Abstract
Introduction
With newer biologics, the achievement of complete skin clearance has become an attainable treatment goal for patients with plaque psoriasis. We evaluate how improvements in Psoriasis Area and Severity Index (PASI) responses, particularly at incremental improvements approaching complete skin clearance (PASI 100), translate into improvements in health-related quality of life (HRQoL) and patient-perceived symptoms.
Methods
Data from the BE RADIANT phase 3b trial (NCT03536884) and its open-label extension (OLE), pooled across all study visits and treatments over 16 weeks (randomised patients) and 2 years (patients entering the OLE), were analysed using mixed-effects logistic regression models. Proportions of patients achieving a Dermatology Life Quality Index (DLQI) of 0/1, DLQI item scores of 0, and Psoriasis Symptoms and Impacts Measure (P-SIM) item scores of 0 for itching, scaling, and skin pain at specific PASI improvement levels were estimated.
Results
Seven hundred and forty-three patients were randomised to treatment; 654 entered the OLE. Using 16-week pooled data, there were incremental improvements in the proportions of patients estimated by our model to achieve DLQI 0/1 with PASI 100 compared with 95% (PASI = 95%) and 90% (PASI = 90%) improvements in PASI (93.0%, 89.3%, and 83.8% achieving DLQI 0/1, respectively). Estimated proportions achieving DLQI item scores of 0 had the greatest increases at higher PASI improvement levels for Items 1 (itchy, sore, painful, or stinging skin), 2 (embarrassment), and 4 (choice of clothing). Estimated proportions of patients achieving P-SIM = 0 were also higher for PASI 100 (itching: 61.7%; scaling: 82.2%; skin pain: 96.9%) than for PASI = 95% (50.8%; 72.3%; 95.7%) and PASI = 90% (39.8%; 59.5%; 94.0%). Similar benefits of incremental PASI improvements were estimated using 2-year data.
Conclusions
Complete skin clearance translated into the greatest benefits to HRQoL and patient-perceived symptoms, over and above skin clearance between 90% and 100%, highlighting the importance of targeting PASI 100 as a treatment outcome for patients with psoriasis.
Trial Registration Number
Complete skin clearance is associated with the greatest benefits to health-related quality of life and perceived symptoms for patients with psoriasis: KeyResults and Conclusions (MP4 72828 kb)
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01261-6.
Keywords: Biologics, Clinical trials, Psoriasis, Quality of life
Key Summary Points
| Why carry out this study? |
| The introduction of newer biologic therapies has made the achievement of complete skin clearance an attainable goal for a growing number of patients. |
| Improvement of health-related quality of life (HRQoL) is a major objective in the management of psoriasis and should be considered alongside clinical efficacy parameters such as skin clearance. |
| In this analysis we evaluated how improvements in Psoriasis Area and Severity Index (PASI) responses, particularly at incremental improvements approaching complete skin clearance (PASI 100), translate into improvements in HRQoL and patient-perceived symptoms. |
| What was learned from the study? |
| Incremental PASI improvements corresponded with more patients achieving benefits in HRQoL and perceived symptoms, including at the highest levels of skin clearance between 90% (PASI 90) and 100% (PASI 100). |
| These findings indicate that patients can experience further benefits to HRQoL and perceived symptoms even beyond those achieved at the commonly used PASI 90 treatment goal and highlight the importance of complete skin clearance, or PASI 100, as a treatment outcome for patients with plaque psoriasis. |
Digital Features
This article is published with digital features, including a video to facilitate understanding of the article. To view digital features for this article go to 10.6084/m9.figshare.26780089.
Introduction
Plaque psoriasis is a chronic, systemic, inflammatory skin disease resulting in debilitating physical symptoms, such as itchy, sore, painful, and scaly skin, and is associated with increased rates of psoriatic arthritis and cardiometabolic diseases [1, 2]. For patients with mild psoriasis, topical agents, including corticosteroids, vitamin D analogues, and calcineurin inhibitors, as well as phototherapy, remain the mainstay of treatment, whilst for moderate to severe psoriasis biologics are recommended as the first-line treatment option [1]. Over the past 2 decades, there have been major advances in the treatment of plaque psoriasis; the introduction of newer biologic therapies, which target and modify the underlying dysregulated inflammatory response, has made the achievement of complete skin clearance (PASI 100; 100% improvement from baseline in Psoriasis Area and Severity Index) an attainable goal for a growing number of patients [3].
In addition to physical symptoms of psoriasis, patients can experience stigmatisation, embarrassment, and psychological strain, all of which impact their emotional well-being and overall health-related quality of life (HRQoL) [4]. The combination of physical and psychological impairment can accumulate over the course of patients’ lives and may have a significant impact during critical periods, such as adolescence or early adulthood, when patients are consolidating their personality, establishing social contacts, and planning for higher education or their career [5, 6]. As a result, improvement of HRQoL is a major objective in the management of psoriasis and should be considered alongside clinical efficacy parameters such as skin clearance to provide a comprehensive view of treatment outcomes [4, 7–9]. Understanding the relationship between different levels of clinical response and HRQoL is important in determining whether psoriasis treatments that lead to physical disease control further translate into patient-perceived improvements in their daily life and well-being [4, 9].
Patient-reported outcome measures, such as the Dermatology Life Quality Index (DLQI), have been developed to assess HRQoL in patients with dermatological conditions. This measure, however, is not specific to psoriasis and may not fully capture all relevant aspects of patient experiences for this disease [10, 11]. Psoriasis-specific tools also exist, such as the Psoriasis Symptoms and Impacts Measure (P-SIM), a valid, reliable, well-defined patient-reported outcome tool that was developed to capture key signs, symptoms (including itching, scaling, and skin pain), and impacts of psoriasis [9, 12].
A previous review has highlighted the advantages of complete skin clearance compared to high levels of efficacy without complete skin clearance; additional benefits of complete skin clearance included greater improvements in patient-reported symptoms of psoriasis, a higher proportion of symptom-free days, and significant improvements in HRQoL [3]. Furthermore, up to 95% of patients consider clear skin as a ‘highly important’ outcome of their treatment [13, 14]. This indicates the value of complete skin clearance to patients over other key treat-to-target outcomes, such as PASI 90 and absolute PASI ≤ 2 [8]. However, the exact relationship between very high levels of skin clearance and resulting improvements in HRQoL has not been described.
