Abstract
Pancreatic cancer is among the most immune-resistant tumor types due to its unique tumor microenvironment and low cancer immunogenicity. Single-agent immune modulators have thus far proven clinically ineffective. However, a growing body of evidence suggests that combination of these modulators with other strategies could unlock the potential of immunotherapy in pancreatic cancer. Herein, we describe the case of a 59-year-old male with metastatic pancreatic ductal adenocarcinoma, referred to our center to receive immunotherapy (serplulimab, a novel anti-PD-1 antibody) combined with chemotherapy (gemcitabine/nab-paclitaxel). During the initial three treatment cycles, the patient was assessed as having stable disease (SD) according to RECIST 1.1 criteria. However, following two additional cycles of combination therapy, the primary tumor mass increased from 4.9 cm to 13.2 cm, accompanied by the development of new lung lesions, ascites, and pelvic metastases. He succumbed to respiratory failure one month later. Retrospective analysis revealed that the patient had MDM4 amplification, identified as a high-risk factor for hyperprogressive disease (HPD). To our knowledge, this is the first reported case of HPD in pancreatic cancer with multiple metastases treated using combination therapy. We investigated the potential mechanisms and reviewed the latest literature on predictive factors for HPD. These findings suggest that while chemotherapy combined with immunotherapy may hold promise for treating pancreatic cancer, it is imperative to identify and closely monitor patients with high-risk factors for HPD when using immunotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-024-01420-3.
Keywords: Pancreatic cancer, Combination therapy, MDM4 amplification, Hyperprogressive disease, Case report
Introduction
Immune checkpoint inhibitors (ICIs), including programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) inhibitors, have revolutionized the treatment of multiple cancers, especially non-small cell lung cancer and melanoma [1–3]. Generally, approximately 25% of cancer patients show a favorable response to these ICIs. However, with the exception of the < 1% of patients with microsatellite instability-high (MSI-H) tumors, almost all pancreatic cancer patients are refractory to ICIs. To address this issue, combination therapies, including chemotherapy combined with ICIs, have been attempted and validated in preclinical and clinical trials, potentially improving response rates and prognosis [4, 5]. HPD is an adverse outcome of immunotherapy characterized by accelerated tumor growth and poor prognosis, occurring in 6% to 43% of cancer patients [6, 7]. Previous studies have reported that patients with HPD show shorter overall survival compared with those with natural progressive disease (PD) [8, 9]. The most studied cancer types associated with HPD are non-small-cell lung cancer and gastric cancer, primarily occurring in the context of immunotherapy alone [1, 10]. While HPD in pancreatic cancer is rarely reported, it is even less documented in combination therapy contexts.
AS for reports of HPD in pancreatic cancer, there is a case of a 63-year-old male who was found to have pancreatic cancer with multiple liver metastases at initial diagnosis [11]. The patient experienced a partial response to gemcitabine/nab-paclitaxel but showed marked tumor progression after switching to pembrolizumab (PD-1 monoclonal antibody), consistent with HPD. This case suggests that the gemcitabine/nab-paclitaxel regimen could be a promising approach for patients with pancreatic cancer and multiple metastases. Moreover, immunotherapy should be considered in combination with initial treatment rather than as a salvage therapy.
Herein, we present the case of a pancreatic cancer patient with multiple metastases (liver, septal angle, abdominal, and retroperitoneal) who received gemcitabine/nab-paclitaxel chemotherapy combined with serplulimab immunotherapy. The patient initially showed a favorable response to the combination treatment but subsequently developed HPD and rapidly deteriorated.
Case report
Clinical presentation
A 59-year-old man presented with multiple masses in the pancreatic body/tail and multiple liver hypodensities, along with cardiac septal angle, abdominal, and retroperitoneal multiple enlarged lymph nodes and implant metastases (Fig. 1a, d). Biopsy of a liver mass revealed poorly differentiated pancreatic ductal adenocarcinoma. Next-generation sequencing showed a tumor mutation burden of 2.9 mutations/Mb and identified KRAS p.G12R and TP53 p.Y205F mutations. Notably, MDM4 copy number variation was also detected (Table 1). The patient was treated with gemcitabine (1000 mg/m2) plus nab-paclitaxel (125 mg/m2) on days 1 and 8, and one dose of serplulimab (200 mg) on day 1, every 3 weeks for one course. The patient’s Eastern Cooperative Oncology Group (ECOG) performance status was 0.
