Abstract
Background
The main objective is to discuss why treatment of non-prostate cancers with [177Lu]Lu-PSMA-radioligand achieved only low tumor dose in most published cases, despite high uptake on PSMA PET. We use a patient with renal cell carcinoma as an illustrative example. Furthermore, we discuss how the problem with early washout and low tumor dose might be overcome by using a radionuclide with shorter half-life, matching the target binding residence time.
Case presentation
[68Ga]Ga-PSMA-11 PET/CT of a 56-year old man with metastatic renal cell carcinoma showed high lesion uptake. One dose of 6.9 GBq [177Lu]Lu-PSMA-I&T was administrated. Post-therapy dosimetry was performed with SPECT/CT and whole-body planar imaging after 5, 24 and 48 h. Doses to target lesions were only 0.2–0.5 Gy. No treatment effect was achieved.
Conclusion
Rapid tumor washout of [177Lu]Lu-PSMA-I&T and low tumor dose despite high uptake of [68Ga]Ga-PSMA-11 are most likely caused by localization of PSMA-receptors on neovasculature rather than on the tumor cells, and unlike in prostate cancer cells, the PSMA-RL / PSMA-receptor complex is not internalized. To overcome the problem with early washout, the use of a radionuclide with shorter half-life matching the target binding residence time will be needed.
Keywords: [177Lu]-PSMA-RL, Non-prostate cancer, Neovasculature, Tumor retention, Internalization
Background
«All that glitters is not gold»
William Shakespeare,
(Merchant of Venice)
Treatment of metastatic castration-resistant prostate cancer with [177Lu]Lu-PSMA-radioligand ([177Lu]Lu-PSMA-RL) is safe and effective [1]. A prerequisite for treatment is high tumor uptake on [68Ga]Ga- or [18F]F-PSMA-radioligand PET (PSMA PET), which reflects high expression of PSMA-receptors on the prostate cancer cells.
Multiple articles have reported high uptake on PSMA PET in a number of non-prostate, solid cancers, and treatment with [177Lu]Lu-PSMA-RL has been suggested, e.g. glioblastoma, differentiated and medullary thyroid cancer, triple negative breast cancer, hepatocellular carcinoma and renal cell carcinoma (RCC) [1–3]. In contrast to prostate cancer, the high tumor uptake on PSMA PET in these tumors is primarily caused by a high expression of PSMA-receptors on the endothelial cells in neovasculature rather than on the cancer cells [1–3].
In this article we report the results of post-treatment tumor dosimetry based on the gamma emission of lutetium-177 after a single dose of [177Lu]Lu-PSMA-I&T of a patient with metastatic RCC. We discuss likely explanations for why we achieved only low tumor dose despite high uptake on PSMA PET. Furthermore, we discuss whether our considerations might be valid also for other solid cancers showing high uptake on PSMA PET but early washout and low tumor dose.
Case presentation
Methods
A 56-year old male with metastatic chromophobe RCC did not respond to standard treatment. When unapproved treatment with the approved (“off-label”) drugs sacituzmab govicetan-hziy followed by enfortumabvedotin-ejfv both failed, experimental treatment with [177Lu]Lu-PSMA-I&T was considered. PSMA-PET showed high uptake in numerous lesions (Fig. 1). A single dose of 6.9 GBq [177Lu]Lu-PSMA-I&T (Curium OY, Finland) was administered. Post-therapy dosimetry for target lesions based was performed on the gamma emission from lutetium-177 with SPECT/CT (GE Discovery 670, GE HealthCare, Cincinnati, Ohio, USA) after 5, 24 and 48 h, in close accordance with the EANM dosimetry committee recommendations [4]. In addition to post-therapy SPECT/CT after 5, 24 and 48 h, whole-body planar images were also performed. After the 48 h SPECT/CT, a dynamic [68Ga]Ga-PSMA-11 PET/CT (Biograph Vision, Siemens Healthineers, Forchheim, Germany) was acquired for 3.8 h to provide the initial [68Ga]Ga-PSMA-11 pharmacokinetics. The patient did not receive any anticancer drugs within the 6 weeks before or during treatment with [177Lu]Lu-PSMA-I&T.