Here, we evaluated how improvements in PASI responses, in particular incremental improvements at very high levels of response between 90 and 100% improvement from baseline, translate into improvements in HRQoL and patient-perceived symptoms specific to psoriasis, irrespective of treatment received. We report analyses using data over up to 2 years from patients with moderate to severe plaque psoriasis from the BE RADIANT phase 3b clinical trial of bimekizumab, a dual inhibitor of IL-17A and IL-17F which has demonstrated substantial efficacy in treating psoriasis [15, 16].
Methods
In this post hoc analysis, we used mixed-effects logistic regression models to assess the relationship between incremental improvements in skin clearance and improvements in HRQoL, irrespective of treatment received by patients. Inputs for the models were taken from all treatment arms of the BE RADIANT (NCT03536884) phase 3b trial of bimekizumab in psoriasis.
Patients
Data from the first 96 weeks of the BE RADIANT trial were analysed. Full inclusion and exclusion criteria for BE RADIANT have been published previously [15, 16]. Eligible patients were ≥ 18 years of age with moderate to severe plaque psoriasis at baseline (defined as absolute PASI ≥ 12, body surface area [BSA] affected by psoriasis ≥ 10%, and Investigator’s Global Assessment [IGA] score ≥ 3) and were eligible for systemic psoriasis therapy and/or phototherapy.
Study Design
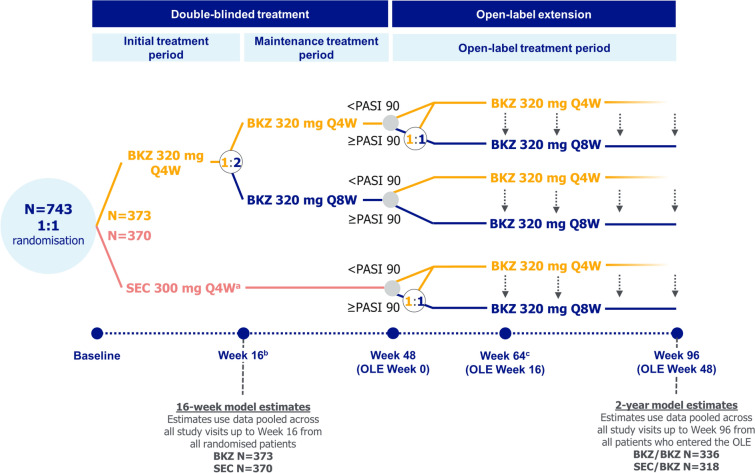
The full study design for the 48-week double-blinded period of BE RADIANT, which compared bimekizumab versus secukinumab, and the first 48 weeks of its open-label extension (OLE), has been reported previously [15, 16]. Patients were initially randomised to bimekizumab or secukinumab. Patients randomised to bimekizumab received 320 mg dosed every 4 weeks (Q4W) to Week 16, then Q4W or every 8 weeks (Q8W) to Week 48; patients randomised to secukinumab received 300 mg weekly to Week 4, then Q4W to Week 48 (Fig. 1).
Fig. 1.
BE RADIANT study design. aSEC 300 mg was administered at baseline, Weeks 1, 2, 3, and 4, then Q4W for the remainder of the double-blinded treatment period; bre‑randomisation at Week 16 was due to a protocol amendment; some patients had already passed Week 16 at the time of protocol amendment implementation; cfollowing a protocol amendment, all patients receiving BKZ 320 mg Q4W in the OLE period were switched to BKZ 320 mg Q8W at Week 64 or the next scheduled clinic visit. BKZ bimekizumab, OLE open-label extension, PASI 90 ≥ 90% improvement from baseline in Psoriasis Area and Severity Index, Q4W every 4 weeks, Q8W every 8 weeks, SEC secukinumab
The OLE began at Week 48, in which all patients received open-label bimekizumab 320 mg dosed Q4W or Q8W. Dose frequency in the OLE was dependent on PASI response at Week 48 and treatment and dose frequency at the end of the double-blinded period. Following a protocol amendment, all patients receiving bimekizumab 320 mg Q4W were switched to bimekizumab Q8W at Week 64 or the next scheduled clinic visit.
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by an Independent Review Board and Independent Ethics Committee. All participants provided informed written consent.
Outcomes
The PASI is a clinician-evaluated tool commonly used to assess the severity of psoriasis, measuring the average redness, thickness, and scaliness of psoriatic lesions weighted by area of involvement [17]. PASI divides the body into four regions (head and neck, trunk, upper limbs, and lower limbs), which are individually scored before being combined to give the weighted overall score from 0 to 72 [18]. Relative PASI (the percentage improvement in PASI from baseline) is also used to define disease activity endpoints, such as PASI 100, which represents a 100% improvement in PASI from baseline, or complete skin clearance [8].
The DLQI consists of ten items asking patients questions regarding how their skin disease impacts various aspects of their daily life. Each item is scored by the patient from 0, indicating ‘not at all’ (all items) or ‘not relevant’ (items 3–10 only), to 3, indicating ‘very much’ or ‘prevented’ (item 7 only) [19]. In some cases, an item score of ‘not relevant’ may mean that the activity in question is not possible, sometimes due to severe disease, rather than there being no impact of disease on the activity. The DLQI ranges from 0–30, with a total score of 0 or 1 defined as ‘no impact of skin disease on a patient’s life’ [20]. The DLQI, however, is not specific to psoriasis and aspects captured by each item may differ in terms of how burdensome they are to patients with psoriasis. Therefore, in addition to the total score, all item scores are considered individually in these analyses. DLQI Item 1 (‘over the last week, how itchy, sore, painful, or stinging has your skin been’) is specific to skin symptoms commonly seen in psoriasis and so was considered to be of particular relevance for analysis here.
The 14-item P-SIM is a novel, reliable, well-defined patient-reported outcome tool developed to capture key signs, symptoms, and life impacts specific to plaque psoriasis. Items are scored on an 11-point numeric rating scale from 0 to 10 by patients, with 0 meaning ‘no sign, symptom, or impact’ and 10 meaning ‘very severe sign, symptom, or impact’ [9, 12]. In the BE RADIANT trial, 3 of the 14 P-SIM items were assessed and are therefore reported in these analyses (itching, scaling, and skin pain). P-SIM items were scored based on symptoms experienced in the 24 h prior to the visit at which it was assessed. Data collection schedules are available in Table S1.
Statistical Analysis
We used mixed-effects logistic regression models to assess the relationship between skin clearance (measured via PASI) and achievement of DLQI 0/1, DLQI item scores of 0, or a score of 0 in P-SIM itching, scaling, and skin pain items. The models included PASI percentage change from baseline and baseline DLQI, DLQI item scores, or P-SIM item scores as covariates, respectively, with a patient-level random intercept to account for repeated observations at the patient level.