Fig. 1.
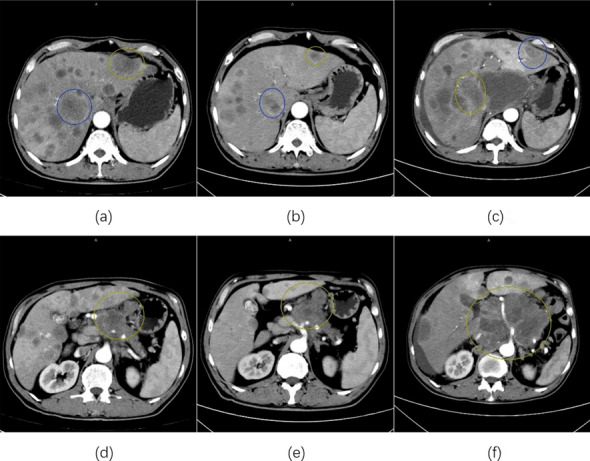
Representative CT during pancreatic cancer progression. a, d CT scans obtained before initiation of serplulimab, which correspond to the first triangle in the right graph. b, e CT scans after 3 cycles of serplulimab, which correspond to the second triangle in the right graph. c, f CT scans after 5 cycles of serplulimab, which correspond to the third triangle in the right graph. CT Computed Tomography
Table 1.
Characteristics of the patient in this case report
| History of primary diagnosis and medical history | |
|---|---|
| Gender, age | Male, 59 years |
| Tumor status | Pancreatic ductal carcinoma |
| Tumor diameter: 5.7 cm with multiple enlarged lymph nodes around the focal, abdominal areas | |
| Multiple liver metastases up to 5.6 cm diameter | |
| Immunohistochemistry | CDX2 (−) |
| CK19 ( +) | |
| CK20 (−) | |
| CK7 ( +) | |
| Her-2 (−) | |
| Claudin18.2 (−) | |
| S-100P (−) | |
| Ki-67: 90% | |
| PD-1: CPS = 5, TPS = 0 | |
| PD-L1(22C3): CPS = 5, TPS = 2 | |
| PD-L1(EIL3N): CPS = 2, TPS = 0 | |
| Molecular profile | KRAS p.G12R (35.03%), TP53 p.Y205F (56.79%), KMT2D p.R1586C (53.96%), MDM4 CNV (amplification) (4), PTPRS p.G510R (27.32%). 2.9 Muts/Mb |
| Performance status | ECOG 0 |
| Medical history | None |
| Family history | None |
| Psychological history | Married, 1 child |
Progressive disease during serplulimab treatment
After three regular treatment cycles, restaging tomographic scans showed stable disease (SD) according to RECIST 1.1 criteria (Fig. 1b, e). However, after two additional doses of serplulimab, CT scans revealed significant tumor progression, with primary masses increasing from 4.9 cm to 13.2 cm, new lungs lesions, ascites, and pelvic metastases (Fig. 1c, f). Laboratory tests showed elevated serum lactate dehydrogenase (LDH) levels (1456 IU/L, normal range: 109–245 IU/L, Fig. 2). The patient’s clinical condition rapidly deteriorated, with an ECOG score of 2. Due to disease progression, anti-tumor treatment was withdrawn, and anti-infective therapy with supportive care was administered. One month later, the patient died of respiratory failure (Fig. 3).
