Abstract
Background
Psoriasis, a chronic, immune-mediated, inflammatory disease, affects 2‒3% of the population. Tyrosine kinase 2 (TYK2) mediates cytokine signaling involved in adaptive [interleukin (IL)-12, IL-23] and innate (type-I interferons) immune responses; IL-23–driven T-helper (Th)17 pathways play a key role in chronic inflammation in psoriasis. In a phase 2 trial, deucravacitinib, an oral, selective, allosteric TYK2 inhibitor, reduced IL-23/Th17 and type-I interferon pathway expression in the skin of patients with moderate to severe plaque psoriasis, reductions that were accompanied by clinical improvement of psoriatic lesions.
Objectives
The aim of this study was to identify biomarkers of psoriatic disease in serum from patients enrolled in the phase 2 trial and to assess the effects of deucravacitinib on those biomarkers.
Methods
Serum biomarkers from Olink proteomics and other quantitative assays were evaluated for a pharmacodynamic response to deucravacitinib treatment and correlation with psoriasis disease activity measures.
Results
Serum biomarkers associated with the IL-23/Th17 pathway [IL-17A, IL-17C, IL-19, IL-20, beta-defensin, and peptidase inhibitor 3 (PI3)] were upregulated in patients with psoriasis versus healthy controls. Deucravacitinib treatment reduced IL-17A (adjusted mean change from baseline at Day 85; 12 mg once daily versus placebo; −0.240 versus −0.067), IL-17C (−14.850 versus −1.664), IL-19 (−96.445 versus −8.119), IL-20 (−0.265 versus −0.064), beta-defensin (−65,025.443 versus −7553.961), and PI3 (−14.005 versus −1.360) expression. Reductions in serum biomarker expression occurred in a dose- and time-dependent manner, with significant reductions from baseline seen with deucravacitinib doses ≥ 3 mg twice daily (P ≤ 0.05). Biomarker expression correlated with disease activity measures such as Psoriasis Area and Severity Index (PASI) at baseline. Biomarker expression also correlated with PASI scores at Week 12.
Conclusion
IL-23/Th17 pathway expression in the serum of patients with psoriasis is an indicator of disease activity and response to deucravacitinib treatment.
Trial registration number
Keywords: Cytokine, Deucravacitinib, Pharmacodynamics, Psoriasis, Tyrosine kinase 2
Plain Language Summary
Plaque psoriasis is a long-term disease that causes inflammation, scaling, and itching of the skin. Compared with healthy volunteers without psoriasis, patients with psoriasis have higher amounts of certain biomarkers (molecules that indicate what is happening in the body) in their blood that are associated with inflammation. Higher amounts of these biomarkers are also associated with more severe psoriasis. In a study of patients with psoriasis, those who received the oral drug deucravacitinib had lower amounts of biomarkers after 12 weeks of treatment compared with patients who received a placebo (a lookalike pill that contains no medicine). Patients who were treated with deucravacitinib also saw an improvement in their psoriasis after 12 weeks compared with patients who received placebo.
Key Summary Points
| Why carry out this study? |
| Tyrosine kinase 2 (TYK2) mediates signaling by cytokines involved in psoriasis pathogenesis. |
| Serum biomarkers associated with the interleukin-23/T-helper-17 pathways are higher in patients with psoriasis versus healthy controls. |
| Deucravacitinib, an oral, selective, allosteric TYK2 inhibitor, was evaluated at various doses in a previously reported phase 2 trial of patients with psoriasis and healthy controls to determine serum biomarker expression. |
| What was learned from the study? |
| Deucravacitinib reduced biomarker expression in a phase 2 trial of patients with psoriasis. |
| Biomarker levels were correlated with psoriasis disease activity as measured by Psoriasis Area and Severity Index at both baseline and Week 12. |
Introduction
Psoriasis is a chronic, immune-mediated, inflammatory disease affecting 2‒3% of the worldwide population [1]. Preclinical studies demonstrated that psoriasis pathogenesis is triggered via activation of plasmacytoid dendritic cells and induction of type-I interferons (IFNs) in the skin, although an anti-IFN-α monoclonal antibody exhibited no activity in a clinical trial of patients with chronic plaque psoriasis [2, 3]. Tyrosine kinase 2 (TYK2) mediates signaling of inflammatory cytokines required for adaptive [interleukin (IL)-12, IL-23] and innate (type-I IFN) immune responses [4, 5]; IL-23–driven T-helper (Th)17 pathways play a crucial role in chronic inflammation in psoriasis and other inflammatory conditions [6, 7]. Cytokines of the IL-23/Th17 pathway, including IL-23 and IL-17, are expressed in psoriatic lesions [8]. IL-23 appears to be a central driver of IL-17 expression, and blockade of either IL-23 or IL-17 rapidly alleviates psoriasis symptoms [9]. Expression of IL-23/Th17-related cytokines such as IL-19 and IL-20 is also increased in psoriatic lesions [8, 10]. IL-19 expression correlates with psoriasis disease activity and response to antipsoriatic therapies [11]. Beta-defensin, a downstream product of the Th17 pathway in psoriatic disease, is also associated with disease activity [8, 12].