Data to be inputted into the models were first taken from all randomised patients pooled across all study visits up to Week 16, regardless of whether they were treated with bimekizumab or secukinumab. Models were also then run using data pooled across all study visits and treatments over 2 years (up to Week 96) for patients who received ≥ 1 dose of study treatment in the OLE. Model analyses used observed case data from the relevant patient population.
Using observed patient data, the models estimated the proportions of patients who achieve DLQI 0/1, DLQI item scores of 0, and a P-SIM score of 0 for the itching, scaling, and skin pain items at specific PASI improvement levels (e.g. PASI = 75%, PASI = 90%). The model estimates are presented here alongside 95% confidence intervals (CIs). In this report, PASI improvement levels correspond with discrete levels of PASI achievement rather than bands of improvement (e.g., when reporting results for PASI = 90%, this refers to exactly 90% improvement in PASI from baseline rather than ≥ 90% improvement).
Model estimates for proportions of patients achieving DLQI 0/1, DLQI item scores of 0, and P-SIM item scores of 0 were obtained with the value of the covariate corresponding to the baseline scores for each measure set equal to the baseline median for the respective population, therefore reflecting a typical patient enrolled in BE RADIANT and its OLE.
Results
Patient Disposition and Baseline Characteristics
In BE RADIANT, 743 patients were randomised to treatment: 373 to bimekizumab and 370 to secukinumab. Mean and median DLQI were both 11.0; mean and median DLQI Item 1 scores were both 2.0. Mean and median baseline P-SIM scores were 6.7 and 7.0, respectively, for itching; 6.7 and 7.0 for scaling; and 4.6 and 5.0 for skin pain.
Six hundred and fifty-four of 743 randomised patients entered the OLE (336 bimekizumab-randomised; 318 secukinumab-randomised). Baseline DLQI and P-SIM scores for this group were similar to those for all randomised patients (Table 1).
Table 1.
Baseline characteristics
| BE RADIANT randomised patientsa (N = 743) | BE RADIANT OLE patientsb (N = 654) | |
|---|---|---|
| Age (years), mean ± SD | 45.0 ± 14.5 | 45.0 ± 14.4 |
| Male, n (%) | 486 (65.4) | 436 (66.7) |
| White, n (%) | 695 (93.5) | 613 (93.7) |
| Weight (kg), mean ± SD | 89.5 ± 20.7 | 89.7 ± 20.3 |
| Duration of psoriasis (years), mean ± SD | 17.8 ± 12.7 | 18.0 ± 12.6 |
| PASI, mean ± SD | 20.0 ± 7.1 | 19.9 ± 7.0 |
| IGA score, n (%) | ||
| 3: Moderate | 508 (68.4) | 448 (68.5) |
| 4: Severe | 233 (31.4) | 204 (31.2) |
| BSA (%), mean ± SD | 24.3 ± 14.9 | 24.2 ± 14.8 |
| DLQI, mean ± SD | 11.0 ± 6.9 | 11.0 ± 7.0 |
| DLQI, median | 11.0 | 10.0 |
| DLQI (Item 1), n (%) | ||
| 0 | 20 (2.7) | 18 (2.8) |
| 1 | 171 (23.0) | 156 (23.9) |
| 2 | 314 (42.3) | 275 (42.0) |
| 3 | 238 (32.0) | 205 (31.3) |
| DLQI item scores, median | ||
| 1: Itchy, sore, painful, or stinging | 2.0 | 2.0 |
| 2: Embarrassed | 2.0 | 2.0 |
| 3: Shopping, gardening | 1.0 | 1.0 |
| 4: Choice of clothing | 2.0 | 2.0 |
| 5: Social or leisure activities | 1.0 | 1.0 |
| 6: Sport | 0.0 | 0.0 |
| 7: Prevent/problem with working or studying | 0.0 | 0.0 |
| 8: Problems with partners or friends | 1.0 | 1.0 |
| 9: Sexual difficulties | 0.0 | 0.0 |
| 10: Treatment | 1.0 | 1.0 |
| P-SIM item score, mean ± SD | ||
| Itching | 6.7 ± 2.7 | 6.7 ± 2.8 |
| Scaling | 6.7 ± 2.4 | 6.7 ± 2.4 |
| Skin pain | 4.6 ± 3.2 | 4.6 ± 3.2 |
| P-SIM item score, median | ||
| Itching | 7.0 | 7.0 |
| Scaling | 7.0 | 7.0 |
| Skin pain | 5.0 | 5.0 |
| Any prior systemic therapy, n (%) | 540 (72.7) | 478 (73.1) |
| Prior biologic therapy, n (%) | 245 (33.0) | 219 (33.5) |
BSA body surface area, DLQI Dermatology Life Quality Index, IGA Investigator's Global Assessment, OLE open-label extension, PASI Psoriasis Area and Severity Index, P-SIM Psoriasis Symptoms and Impacts Measure, Q4W every 4 weeks, Q8W every 8 weeks, SD standard deviation
aPatients were initially randomised to bimekizumab 320 mg Q4W to Week 16, then Q4W or Q8W to Week 48, or to secukinumab 300 mg weekly to Week 4, then Q4W to Week 48
bBE RADIANT OLE patients completed the double-blinded maintenance treatment period and received ≥ 1 dose of bimekizumab treatment in the OLE
DLQI 0/1 at Different PASI Improvement Levels
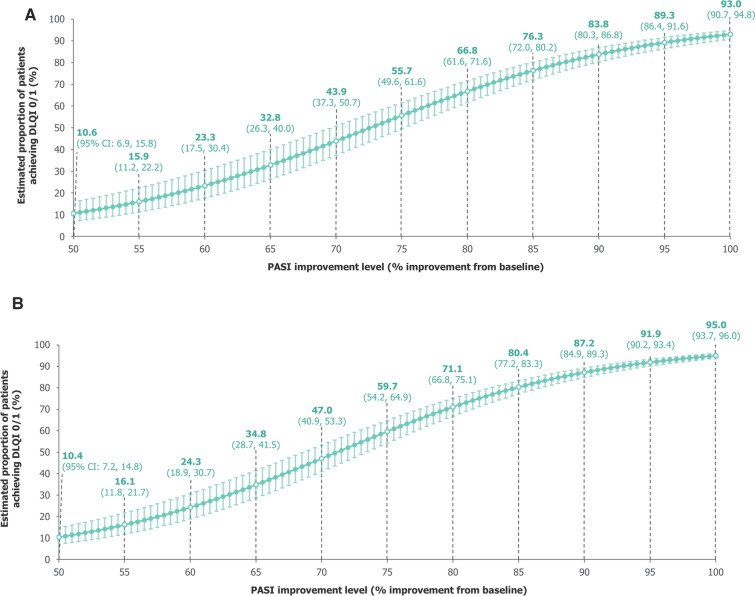
Using data pooled over 16 weeks, our model estimated the proportion of patients achieving DLQI 0/1 to be 93.0% (95% CI: 90.7%, 94.8%) with PASI 100, 89.3% (86.4%, 91.6%) with PASI = 95%, 83.8% (80.3%, 86.8%) with PASI = 90%, and 55.7% (49.6%, 61.6%) with PASI = 75% (Fig. 2A). Estimates were similar using data pooled over 2 years (Fig. 2B).