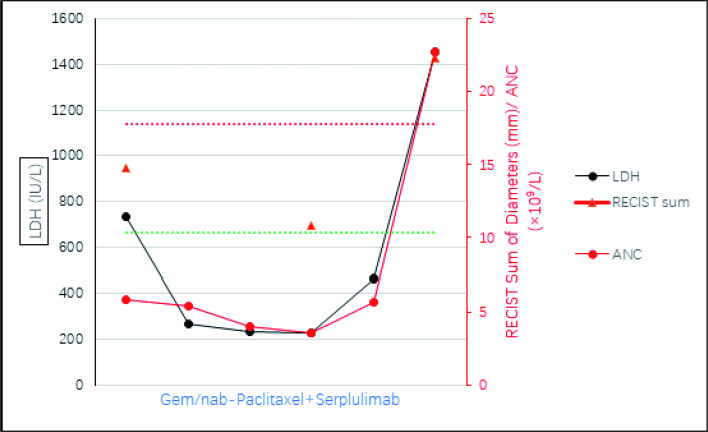
Fig. 2.
Serum index dynamics during pancreatic cancer progression. Graph showing changes in LDH (black y axis and dots), ANC (red y axis and dots), and RECIST sum of diameters (red y axis and triangles) while receiving therapy. The red and green dotted lines indicate the thresholds for progressive disease (+ 20%) and partial response (-30%), respectively
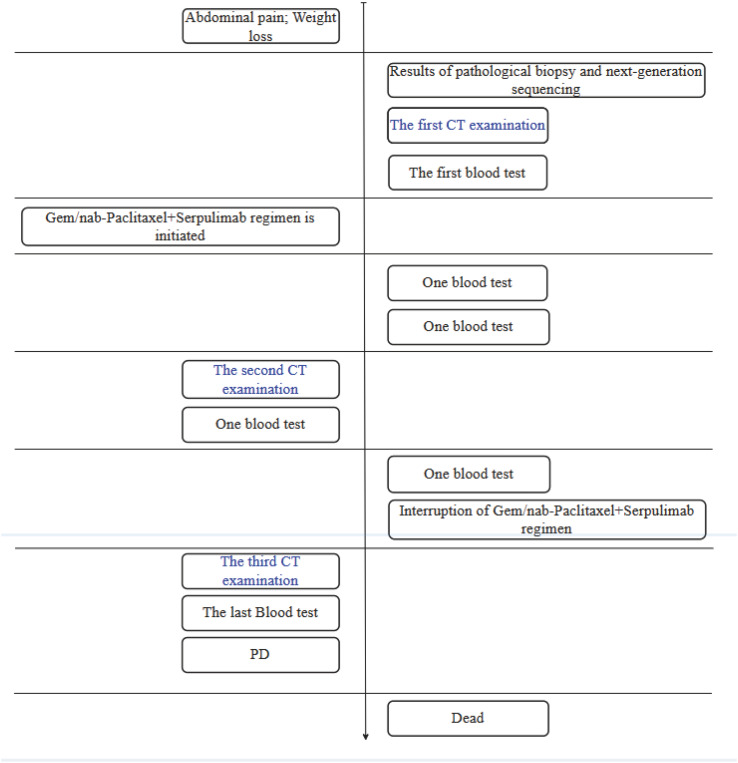
Fig. 3.
Flow chart of diagnosis, treatment and examination
Diagnosis of HPD
Parameters such as tumor growth rate (TGR) > 2, tumor growth kinetics (TGK) > 2, and time to treatment failure (TTF) < 2 months are commonly used to define and quantify HPD. However, obtaining pre-immunotherapy parameters like TGR or TGK can be challenging in clinical practice, particularly for cancers like non–small-cell lung cancer and renal cell carcinoma where pre-baseline CT imaging data may be lacking since immunotherapy has been approved as first-line therapy in these cancers. Additionally, relying solely on TGR or TGK of target lesions may overestimate or underestimate HPD, as nontarget and new lesions also reflect tumor burden kinetics. Consistently, Park et al. suggested that measurement of tumor growth acceleration based on tumor kinetics alone is insufficient, and TTF within 2 months might be too arbitrary and not sufficiently quantitative [12]. Therefore, based on the majority of current definitions with appropriate modifications, our patient met the following criteria for HPD:
1. Progressive disease (PD) by RECIST 1.1 at second therapeutic effect evaluation (the first therapeutic effect evaluation time defined as the CT scans time of 3 cycles treatment completion, the second defined as 5 cycles treatment).