The IL-23/Th17 pathway plays a central role in regulating the function and balance of Th17 and regulatory T (Treg) cells, CD4 + T-cell subsets that promote or suppress immune-mediated inflammatory responses [13, 14]. In conjunction with signaling factors such as transforming growth factor-β (TGF-β), IL-6, IL-21, and IL-1β, IL-23 stimulates the differentiation of T cells into Th17 cells, which secrete IL-17 and various additional proinflammatory effector molecules, including IL-23, as part of a positive feedback loop, and contribute to tissue damage and inflammation [13, 14]. T cells can also differentiate into Treg cells that secrete IL-10 and TGF-β to maintain self-tolerance and suppress inflammation [13, 14]. Among all Th cell subtypes, Th17 and Treg cells are regarded as the subtypes with the greatest plasticity; in the presence of appropriate cytokine stimulation, proinflammatory Th17 cells can be converted into immunosuppressive Treg cells [13, 14]. IL-23 also influences the development and function of Treg-expressing IL-17 (Trem) cells, which represent intermediate forms in the transformation between Th17 and Treg states [15]. Therefore, IL-23 affects Th17 and Treg balance, contributing to both immune homeostasis and immune-mediated disease processes.
Deucravacitinib, an oral, selective, allosteric TYK2 inhibitor, is approved in the USA and other countries for the treatment of adults with moderate to severe plaque psoriasis who are candidates for systemic therapy [16–21]. Deucravacitinib binds to the regulatory (pseudokinase) domain of TYK2 rather than to the more conserved catalytic domain where Janus kinase (JAK) 1, 2, and 3 inhibitors bind, driving the selectivity of deucravacitinib for TYK2 [5]. In cell-based assays, deucravacitinib demonstrated > 100-fold greater selective inhibition of TYK2 over JAK1 and 3, and > 2000-fold greater selective inhibition of TYK2 over JAK2 [5, 22]. In a biomarker substudy of a phase 2 trial, dose-dependent, deucravacitinib-mediated inhibition of TYK2 was associated with decreases in IL-23/Th17 and type-I IFN pathway biomarkers in the skin of patients with moderate to severe plaque psoriasis [23]. The selectivity of deucravacitinib for TYK2 was confirmed by the lack of JAK1, 2, and 3 inhibition in blood from these patients [23].
It is not feasible to require skin biopsy from all patients in a clinical trial or to use molecular analysis of skin biopsy for routine clinical care. Thus, it is desirable to find molecular biomarkers in blood that could be used to monitor response to therapy and identify psoriasis flares or residual disease activity [7, 11].
The objective of the analysis reported here was to identify biomarkers of psoriatic disease in serum and to assess the pharmacodynamic effects of deucravacitinib treatment on those biomarkers in the phase 2 trial.
Methods
Biomarker Analysis Population
This exploratory analysis evaluated serum biomarker expression in patients with psoriasis from a previously completed phase 2 trial, as well as in healthy controls. As previously reported, a global, 12-week, phase 2, randomized, double-blind, placebo-controlled, dose-ranging trial (NCT02931838) evaluated deucravacitinib efficacy and safety in patients with moderate to severe plaque psoriasis [defined as Psoriasis Area and Severity Index (PASI) score ≥ 12, static Physicians Global Assessment score ≥ 3, and body surface area (BSA) involvement ≥ 10% at baseline] for ≥ 6 months [24]. Patients were randomized (1:1:1:1:1:1) to oral placebo or deucravacitinib 3 mg every other day, 3 mg once daily (QD), 3 mg twice daily (BID), 6 mg BID, or 12 mg QD [24].
Serum Biomarker Expression in Patients with Psoriasis and Healthy Controls
Serum biomarkers of primary interest in this analysis were IL-17A, IL-19, beta-defensin, and proteins from Olink proteomics technology (Olink Proteomics, Uppsala, Sweden). IL-17A and IL-19 levels were determined using single molecule array (Simoa®) enzyme-linked immunoassay (ELISA; Quanterix, Billerica, MA, USA), and beta-defensin levels were determined using ELISA. The Olink proteomics analysis evaluated 276 serum biomarkers in three cardiovascular disease and inflammation panels (Olink Cardiovascular II, Olink Cardiovascular III, and Olink Inflammation). Each of the three Olink biomarker panels was analyzed separately. The number of expressed proteins was calculated in each sample, and a protein was considered expressed if the expression was greater than the limit of detection of that protein. After the removal of quality control samples, all data were included in the analysis and flagged values (those below the limit of detection or from samples that failed quality control) were identified. Serum biomarker expression at baseline was compared in patients with psoriasis and in 60 healthy controls. For markers from Olink proteomics, the R package limma (version 3.42.2) was used to calculate the fold change in serum biomarker expression between patients with psoriasis and healthy controls, the corresponding P-values, and the false discovery rate-adjusted P-values (generated only for proteins that underwent multiple testing).