Fig. 2.
Model-estimated proportions of patients achieving DLQI 0/1. A Over 16 weeks. B Over 2 years. A mixed-effects logistic regression model used data pooled across all trial visits and treatments over A 16 weeks and B 2 years of BE RADIANT to estimate the proportions of patients achieving DLQI 0/1 at specific PASI improvement levels. Error bars correspond to 95% CIs. The curves correspond to model estimates calculated with baseline DLQI equal to the baseline median for the respective populations (16 weeks: 11.0 [all randomised patients]; 2 years: 10.0 [patients who entered the OLE]). CI confidence interval, DLQI Dermatology Life Quality Index, OLE open-label extension, PASI Psoriasis Area and Severity Index
DLQI Item Scores = 0 at Different PASI Improvement Levels
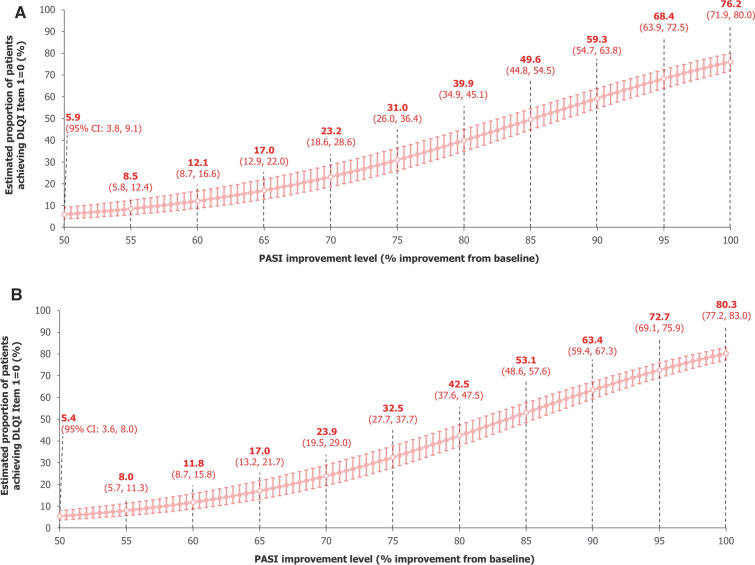
DLQI Item 1 (‘over the last week, how itchy, sore, painful, or stinging has your skin been’) is specific to skin symptoms commonly seen in psoriasis. Using data pooled over 16 weeks, our model estimated the proportion of patients achieving DLQI Item 1 = 0 to be 76.2% (95% CI: 71.9%, 80.0%) with PASI 100, 68.4% (63.9%, 72.5%) with PASI = 95%, 59.3% (54.7%, 63.8%) with PASI = 90%, and 31.0% (26.0%, 36.4%) with PASI = 75% (Fig. 3A). Estimates were similar using data pooled over 2 years (Fig. 3B).
Fig. 3.
Model-estimated proportions of patients achieving DLQI Item 1 (itchy, sore, painful, or stinging skin)a = 0. A Over 16 weeks. B Over 2 years, a mixed-effects logistic regression model used data pooled across all trial visits and treatments over A 16 weeks and B 2 years of BE RADIANT to estimate the proportions of patients achieving DLQI Item 1 = 0 at specific PASI improvement levels. Error bars correspond to 95% CIs. The curves correspond to model estimates calculated with baseline DLQI Item 1 score equal to the baseline median for the respective populations (16 weeks: 2.0 [all randomised patients]; 2 years: 2.0 [patients who entered the OLE]). aQuestion: ‘over the last week, how itchy, sore, painful, or stinging has your skin been’; answer: ‘not at all’ (0), ‘a little’ (1), ‘a lot’ (2), or ‘very much’ (3). CI confidence interval, DLQI Dermatology Life Quality Index, OLE open-label extension, PASI Psoriasis Area and Severity Index
At PASI = 75%, our model estimated proportions of patients achieving DLQI item scores of 0 were lowest for Items 1, 2 (embarrassment), and 4 (choice of clothing), indicating that these items are most burdensome to patients with psoriasis. The greatest increases in model estimates at higher PASI improvement levels were seen for these items too; estimates were consistently high for all other DLQI items, even at PASI = 75%, demonstrating that changes in achievement of DLQI 0/1 were driven by changes in scores for Items 1, 2, and 4 (Figure S1).
P-SIM Item Scores = 0 at Different PASI Improvement Levels
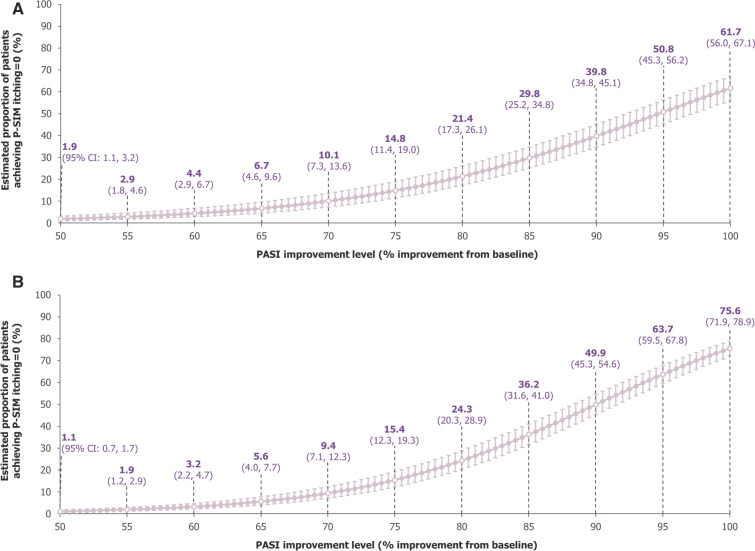
For the itching P-SIM item, large incremental improvements in model estimates for proportions of patients achieving P-SIM = 0 were seen at high levels of skin clearance, indicating the high importance of complete skin clearance for relief of itching in patients with psoriasis. Using data pooled across the first 16 weeks, our model estimated the proportion of patients achieving P-SIM = 0 to be 61.7% (95% CI: 56.0%, 67.1%) with PASI 100, 50.8% (45.3%, 56.2%) with PASI = 95%, 39.8% (34.8%, 45.1%) with PASI = 90%, and 14.8% (11.4%, 19.0%) with PASI = 75% (Fig. 4A). Using data pooled over up to 2 years, at higher levels of PASI improvement, estimated achievement of P-SIM = 0 for itching was numerically higher compared with data pooled over 16 weeks (Fig. 4B).