Fig. 1.
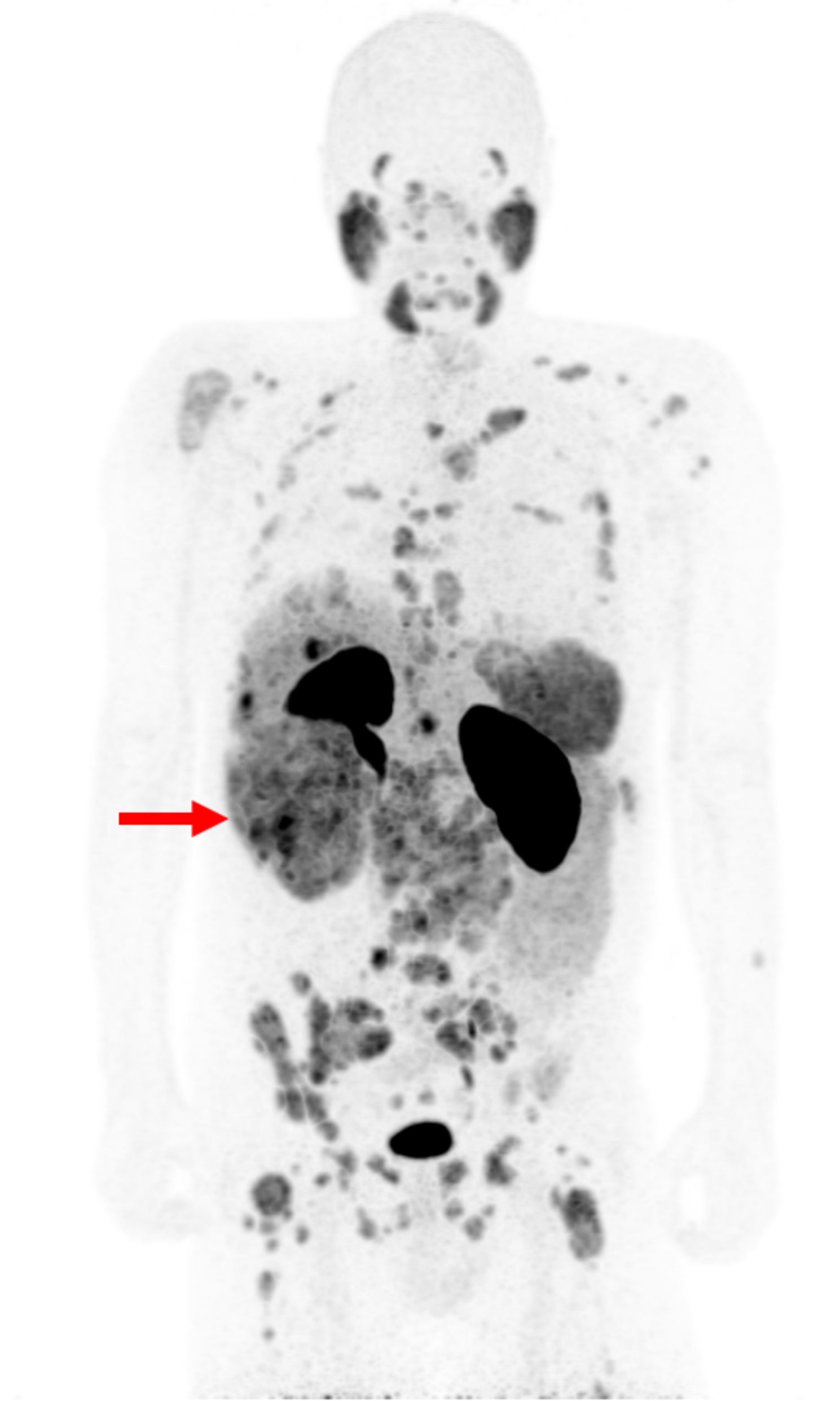
[68Ga]Ga-PSMA-11 PET/CT showing heterogeneous, high uptake in a large kidney tumor (arrow) and numerous metastases to lymph nodes, liver, muscles and bone. SUVmax in primary tumor and metastases: 10 to 18. SUVmax in reference organs: liver 5, parotids 14
The Norwegian Medical Products Agency and The Institutional Review Board had approved the unapproved use of [177Lu]Lu-PSMA-I&T. The patient has given a written consent to the use of images and data for publications.