2. > 50% increase in tumor burden vs. pre-immunotherapy.
3. > twofold increase in tumor size vs. pre-immunotherapy.
4. Spread of the disease to new organs vs. pre-immunotherapy.
5. Clinical deterioration with an ECOG performance status ≥ 2.
Discussion
Immunotherapy has significantly revolutionized the treatment and management of malignant tumors, particularly in malignant melanoma, lung, and renal cancers [13, 14]. However, pancreatic cancer remains challenging due to its immunosuppressive tumor microenvironment (TME) and dense stroma [15]. Research on ICIs, such as PD-1/PD-L1 and CTLA-4 inhibitors, has shown limited efficacy in clinical trials. For instance, the clinical trial NCT02798536, which combined ICIs with chemotherapy or radiation, showed partial responses but low overall response rates (ORRs). Promising results have emerged from combing ICIs with vaccines. The GVAX vaccine, derived from irradiated allogeneic pancreatic cancer cells, upregulates PD-1/PD-L1, indicating a potential synergistic effect with ICIs [16]. Clinical trials combining GVAX and ipilimumab have shown improved median overall survival compared to ipilimumab alone, though not statistically significant. Additionally, new targets like indoleamine 2,3-dioxygenase (IDO) and CCR2 inhibitors are being explored. A phase I trial with an IDO inhibitor plus gemcitabine/nab-paclitaxel in metastatic pancreatic cancer patients showed a 37% ORR. Another trial with the CCR2 inhibitor PF-04136309 and FOLFIRINOX for borderline resectable and locally advanced pancreatic cancer achieved a 49% ORR. Currently, pancreatic cancer immunotherapy focuses on combination strategies, with optimal regimens still under investigation. In addition to exploring treatment combinations, integrating immunonutrition and addressing treatment-induced hypertransaminasemia are emerging areas of interest that may enhance the effectiveness of immunotherapy in pancreatic cancer [17, 18].
Our patient was a 59-year-old male with pancreatic cancer and multiple metastases. The next generation sequencing report suggested potential effectiveness for PD-1 immunotherapy, so the initial treatment strategy was gemcitabine/nab-paclitaxel plus serplulimab immunotherapy. This is the first case of HPD in the context of immunotherapy combined with chemotherapy.
In this case, we mainly replenish the definitions of HPD. Several definitions of HPD are widely accepted. Saâda-Bouzid et al. defined HPD as an increase of at least twofold in the TGK during PD-1/PD-L1 inhibitor therapy (post-TGK) compared with the TGK before PD-1/PD-L1 inhibitors (pre-TGK) [19]. Similarly, Aoki et al. defined HPD as post-TGR/pre-TGR ≥ 2. Such definitions based on tumor growth acceleration require at least three radiologic examinations (pre-baseline, baseline, and posttreatment) [20]. Kato et al. considered TTF < 2 months as one condition of HPD [7]. In general, these definitions rely on radiographic support. In this case, pre-baseline imaging data were lacking, and the interval between CT scans was more than two months. Therefore, currently recognized indicators for identifying HPD such as TGR/TGK and TTF are unavailable. Thus, we used a more comprehensive definition of HPD, which considered the overall tumor burden (including new lesions or nontarget lesions) and clinical presentation (ECOG score). Despite the differences in methods, all definitions highlighted the importance of quantifying tumor burden kinetics.