Fig. 4.
Model-estimated proportions of patients achieving P-SIM itching = 0. A Over 16 weeks. B Over 2 years. A mixed-effects logistic regression model used data pooled across all trial visits and treatments over A 16 weeks and B 2 years of BE RADIANT to estimate the proportions of patients achieving P-SIM itching = 0 at specific PASI improvement levels. Error bars correspond to 95% CIs. The curves correspond to model estimates calculated with baseline P-SIM itching score equal to the baseline median for the respective populations (16 weeks: 7.0 [all randomised patients]; 2 years: 7.0 [patients who entered the OLE]). CI confidence interval, OLE open-label extension, PASI Psoriasis Area and Severity Index, P-SIM Psoriasis Symptoms and Impacts Measure
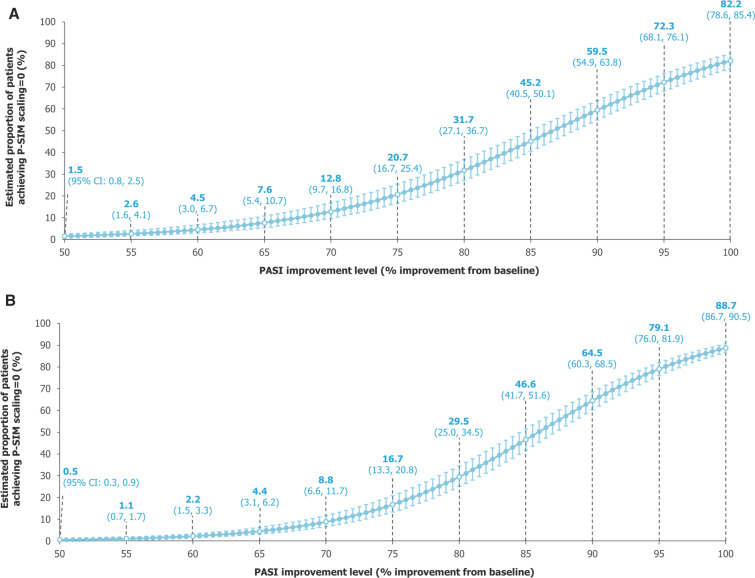
For scaling, our model estimated that the proportions of patients achieving P-SIM = 0 also saw large increases at the highest levels of skin clearance, with 82.2% (78.6%, 85.4%) achieving P-SIM = 0 with PASI 100, 72.3% (68.1%, 76.1%) with PASI = 95%, 59.5% (54.9%, 63.8%) with PASI = 90%, and 20.7% (16.7%, 25.4%) with PASI = 75% (Fig. 5A); at higher PASI improvement levels, estimates using data pooled over 2 years were numerically higher than the 16-week estimates, although the difference was smaller than observed with the itching item (Fig. 5B).
Fig. 5.
Model-estimated proportions of patients achieving P-SIM scaling = 0. A Over 16 weeks. B Over 2 years. A mixed-effects logistic regression model used data pooled across all trial visits and treatments over A 16 weeks and B 2 years of BE RADIANT to estimate the proportions of patients achieving P-SIM scaling = 0 at specific PASI improvement levels. Error bars correspond to 95% CIs. The curves correspond to model estimates calculated with baseline P-SIM scaling score equal to the baseline median for the respective populations (16 weeks: 7.0 [all randomised patients]; 2 years: 7.0 [patients who entered the OLE]). CI confidence interval, OLE open-label extension, PASI Psoriasis Area and Severity Index, P-SIM Psoriasis Symptoms and Impacts Measure
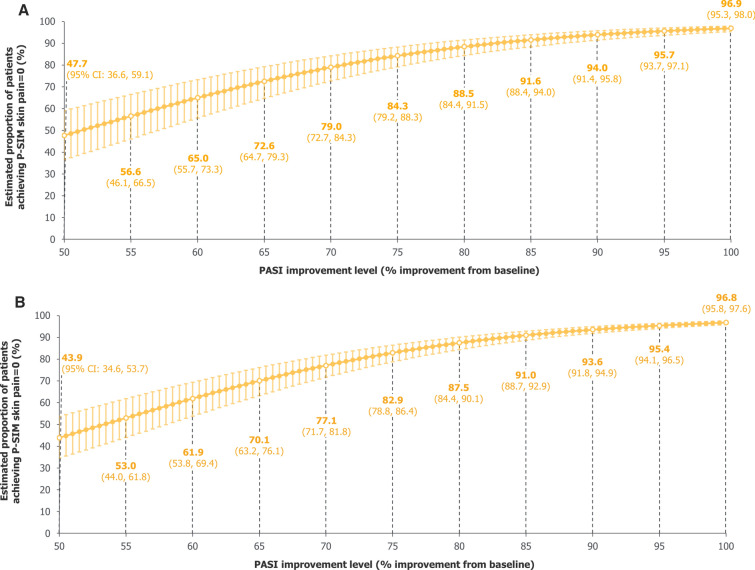
For skin pain, improvements in estimated proportions of patients achieving P-SIM = 0 were also seen with incremental improvements in skin clearance, although these estimates were consistently higher than for the itching and scaling items using both data pooled over 16 weeks and 2 years (Fig. 6).
Fig. 6.