Results
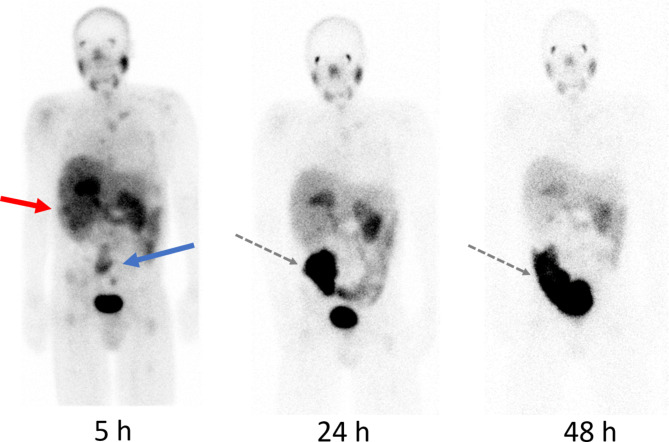
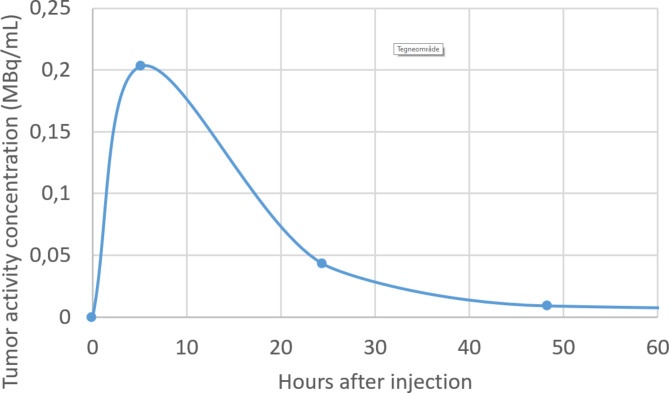
Calculated doses to target lesions were 0.2–0.5 Gy. The dynamic PSMA PET showed that about 20% of [68Ga]Ga-PSMA-11 was washed out from the tumors within 3.8 h. Post-therapy planar images after 5, 24 and 48 h are shown in Fig. 2. No treatment effect was achieved; the disease continued to progress as confirmed by MRI at 3 months, and the patient died from the disease 2 months later.
Fig. 2.
Post-therapy planar gamma camera images acquired 5, 24 and 48 h after injection of 6.9 GBq [177Lu]Lu-PSMA-I&T. Tracer uptake in the primary tumor and metastatic lesions on the 5 h image corresponds to the pre-therapeutic PSMA PET (Fig. 1). Primary tumor (red arrow) and retroperitoneal lymph node metastases (blue arrow). There is very low tumor retention after 24 and almost complete washout after 48 h (Fig. 3). High intestinal uptake (black dotted arrow) is caused by intestinal dilation caused by opioid treatment
Fig. 3.
Graph showing mean activity retention (MBq/mL) in target lesions at 5, 24 and 48 hours after injection of 6.9 GBq [177Lu]Lu-PSMA-I&T
Discussion
In this patient treated with a single administration of 6.9 GBq [177Lu]Lu-PSMA-I&T for metastatic RCC, doses to target lesions were 0.2–0.5 Gy, far too low for treatment effect. For comparison, a median dose of 14.1 Gy was needed for adequate treatment response in prostate cancer [5]. A 3.8 h dynamic PSMA PET performed after 48 h is not according to our standard dosimetry procedure. It was performed because of the early washout to provide information on initial [68Ga]Ga-PSMA-11 pharmacokinetics. The dynamic study showed a 20% washout from target lesions during a 3.5 h acquisition, indicating that the washout may have been even faster than the curve in Fig. 2 indicates. However, it must be clarified that the PSMA PET and the treatment/post-therapy dosimetry were performed with two different radioligands with somewhat different chemical structure and pharmacokinetics despite identical urea receptor binding motif (PSMA-11 vs. PSMA-I&T). An optimal theranostic approach would be using the same ligand for PET imaging and treatment, e.g. a radiohybrid ligand [6].