The patient initially responded favorably to the combination regimen, although he ultimately developed HPD. We considered that our patient showed clinical benefits from the initial three treatment cycles according to irRC and iRECIST, which proposed that patients with SD belong to the clinical benefit group [21, 22]. This benefit effect may be due to the immunomodulatory effects of chemotherapy. Research indicated that chemotherapy can enhance immunity by increasing the antigenicity and immunogenicity of tumor cells, upregulating major histocompatibility complex molecules and PD-L1 expression, increasing CD8 +cell numbers, and depleting tumor-infiltrating Treg cells [23–25]. These findings provide a rationale for the combination of chemotherapy and immunotherapy. Results of clinical trial (NCT03214250, NCT03611556) indicated that chemotherapy combined with PD-1 immunotherapy is a promising approach for pancreatic cancer with multiple metastases. [26, 27]
After continuing two cycles of treatment, our patient showed HPD and died shortly. Retrospectively, he has MDM4 amplification, which has been shown to be associated with HPD. [7] (Figs. 4, 5, Table 1) MDM4 can act alone or with MDM2 to negatively regulate p53 in multiple ways [28–32]. Peng et al. assumed the increase of IFN-γ levels after immunotherapy may trigger JAK/STAT signaling, which upregulates the interferon regulatory factor (IRF)-8 gene. This gene binds to the promoter of MDM4, favoring MDM4 expression [33–35]. Furthermore, MDM4 can inhibit members of E2F and SMAD transcription-factor families and induce chromosomal instability [36, 37]. These MDM4 activities may contribute to HPD. Interestingly, higher level of IFN-γ seem to promote cancer [38, 39]. Recently, MDM4 has been identified as a promising target for cancer treatment, and effective inhibitors have been developed, mainly through three pathways: direct inhibition of p53-MDM4 interaction, inhibition of MDM4 expression, and degradation of MDM4 protein [40–42]. In a preclinical trial, degrading MDM4 to synergize anti-PD-1 immunotherapy was a potentially viable therapeutic strategy [43]. Overall, the amplification of MDM4 may be the main cause of HPD in this patient.
Fig. 4.
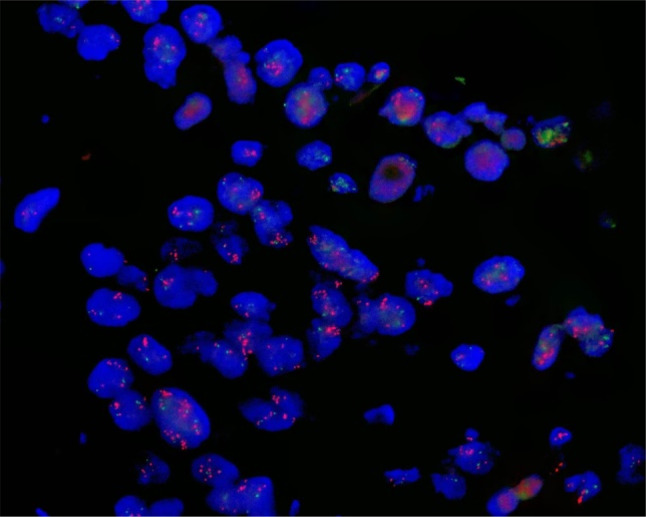
FISH results show MDM4 is amplified. Interphase FISH analysis indicates that MDM4 DNA is expressed in the nucleus. MDM4 in red. FISH fluorescence in situ hybridization
Fig. 5.
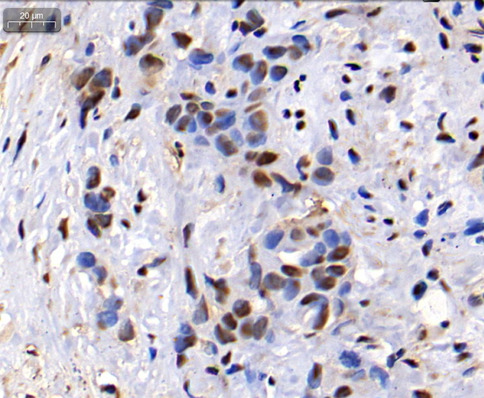
IHC results indicate MDM4 is positive. The results of immunohistochemistry showed that MDM4 protein is expressed in the nucleus and cytoplasm, mainly in the nucleus. Nuclei in blue and MDM4 in brown
Laboratory data including absolute neutrophil count (ANC) and lactate dehydrogenase (LDH) appear to be predictive factors of HPD. [44–46] Among 34 melanoma patients, 11 patients with partial response had a mean reduction of − 27.3%, and 15 patients with progressive disease had a mean increase of + 39% compared to elevated baseline LDH. In our case, the baseline level of ANC and LDH and their changes during treatment appear similar to those of melanoma patients mentioned above (Fig. 2), with increase of 140% and 120%, respectively. ANC was found to induce the release of premature myeloid cells, and more importantly, resistance to ICI treatment is related to the recruitment of myeloid-derived suppressor cells (MDSCs) [47]. Similarly, LDH-associated lactate can also promote the recruitment of MDSCs, inhibiting both innate and adaptive immunity [48]. Notably, the serum CA-199 decreased throughout the course of treatment, even during hyperprogressive periods (supplemental material).