Model-estimated proportions of patients achieving P-SIM skin pain = 0. A Over 16 weeks. B Over 2 years. A mixed-effects logistic regression model used data pooled across all trial visits and treatments over A 16 weeks and B 2 years of BE RADIANT to estimate the proportions of patients achieving P-SIM skin pain = 0 at specific PASI improvement levels. Error bars correspond to 95% CIs. The curves correspond to model estimates calculated with baseline P-SIM skin pain score equal to the baseline median for the respective populations (16 weeks: 5.0 [all randomised patients]; 2 years: 5.0 [patients who entered the OLE]). CI confidence interval, OLE open-label extension, PASI Psoriasis Area and Severity Index, P-SIM Psoriasis Symptoms and Impacts Measure
Discussion
In these analyses, we showed that incremental improvements in skin clearance at the highest levels of PASI improvement between 90 and 100% translated into additional benefits in HRQoL and patient-perceived symptoms specific to psoriasis, with the greatest benefits seen with complete skin clearance (PASI 100), using estimates from mixed-effects logistic regression models looking across all treatment arms in BE RADIANT.
Moderate incremental improvements in model estimates for the attainment of DLQI 0/1 (no impact on patient’s life) were seen at the highest levels of skin clearance between 90% and 100%, using data pooled over both 16 weeks and 2 years. These results are in line with previous studies, indicating that complete skin clearance is highly valued by patients over and above near-complete skin clearance for its impact on HRQoL. Indeed, incremental improvement from near-complete (PASI 90– < 100) to complete skin clearance (PASI 100) has previously been demonstrated to result in 11%–18% differences in proportions of patients reporting DLQI 0/1 [21, 22].
The DLQI is not specific to psoriasis, and for many of the items analysed here, estimated proportions of patients with DLQI item score = 0 were high regardless of PASI improvement level. Meanwhile, estimated proportions of patients achieving scores of 0 for DLQI Items 1, 2, and 4, considered of particular relevance for psoriasis, substantially increased at higher PASI improvement levels. Importantly, these results indicate that Items 1, 2, and 4 have the highest impact on changes to HRQoL for patients with psoriasis. DLQI Item 1 (itchy, sore, painful, or stinging skin) had the greatest incremental improvements at higher PASI improvement levels; however, even with complete skin clearance (PASI 100), the model-estimated proportion of patients reporting no symptom impact was 76.2%, indicating that about a quarter of patients still report itching, soreness, pain, or stinging despite clear skin, in line with the P-SIM itching item results reported here and DLQI results in other studies [23].
Although 60%–75% of patients were estimated by our model to achieve itch resolution alongside complete skin clearance, estimated proportions of patients achieving P-SIM = 0 for the itching item were relatively low at high PASI improvements compared with other items, aligned with the high baseline scores in this item. The large increases in estimated proportions achieving P-SIM = 0 observed with incremental PASI improvements are consistent with previous reports that itching is the most important factor contributing to disease severity for patients [24]. Furthermore, using 2‑year pooled data including later visits where subjects may have had sustained periods of skin clearance, higher estimated proportions achieving P-SIM = 0 for itching with complete skin clearance were reported compared with 16-week pooled data (75.6% vs 61.7%). Nevertheless, these data suggest that residual itching persists over time, even with complete skin clearance, in about 25% of patients. Future research should explore why itching persists in some patients even after complete skin clearance is achieved and seek granularity on the sub-components of itching that persist.
For the skin pain P-SIM item, the curves produced from the model estimates were flatter and consistently higher than those for itching or scaling, potentially owing to the lower median score at baseline, which was used in the model to calculate the estimates. This ultimately suggests that, for a typical patient in these populations, pain was less burdensome than itching or scaling; hence, the level of skin clearance at which patients are estimated to experience no effect of the symptom was lower. This may be due to pain resulting more directly from inflammation rather than skin symptoms themselves; however, it is acknowledged that skin pain is complex and poorly defined [25] and requires further investigation to understand the differential quantitative and qualitative attributes that define pain.
PASI 90 has become widely accepted as the current universal treatment goal in psoriasis [26]. The ability of novel biologics such as bimekizumab to provide rapid, complete skin clearance, which is maintained with long-term use, represents an opportunity to raise treatment expectations for patients with psoriasis. Bimekizumab has previously demonstrated superior efficacy versus placebo, secukinumab, adalimumab, and ustekinumab in phase 3/3b trials. In these trials, bimekizumab treatment also led to greater proportions of patients achieving PASI 100 at Week 16 versus placebo- (68.2% vs 1.2%), secukinumab- (61.7% vs 48.9%), adalimumab- (60.8% vs 23.9%), and ustekinumab-treated patients (58.6% vs 20.9%), respectively (non-responder imputation [NRI]) [16, 27–29]. These high levels of skin clearance have been demonstrated to be durable over 3 years [30]. Taken together with the data presented here, this suggests that higher treatment goals, including PASI 100, could be used routinely in clinical practice to provide additional benefits for patients with psoriasis.
The large sample size, using model estimates with up to 2 years of data, represents a strength of this study. Additionally, the modelling approaches used here were relatively simple, aiding ease of interpretation of the results. However, this simplicity may not provide as good a fit as other, more complex models. Limitations of these analyses include that data were not equally available for each incremental level of PASI improvement; greater availability of data at high levels of PASI improvement may have impacted the strength of the trend seen here. Furthermore, the 2-year analyses only included patients who entered the OLE as opposed to all randomised patients in the 16-week analyses; patients who responded less well to study treatments may have opted not to enrol in the OLE, and patients remaining in the study into the OLE may have been more likely to report higher levels of skin clearance, contributing to the unequal distribution of data for different PASI response levels; this patient population may also reflect patients who are more optimistic that their disease will improve, which may lead to higher rates of patient-reported benefits to their HRQoL and perceived symptoms. Moreover, these models made predictions with the covariate corresponding to the baseline score of the outcome measure set equal to the baseline median, therefore reflecting a typical patient enrolled in BE RADIANT and its OLE in terms of their perceived symptoms and HRQoL at baseline. As such, these findings may be limited in their generalisability to real-world populations because of stringent patient eligibility criteria.
Conclusion
Incremental PASI improvements corresponded with more patients achieving benefits in HRQoL and perceived symptoms; this trend was seen at the highest levels of skin clearance between 90 and 100%, indicating that patients can experience further benefits in HRQoL and patient-perceived symptoms beyond those achieved at the commonly used PASI 90 treatment goal. These findings highlight the importance of complete skin clearance, or PASI 100, as a treatment outcome for patients with plaque psoriasis to achieve the greatest benefits to HRQoL and perceived symptoms.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients, the investigators, and their teams who took part in this study.
