google Scholar]
- 12.Montanari F., Donati T., Farina P., Tshomba Y. Surgical treatment of a chronic thoracoabdominal dissection with false lumen thrombosis and true lumen compression determining multivisceral ischemia. Perfusion. 2024;39:415–419. doi: 10.1177/02676591221137030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monnot A., Dusseaux M.M., Godier S., Plissonnier D. Passive temporary visceral shunt from the axillar artery as an adjunct method during the open treatment of thoracoabdominal aortic aneurysm. Ann Vasc Surg. 2016;36:127–131. doi: 10.1016/j.avsg.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Whitlock R.S., Coselli J.S. Review: perspectives on renal and visceral protection during thoracoabdominal aortic aneurysm repair. Indian J Thorac Cardiovasc Surg. 2019;35(Suppl 2):179–185. doi: 10.1007/s12055-018-0757-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picone A.L., Green R.M., Ricotta J.R., May A.G., DeWeese J.A. Spinal cord ischemia following operations on the abdominal aorta. J Vasc Surg. 1986;3:94–103. [PubMed] [Google Scholar]
- 16.Ballard J.L., Abou-Zamzam A.M., Jr., Teruya T.H. Type III and IV thoracoabdominal aortic aneurysm repair: results of a trifurcated/two-graft technique. J Vasc Surg. 2002;36:211–216. doi: 10.1067/mva.2002.125031. discussion 216. [DOI] [PubMed] [Google Scholar]