Altogether, we may use a modified definition of HPD to evaluate similar cases in pancreatic cancer. This also reflects that the current definition of HPD is insufficient for clinical application. This report raises some new questions: Firstly, we do not know whether the phenomenon of HPD is due to the combination therapy pattern; Secondly, we do not know what role MDM4 plays in HPD. There is still much research needed in the field of immunotherapy for pancreatic cancer, and research into HPD is still in its infancy. We believe that with the deepening of related research, the above questions will be answered, and the development of immunotherapy for pancreatic cancer will be promoted.
Supplementary Information
Author contributions
Wang wenquan and Liu liang: Responsible for the design and conception of the entire study, overseeing the data collection and analysis process.As the corresponding author, he/she is in charge of coordinating communications during the peer review process. Wang yazhou and Peng maozhen: Participated in the selection of cases and data collection, conducted detailed collation of patient clinical information, drafted the initial manuscript, and subsequent revisions, and provided professional input on statistical analysis within the study. Xu yaolin and ying ying: Assisted with the literature review section, providing in-depth research support for the background information of the case, and contributed insights on pathophysiological mechanisms in the discussion chapter. Tang linhui: Mainly responsible for creating images and tables, ensuring the accuracy and standardization of their content, and participated in the editing and proofreading of the paper format. Xu huaxiang and He junyi: Although not directly involved in the implementation of this study, provided valuable suggestions and feedback during the research process, particularly playing a key role in ethical review and patient privacy protection.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82272929、82273382、81827807、81972218、81972257、82173116), Shanghai ShenKang Hospital Development Centre Project (SHDC2020CR2017B), Program of Shanghai Academic/Technology Research Leader (23XD1400600), Science and Technology Planning Project of Yunnan Province (202305AF150148), and Shanghai Municipal Health Commission (20224Y0307). The funding agencies had no role in the study design, data collection and analyses, decision to publish, or preparation of the manuscript.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Tumor index data and adjuvant therapy response evaluation data are referred to excel Table 1.
Declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Ethical Committee of Zhongshan Hospital affiliated to Fudan University. All the experimental protocol for involving human data was in accordance with the guidelines of our institutional Declaration of Helsinki in the manuscript, and written informed consent from the study patient was obtained.
Consent for publication
Written informed consent for the publication of this case report and any accompanying images was obtained from the patient’s legal guardian.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ya-Zhou Wang and Mao-Zhen Peng contributed equally to this work.
Contributor Information
Liang Liu, Email: liuliang.zlhospital@fudan.edu.cn.
Wen-Quan Wang, Email: wang.wenquan@zs-hospital.sh.cn, Email: wenquanwang09@fudan.edu.cn.