Medical Writing, Editorial and Other Assistance
The authors also acknowledge Joe Dixon, PhD, UCB, Slough, UK, for publication coordination and Emma Francis-Gregory, BA, Alexa Holland, MSc, and Daniel Smith, MA, Costello Medical, UK, for medical writing and editorial assistance based on the authors’ input and direction. Support for third-party writing assistance for this article was funded by UCB in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
Author Contributions
Substantial contributions to study conception and design: Matthias Augustin, Alice B. Gottlieb, Mark Lebwohl, Andreas Pinter, Richard B. Warren, Luis Puig, Rhys Warham, Jérémy Lambert, Susanne Wiegratz, Balint Szilagyi, Andrew Blauvelt; substantial contributions to analysis and interpretation of the data: Matthias Augustin, Alice B. Gottlieb, Mark Lebwohl, Andreas Pinter, Richard B. Warren, Luis Puig, Rhys Warham, Jérémy Lambert, Susanne Wiegratz, Balint Szilagyi, Andrew Blauvelt; drafting the article or revising it critically for important intellectual content: Matthias Augustin, Alice B. Gottlieb, Mark Lebwohl, Andreas Pinter, Richard B. Warren, Luis Puig, Rhys Warham, Jérémy Lambert, Susanne Wiegratz, Balint Szilagyi, Andrew Blauvelt; final approval of the version of the article to be published: Matthias Augustin, Alice B. Gottlieb, Mark Lebwohl, Andreas Pinter, Richard B. Warren, Luis Puig, Rhys Warham, Jérémy Lambert, Susanne Wiegratz, Balint Szilagyi, Andrew Blauvelt.
Funding
This study was sponsored by UCB. This article was based on the original study BE RADIANT (NCT03536884), sponsored by UCB. This research was supported by the NIHR Manchester Biomedical Research Centre (NIHR203308). The journal’s Rapid Service Fee was also funded by UCB.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Underlying data from this manuscript may be requested by qualified researchers six months after product approval in the US and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymised individual patient-level data and redacted trial documents, which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password protected portal.
Declarations
Conflict of Interest
Matthias Augustin: Consulting fees from AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Centocor, Eli Lilly and Company, GSK, Hexal, Janssen, LEO Pharma, Medac, Merck, MSD, Mundipharma, Novartis, Pfizer, Sandoz, UCB, and Xenoport. Alice B. Gottlieb: Research/educational grants from AnaptysBio, Bristol Myers Squibb, Highlights Therapeutics, Janssen, MoonLake Immunotherapeutics, Novartis, and UCB, (all paid to Mount Sinai School of Medicine); honoraria as an advisory board member and consultant for Amgen, AnaptysBio, Avotres Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, DICE Therapeutics, Eli Lilly and Company, Highlights Therapeutics, Janssen, Novartis, Sanofi, UCB, and Xbiotech. Mark Lebwohl: Employee of Mount Sinai and receives research funds from AbbVie, Amgen, Arcutis, Avotres Therapeutics, Boehringer Ingelheim, Cara Therapeutics, Dermavant, Eli Lilly and Company, Incyte, Inozyme, Janssen Research & Development, LLC, Ortho Dermatologics, Pfizer, Sanofi-Regeneron, and UCB; consultant for Almirall, AltruBio Inc., AnaptysBio, Apogee, Arcutis Inc., AstraZeneca, Atomwise, Avotres Therapeutics, Boehringer Ingelheim, Brickell Biotech, Bristol Myers Squibb, Castle Biosciences, Celltrion, CorEvitas, Dermavant, EPI, Evommune Inc., Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Galderma, Genentech, Incyte, LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Sanofi-Regeneron, Seanergy, Strata, Takeda, Trevi, and Verrica. Mark Lebwohl is an Editorial Board member of Dermatology and Therapy. He was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Andreas Pinter: Investigator and/or speaker and/or advisor for AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Celgene, Eli Lilly and Company, Galderma, GSK, Hexal, Janssen, LEO Pharma, MC2, Medac, Merck Serono, Mitsubishi Pharma, MSD, MoonLake Immunotherapeutics, Novartis, Pfizer, Regeneron, Roche, Sandoz, Schering-Plough, Tigercat Pharma, and UCB. Richard B. Warren: Consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, DICE Therapeutics, Eli Lilly and Company, GSK, Janssen, LEO Pharma, Meiji Pharma, Novartis, Pfizer, RAPT Therapeutics, Sanofi, Sun Pharma, UCB, and Union; research grants to his institution from AbbVie, Almirall, Amgen, Celgene, Eli Lilly and Company, Janssen, LEO Pharma, Novartis, Pfizer, and UCB; honoraria from AbbVie, Almirall, Bristol Myers Squibb, Eli Lilly and Company, Galderma, Janssen, and Novartis. Luis Puig: Received consultancy/speaker’s honoraria from and/or participated in trials sponsored by AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Fresenius Kabi, Horizon, Janssen, LEO Pharma, Novartis, Pfizer, Sandoz, STADA, Sun Pharma, and UCB. Luis Puig is an Editorial Board member of Dermatology and Therapy. He was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions. Rhys Warham: Veramed statistical consultant for UCB. Jérémy Lambert, Susanne Wiegratz, Balint Szilagyi: Employees and shareholders of UCB. Andrew Blauvelt: Has served as a speaker (received honoraria) for Eli Lilly and Company, and UCB; served as a scientific adviser (received honoraria) for AbbVie, Abcentra, Aclaris, Affibody, Aligos, Almirall, Alumis, Amgen, Anaptysbio, Apogee, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Celldex, CTI BioPharma, Dermavant, EcoR1, Eli Lilly and Company, Escient, Evelo, Evommune, Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, LEO Pharma, Lipidio, Microbion, Merck, Monte Rosa Therapeutics, Nektar, Novartis, Overtone Therapeutics, Paragon, Pfizer, Q32 Bio, Rani, Rapt, Regeneron, Sanofi Genzyme, Spherix Global Insights, Sun Pharma, Takeda, TLL Pharmaceutical, TrialSpark, UCB, Union, Ventyx, Vibliome, and Xencor; acted as a clinical study investigator (institution has received clinical study funds) for AbbVie, Acelyrin, Allakos, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Concert, Dermavant, DermBiont, Eli Lilly and Company, Evelo, Evommune, Galderma, Incyte, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, Takeda, UCB, and Ventyx; owns stock in Lipidio and Oruka.
Ethical Approval
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by an Independent Review Board and Independent Ethics Committee. All participants provided informed written consent.
References
- 1.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–60. [DOI] [PubMed] [Google Scholar]
- 2.Kimmel GW, Lebwohl M. Psoriasis: Overview and diagnosis. Evidence-Based Psoriasis. 2018:1–16.