The low tumor dose corresponds with most other reports on treating solid malignant tumors, other than prostate, with [177Lu]Lu-PSMA-RL, despite high uptake on PSMA PET [7–9]. Zang et al. also reported rapid tumor washout of [177Lu]Lu-PSMA-I&T from RCC and no treatment effect was achieved [7]. Hirmas et al. reported two patients with hepatocellular carcinoma that showed high tumor uptake on [68Ga]Ga-PSMA-11 PET/CT, but achieved only 0.1 and 0.6 Gy when treated with one cycle of 6.9 GBq [177Lu]Lu-PSMA-617 [8]. Three patients with glioblastoma reported by Graef et al. treated with a median activity of 6.03 GBq [177Lu]Lu-PSMA-RL achieved a median tumor dose of only 0.56 Gy despite high uptake on PSMA PET/MRI [9]. One exception is a case report on a patient with glioblastoma in which a tumor dose of 14 Gy was achieved after treatment with 8.39 GBq [177Lu]Lu-PSMA-617 [10]. A few reports are published on temporary stable disease and pain relief in a few patients with salivary gland cancer [1]. However, in salivary gland cancers the PSMA uptake is not fully described in the literature. The binding may not be restricted to neovasculature, but specific as well as non-specific binding to the cancer cells may be present [11].
It is important to point out that few articles, mainly case reports, of short retention of PSMA-RL and low tumor doses in non-prostate malignancies have been published. It is reasonable to assume that a publication bias against negative treatment attempts may exist. Notably, most patients treated so far have been heavily pretreated with very advanced disease [7–9]. We suggest that further attempts of therapy with [177Lu]Lu-PSMA-RL of non-prostate cancers showing high uptake on PSMA PET should best be done as pilot studies after pre-treatment dosimetry. PSMA-RL used for dosimetry, however, will need to be labeled with a radionuclide with longer physical half-life (T1/2) than fluorine-18 or gallium-68, e.g. copper-64 (T1/2=12.7 h).
Unlike in prostate cancer, in the neovasculature the PSMA-RL / PSMA-receptor complex might not be internalized. One article reported on internalization in endothelial cells, however, they tested monoclonal antibodies and nanoparticles [12]. We have not found any other article reporting on internalization in endothelial cells of neovasculature. We might speculate that absence of internalization may be the main reason for short tumor retention time despite high density of PSMA-receptors on the endothelial cells. This does not necessarily mean that PSMA-receptors on non-prostate cancers are not a potential target for radioligand treatment. It may, however, indicate that the radionuclides clinically used today, lutetium-177 (T1/2=6.7 days) and actinium-225 (T1/2=10 days), have too long half-lives and are thus suboptimal. A very important basic principle for targeted radionuclide therapy is that the half-life of the radionuclide should match the biological tumor/receptor residence time to provide efficient tumor dose and to reduce toxicity by off target accumulation of longer-lived radionuclides [13, 14]. Thus, radionuclides with relatively short physical half-life may be needed for successful PSMA-RL therapy targeting PSMA-receptors on neovasculature, e.g. the alpha emitter astatine-211 (T1/2 =7.2 h), or the beta-/alpha emitter lead-212 (T1/2=10.7 h) [14, 15].
It is worth noting that the distance between capillaries of neovasculature is shorter than the range of alpha particles, which means that alpha particles emitted from radioligands targeting neovasculature should be able to kill not only endothelial cells but cancer cells and supporting cells in the tumor microenvironment as well [16]. Alpha particles have such a short range that they may potentially kill micrometastases of prostate cancer. However, this may not work for other malignancies, as small micrometastases may not yet have neovasculature [16].