References
- 1.Hellmann MD, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–31. 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 2.Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:1270–1. 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 3.Reck M, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33. 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 4.Ye Y, Zheng S. Successful immunotherapy for pancreatic cancer in a patient with TSC2 and SMAD4 mutations: a case report. Front Immunol. 2021;12:785400. 10.3389/fimmu.2021.785400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, et al. Case report: anlotinib combined with PD-1 inhibitor and sequential GA regimen or FOLFIRINOX Chemotherapy in treatment of KRAS G12V mutated pancreatic ductal adenocarcinoma with liver metastasis: a case and literature review. Front Immunol. 2022;13:1016647. 10.3389/fimmu.2022.1016647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forschner A, et al. MDM2, MDM4 and EGFR amplifications and hyperprogression in metastatic acral and mucosal melanoma. Cancers. 2020. 10.3390/cancers12030540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato S, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res. 2017;23:4242–50. 10.1158/1078-0432.Ccr-16-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champiat S, et al. Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res. 2017;23:1920–8. 10.1158/1078-0432.Ccr-16-1741. [DOI] [PubMed] [Google Scholar]
- 9.Ferrara R, et al. Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol. 2018;4:1543–52. 10.1001/jamaoncol.2018.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CG, et al. Hyperprogressive disease during PD-1 blockade in patients with advanced gastric cancer. Eur J Cancer. 2022;172:387–99. 10.1016/j.ejca.2022.05.042. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, et al. Pancreatic cancer progression in a patient with lynch syndrome receiving immunotherapy: a cautionary tale. J Natl Compr Canc Netw. 2021;19:883–7. 10.6004/jnccn.2021.7049. [DOI] [PubMed] [Google Scholar]
- 12.Park HJ, et al. Definition, incidence, and challenges for assessment of hyperprogressive disease during cancer treatment with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2021;4: e211136. 10.1001/jamanetworkopen.2021.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol. 2022;40:586–97. 10.1200/jco.21.01497. [DOI] [PubMed] [Google Scholar]
- 14.Bi K, et al. Tumor and immune reprogramming during immunotherapy in advanced renal cell carcinoma. Cancer Cell. 2021;39:649-661.e645. 10.1016/j.ccell.2021.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Federico A, et al. Immunotherapy in pancreatic cancer: why do we keep failing? A focus on tumor immune microenvironment predictive biomarkers and treatment outcomes. Cancers. 2022. 10.3390/cancers14102429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Federico A, et al. Hacking pancreatic cancer: present and future of personalized medicine. Pharmaceuticals. 2021. 10.3390/ph14070677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Luca R, et al. Immunonutrition and prehabilitation in pancreatic cancer surgery: a new concept in the era of ERAS® and neoadjuvant treatment. Eur J Surg Oncol. 2023;49:542–9. 10.1016/j.ejso.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Rizzo A, et al. Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: the MOUSEION-05 study. Cancer Immunol Immunother. 2023;72:1381–94. 10.1007/s00262-023-03366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saada-Bouzid E, et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol. 2017;28:1605–11. 10.1093/annonc/mdx178. [DOI] [PubMed] [Google Scholar]
- 20.Aoki M, et al. Hyperprogressive disease during nivolumab or irinotecan treatment in patients with advanced gastric cancer. ESMO Open. 2019;4: e000488. 10.1136/esmoopen-2019-000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–20. 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 22.Seymour L, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–52. 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng M, Ying Y, Zhang Z, Liu L, Wang W. Reshaping the pancreatic cancer microenvironment at different stages with chemotherapy. Cancers. 2023. 10.3390/cancers15092448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doi T, et al. The JAK/STAT pathway is involved in the upregulation of PD-L1 expression in pancreatic cancer cell lines. Oncol Rep. 2017;37:1545–54. 10.3892/or.2017.5399. [DOI] [PubMed] [Google Scholar]