- 3.Blauvelt A, Wu JJ, Armstrong A, Menter A, Liu C, Jacobson A. Importance of complete skin clearance in psoriasis as a treatment goal: implications for patient-reported outcomes. J Drugs Dermatol. 2020;19(5):487–92. [PubMed] [Google Scholar]
- 4.Augustin M, Radtke MA. Quality of life in psoriasis patients. Expert Rev Pharmacoecon Outcomes Res. 2014;14(4):559–68. [DOI] [PubMed] [Google Scholar]
- 5.Romiti R, Magalhães RF, Duarte GV. Cumulative life course impairment in patients with dermatological diseases, with a focus on psoriasis. An Bras Dermatol. 2024;99(2):269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommer R, Mrowietz U, Gaarn Du Jardin K, Kasujee I, Martini E, Daudén E, et al. Implementing well‐being in the management of psoriasis: an expert recommendation. J Eur Acad Dermatol Venereol 2024;38(2):302–10. [DOI] [PubMed]
- 7.Armstrong AW, Siegel MP, Bagel J, Boh EE, Buell M, Cooper KD, et al. From the medical board of the National psoriasis foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76(2):290–8. [DOI] [PubMed] [Google Scholar]
- 8.Mahil SK, Wilson N, Dand N, Reynolds NJ, Griffiths CEM, Emsley R, et al. Psoriasis treat to target: defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br J Dermatol. 2020;182(5):1158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren RB, Gottlieb AB, Merola JF, Garcia L, Cioffi C, Peterson L, et al. Psychometric Validation of the Psoriasis Symptoms and Impacts Measure (P-SIM), a novel patient-reported outcome instrument for patients with plaque psoriasis, using data from the BE VIVID and BE READY phase 3 trials. Dermatol Ther (Heidelb). 2021;11(5):1551–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basra MKA, Fenech R, Gatt RM, Salek MS, Finlay AY. The dermatology life quality index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol. 2008;159(5):997–1035. [DOI] [PubMed] [Google Scholar]
- 11.Lebwohl M, Swensen AR, Nyirady J, Kim E, Gwaltney CJ, Strober BE. The psoriasis symptom diary: development and content validity of a novel patient-reported outcome instrument. Int J Dermatol. 2014;53(6):714–22. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb AB, Warren RB, Augustin M, Garcia L, Cioffi C, Peterson L, et al. Psychometric Validation of the Psoriasis Symptoms and Impacts Measure (P-SIM): a novel patient-reported outcome instrument for patients with plaque psoriasis, using reported data from the BE RADIANT phase 3b trial. Adv Ther. 2021;38(10):5253–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelick J, Shrom D, Sikand K, Renda L, Burge R, Dworkin C, et al. Understanding treatment preferences in patients with moderate to severe plaque psoriasis in the USA: results from a cross-sectional patient survey. Dermatol Ther (Heidelb). 2019;9(4):785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blome C, Gosau R, Radtke MA, Reich K, Rustenbach SJ, Spehr C, et al. Patient-relevant treatment goals in psoriasis. Arch Dermatol Res. 2016;308(2):69–78. [DOI] [PubMed] [Google Scholar]
- 15.Strober B, Paul C, Blauvelt A, Thaçi D, Puig L, Lebwohl M, et al. Bimekizumab efficacy and safety in patients with moderate to severe plaque psoriasis: two-year interim results from the open-label extension of the randomized BE RADIANT phase 3b trial. J Am Acad Dermatol. 2023;89(3):486–95. [DOI] [PubMed] [Google Scholar]
- 16.Reich K, Warren RB, Lebwohl M, Gooderham M, Strober B, Langley RG, et al. Bimekizumab versus Secukinumab in plaque psoriasis. N Engl J Med. 2021;385(2):142–52. [DOI] [PubMed] [Google Scholar]
- 17.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;64 Suppl 2(Suppl 2):ii65–8; discussion ii9–73 [DOI] [PMC free article] [PubMed]
- 18.Jacobson CC, Kimball AB. Rethinking the psoriasis area and severity index: the impact of area should be increased. Br J Dermatol. 2004;151(2):381–7. [DOI] [PubMed] [Google Scholar]
- 19.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–6. [DOI] [PubMed] [Google Scholar]
- 20.Hongbo Y, Thomas CL, Harrison MA, Salek MS, Finlay AY. Translating the science of quality of life into practice: what do dermatology life quality index scores mean? J Invest Dermatol. 2005;125(4):659–64. [DOI] [PubMed] [Google Scholar]
- 21.Strober B, Papp KA, Lebwohl M, Reich K, Paul C, Blauvelt A, et al. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol. 2016;75(1):77-82.e7. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Li Y, Huang D, Zhong X, Yu Q, Liang Y, et al. Quality of life benefit and clinical predictors of complete skin clearance in psoriasis: a multicenter, prospective, real-world study. Dermatology (Basel, Switzerland). 2023;239(5):802–10. [DOI] [PubMed] [Google Scholar]
- 23.Miyagi T, Kanai Y, Murotani K, Okubo Y, Honma M, Kobayashi S, et al. Itch as a critical factor in impaired health-related quality of life in patients with plaque psoriasis achieving clear or almost-clear skin: analysis of the single-arm, open-label, multicenter, prospective ProLOGUE study. JAAD Int. 2022;8:146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Am J Clin Dermatol. 2016;17(1):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee L-L, Huo A-P, Chen S-L. Experiences and coping behaviors of patients with psoriasis: a qualitative study. J Dermatol Treat. 2023;34(1):2193661. [DOI] [PubMed] [Google Scholar]
- 26.Puig L. PASI90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645–8. [DOI] [PubMed] [Google Scholar]
- 27.Warren RB, Blauvelt A, Bagel J, Papp KA, Yamauchi P, Armstrong A, et al. Bimekizumab versus adalimumab in plaque psoriasis. N Engl J Med. 2021;385(2):130–41. [DOI] [PubMed] [Google Scholar]
- 28.Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–98. [DOI] [PubMed] [Google Scholar]
- 29.Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–86. [DOI] [PubMed] [Google Scholar]
- 30.Strober B, Tada Y, Mrowietz U, Lebwohl M, Foley P, Langley RG, et al. Bimekizumab maintenance of response through three years in patients with moderate to severe plaque psoriasis: results from the BE BRIGHT open-label extension trial. Br J Dermatol. 2023;188(6):749–59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Underlying data from this manuscript may be requested by qualified researchers six months after product approval in the US and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymised individual patient-level data and redacted trial documents, which may include: analysis-ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password protected portal.