Important for the tumor retention and thus the tumor dose is the internalization fraction, i.e. the proportion of radioligand bound to receptors on the cell membrane that is internalized. Internalization fraction of the commonly used PSMA-RL in prostate cancer cells, based on in vitro cell experiments, seems to be as low as 10–20% [17]. Thus, relatively short-lived radionuclides like astatine-211 and lead-212 may have an advantage over lutetium-177 and actinium-225 labeled PSMA-RL not only for targeting neovasculature, but for prostate cancer as well [13, 14]. In addition to more optimal half-life, astatine-211 emits only one alpha particle per decay, in contrast to actinium-225, which has multiple alpha-emitting daughters that may escape the target site and result in low tumor dose and unwanted radiation to normal tissue. The advantage of using an alpha emitter for radioligand therapy is the higher biological effectiveness including indirect effects. In addition to the favorable half-life, ligand-labeling is easy for lead-212, and the radionuclide decays to stable lead-208 emitting two alpha- and three beta particles. The mix of both beta and alpha may be an advantage, particularly in heterogenous tumors [15]. Several publications have reported on successful labeling of PSMA-RL with lead-212, as well as astatine-211 [15]. Both astatine-211 and lead-212 can be imaged with SPECT/CT post-therapy for dosimetry, and lead-212 constitutes an attractive theranostic pair with lead-203.
The internalization fraction of radioligands targeting somatostatin receptors (SSTR) and fibroblast activating protein (FAP) also seems to be as low as 10–20% [18, 19]. Thus, the considerations above about radionuclides with shorter half-lives for therapy and the potential advantages of alpha particles, or a combination of alpha and beta should also be considered for treatment with radionuclides targeting SSTR and FAP [20].
Conclusion
Rapid tumor washout of [177Lu]Lu-PSMA-RL from non-prostate cancers, despite high uptake on PSMA PET, is probably due to uptake on the endothelial cells in neovasculature, rather than on the tumor cells, and lack of internalization of PSMA-RL / PSMA-receptor complex in the endothelial cells. To overcome the problem with early washout, the use of radionuclides with shorter half-lives matching the target binding residence time will be needed, i.e. astatine-211 or lead-212.
Acknowledgements
Not applicable.
Abbreviations
- PSMA
Prostate-specific membrane antigen
- PSMA PET
[68Ga]Ga- or [18F]F-PSMA-radioligand
- RL
Radioligand
- RCC
Renal cell carcinoma
- FAP
Fibroblast activating protein
- SSTR
Somatostatin receptors
Author contributions
TVB, JMD, OE, TTTL had the main contribution to the concept. TVB, JMD drafted the work. AS, RS were responsible for the funding and the treatment planning. OE was responsible for planning, execution and analysing the dosimetry. AS, DRJ, BJB, ATK, MKP, RS made a substantial and decisive contributions to the conception. TVB, OE, TTTL, AS, DRJ, BJB, ATK, MKP, RS, JMD contributed in the search for relevant references and were active in critical revising the manuscript. TVB, OE, TTTL, AS, DRJ, BJB, ATK, MKP, RS, JMD agree to be personally accountable for own contribution and to ensure that questions related to the accuracy or integrity of any part of the work are appropriately investigated, resolved, and the resolution documented in the literature. TVB, OE, TTTL, AS, DRJ, BJB, ATK, MKP, RS, JMD have approved the submitted manuscript.
Funding
The unapproved use of [177Lu]Lu-PSMA-I&T was funded by Department of Oncology, Ostfold Hospital, Kalnes, Norway.