- 25.Von Hoff DD, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–54. 10.1200/jco.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padron LJ, et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat Med. 2022;28:1167–77. 10.1038/s41591-022-01829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Du J, et al. PD-1 blockade plus chemoradiotherapy as preoperative therapy for patients with BRPC/LAPC: a biomolecular exploratory, phase II trial. Cell Rep Med. 2023;4:100972. 10.1016/j.xcrm.2023.100972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shadfan M, Lopez-Pajares V, Yuan ZM. MDM2 and MDMX: alone and together in regulation of p53. Transl Cancer Res. 2012;1:88–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Francoz S, et al. Mdm4 and Mdm2 cooperate to inhibit p53 activity in proliferating and quiescent cells in vivo. Proc Natl Acad Sci U S A. 2006;103:3232–7. 10.1073/pnas.0508476103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okamoto K, Taya Y, Nakagama H. Mdmx enhances p53 ubiquitination by altering the substrate preference of the Mdm2 ubiquitin ligase. FEBS Lett. 2009;583:2710–4. 10.1016/j.febslet.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Mancini F, Moretti F. Mitochondrial MDM4 (MDMX): an unpredicted role in the p53-mediated intrinsic apoptotic pathway. Cell Cycle. 2009;8:3854–9. 10.4161/cc.8.23.10089. [DOI] [PubMed] [Google Scholar]
- 32.Klein AM, et al. MDM2, MDMX, and p73 regulate cell-cycle progression in the absence of wild-type p53. Proc Natl Acad Sci U S A. 2021. 10.1073/pnas.2102420118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng W, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209–18. 10.1158/0008-5472.Can-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282:20059–63. 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 35.Waight JD, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123:4464–78. 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadakia M, Brown TL, McGorry MM, Berberich SJ. MdmX inhibits Smad transactivation. Oncogene. 2002;21:8776–85. 10.1038/sj.onc.1205993. [DOI] [PubMed] [Google Scholar]
- 37.Wunderlich M, Ghosh M, Weghorst K, Berberich SJ. MdmX represses E2F1 transactivation. Cell Cycle. 2004;3:472–8. [PubMed] [Google Scholar]
- 38.Benci JL, et al. Tumor interferon signaling regulates a multigenic resistance program to immune checkpoint blockade. Cell. 2016;167:1540-1554.e1512. 10.1016/j.cell.2016.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oyler-Yaniv J, et al. Catch and release of cytokines mediated by tumor phosphatidylserine converts transient exposure into long-lived inflammation. Mol Cell. 2017;66:635-647.e637. 10.1016/j.molcel.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoppmann C, Wang L. Proximity-enabled bioreactivity to generate covalent peptide inhibitors of p53-Mdm4. Chem Commun. 2016;52:5140–3. 10.1039/c6cc01226d. [DOI] [PubMed] [Google Scholar]
- 41.Jiang L, Malik N, Acedo P, Zawacka-Pankau J. Protoporphyrin IX is a dual inhibitor of p53/MDM2 and p53/MDM4 interactions and induces apoptosis in B-cell chronic lymphocytic leukemia cells. Cell Death Discov. 2019;5:77. 10.1038/s41420-019-0157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sang P, et al. α-helix-mimicking sulfono-γ-AApeptide inhibitors for p53-MDM2/MDMX protein-protein interactions. J Med Chem. 2020;63:975–86. 10.1021/acs.jmedchem.9b00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng X, et al. De novo nano-erythrocyte structurally braced by biomimetic Au(I)-peptide skeleton for MDM2/MDMX predation toward augmented pulmonary adenocarcinoma immunotherapy. Small. 2021;17: e2100394. 10.1002/smll.202100394. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki A, et al. Predictive factors for hyperprogressive disease during nivolumab as anti-PD1 treatment in patients with advanced gastric cancer. Gastric Cancer. 2019;22:793–802. 10.1007/s10120-018-00922-8. [DOI] [PubMed] [Google Scholar]
- 45.Cao S, et al. A nomogram for predicting hyperprogressive disease after immune checkpoint inhibitor treatment in lung cancer. Transl Lung Cancer Res. 2022;11:607–16. 10.21037/tlcr-22-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yildirim HC, et al. Blood based biomarkers as predictive factors for hyperprogressive disease. J Clin Med. 2022. 10.3390/jcm11175171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engblom C, Pfirschke C, Pittet MJ. The role of myeloid cells in cancer therapies. Nat Rev Cancer. 2016;16:447–62. 10.1038/nrc.2016.54. [DOI] [PubMed] [Google Scholar]
- 48.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the paper and its Supplementary Information. Tumor index data and adjuvant therapy response evaluation data are referred to excel Table 1.