Data availability
Further data are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Norwegian Medical Products Agency approved the unapproved use of [177Lu]Lu-PSMA-I&T for treatment of renal cell carcinoma in the patient. The Institutional Review Board gave ethical approval of the experimental treatment. The patient gave a consent to the procedure.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing interests
The authors have nothing to disclose / there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang JH, Kiess AP. PSMA-targeted therapy for non-prostate cancers. Front Oncol. 2023;13:1220586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van de Wiele C, Sathekge M, de Spiegeleer B, et al. PSMA expression on neovasculature of solid tumors. Histol Histopathol. 2020;35:919–27. [DOI] [PubMed] [Google Scholar]
- 3.Pozzessere C, Bassanelli M, Ceribelli A, Rasul S, Li S, Prior JO, Cicone F. Renal cell carcinoma: the oncologist asks, can PSMA PET/CT answer? Curr Urol Rep. 2019;20:68. [DOI] [PubMed] [Google Scholar]
- 4.Sjögreen Gleisner K, Chouin N, Gabina PM, et al. EANM dosimetry committee recommendations for dosimetry of 177Lu-labelled somatostatin-receptor- and PSMA-targeting ligands. Eur J Nucl Med Mol Imaging. 2022;49:1778–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Violet J, Jackson P, Ferdinandus J, et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate Cancer: correlations between Pretherapeutic Imaging and whole-body tumor dosimetry with treatment outcomes. J Nucl Med. 2019;60:517–23. [DOI] [PubMed] [Google Scholar]
- 6.Wurzer A, Di Carlo D, Schmidt A, Beck R, Eiber M, Schwaiger M, Wester HJ. Radiohybrid ligands: a Novel Tracer Concept Exemplified by 18F- or 68Ga-Labeled rhPSMA inhibitors. J Nucl Med. 2020;61:735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Schuchardt C, Chen X, Baum RP. Rapid Tumor Washout of 177Lu-PSMA Radioligand in Renal Cell Carcinoma. Clin Nucl Med. 2023;48:732–4. [DOI] [PubMed] [Google Scholar]
- 8.Hirmas N, Leyh C, Sraieb M, et al. 68Ga-PSMA-11 PET/CT Improves Tumor Detection and Impacts Managemepatientstients with Hepatocellular Carcinoma. J Nucl Med. 2021;62:1235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graef J, Bluemel S, Brenner W, et al. [177Lu]Lu-PSMA Therapy as an Individual Treatment Approach for patients with high-Grade Glioma: Dosimetry results and critical Statement. J Nucl Med. 2023;64:892–95. [DOI] [PubMed] [Google Scholar]
- 10.Kunikowska J, Charzyńska I, Kuliński R, Pawlak D, Maurin M, Królicki L. Tumor uptake in glioblastoma multiforme after IV injection of [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2020;47:1605–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rupp NJ, Umbricht CA, Pizzuto DA, et al. First clinicopathologic evidence of a Non-PSMA-Related uptake mechanism for 68Ga-PSMA-11 in salivary glands. J Nucl Med. 2019;60:1270–6. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen DP, Xiong PL, Liu H, et al. Induction of PSMA and internalization of an Anti-PSMA mAb in the vascular compartment. Mol Cancer Res. 2016;14:1045–53. [DOI] [PubMed] [Google Scholar]
- 13.Lau J, et al. Insight into the development of PET radiopharmaceuticals for Oncology. Cancers. 2020;12:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Fakiri M, Ayada N, Müller M, et al. Development and preclinical evaluation of [211At] PSAt-3-Ga: an inhibitor for targeted α-Therapy of prostate Cancer. J Nucl Med. 2024;65:593–9. [DOI] [PubMed] [Google Scholar]
- 15.Jang A, Kendi AT, Johnson GB, Halfdanarson TR, Sartor O. Targeted alpha-particle therapy: a review of current trials. Int J Mol Sci. 2023;24:11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelch ID, Bogle G, Sands GB, Phillips AR, LeGrice IJ, Dunbar PR. Organ-wide 3D-imaging and topological analysis of the continuous microvascular network in a murine lymph node. Sci Rep. 2015;5:16534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Afshar-Oromieh A, Hetzheim H, Kratochwil C, et al. The theranostic PSMA ligand PSMA-617 in the diagnosis of prostate Cancer by PET/CT: Biodistribution in humans, Radiation Dosimetry, and first evaluation of Tumor lesions. J Nucl Med. 2015;56:1697–705. [DOI] [PubMed] [Google Scholar]
- 18.Delbart W, Karabet J, Marin G, et al. Understanding the Radiobiological mechanisms Induced by 177Lu-DOTATATE in comparison to External Beam Radiation Therapy. Int J Mol Sci. 2022;23:12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loktev A, Lindner T, Mier W, et al. A tumor-imaging Method Targeting Cancer-Associated fibroblasts. J Nucl Med. 2018;59:1423–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindner T, Loktev A, Giesel F, Kratochwil C, Altmann A, Haberkorn U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm Chem. 2019;4:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Further data are available from the corresponding author on reasonable request.