Serum Biomarker Expression and Pharmacodynamic Responses to Deucravacitinib Treatment
The serum biomarkers of interest were IL-17A, IL-17C, IL-19, IL-20, beta-defensin, and peptidase inhibitor 3 (PI3). The effect of deucravacitinib treatment on the expression of these serum biomarkers was evaluated in a dose- and time-dependent manner, and the fold change in expression at Days 8, 15, 29, 57 and 85/end of treatment versus Day 1 was determined. A linear mixed-effects model with random intercept for subject was fitted for each biomarker to determine whether the change from baseline was significant for each treatment arm (P < 0.05).
Serum Biomarker Expression and Psoriasis Disease Activity
The relationship between IL-17A, IL-19, beta-defensin, and PI3 expression and the disease activity measures, PASI and BSA involvement, was determined at baseline and during deucravacitinib treatment. Pearson’s correlation analysis was conducted to evaluate the strength and significance of the correlation.
Serum Biomarker Expression and Clinical Outcome
The relationship between IL-17A, IL-19, beta-defensin, and PI3 expression and the clinical activity measure PASI (percent change from baseline; categorized as < 25, 25–50, 50–75, 75–90, 90– < 100, and 100% improvement) was determined at Week 12 in deucravacitinib-treated patients. Serum biomarker levels were compared across patients achieving different degrees of PASI improvement. All statistical analysis was conducted in R programming language (version 4.2.1).
Compliance with Ethics Guidelines
This biomarker study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki. The protocol, amendments, and patient informed consent received appropriate approval by the institutional review board or independent ethics committee prior to the initiation of the trial at the study center. All patients provided written informed consent before any study-related procedures were performed.
Results
Baseline Demographics and Clinical Characteristics
A total of 256 patients from the phase 2 trial and 60 healthy controls (matched for age, gender, and body mass index) were included in this analysis. The baseline demographics and clinical characteristics of the study population were described previously [23].
Comparison of Serum Biomarker Expression in Patients with Psoriasis Versus Healthy Controls
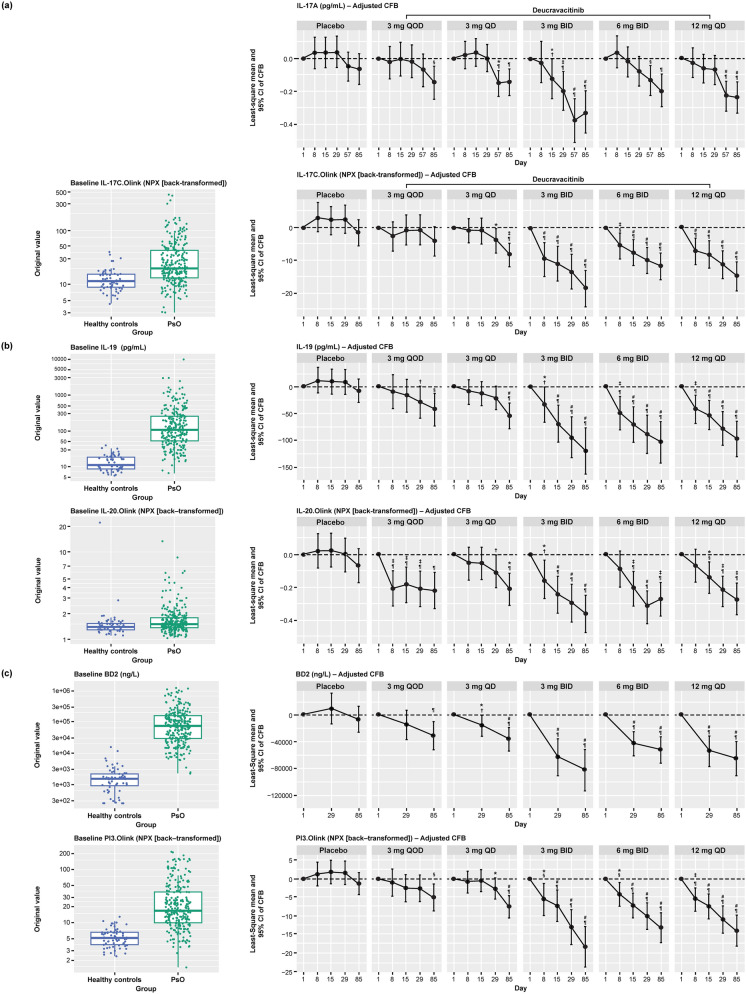
Serum biomarkers from Olink proteomics associated with psoriasis were identified by comparing baseline expression in patients with psoriasis versus healthy controls. Upregulated biomarkers in patients with psoriasis were consistent with the Th17 phenotype of psoriatic disease and psoriasis-related proteins, and included IL-17A, IL-17C, IL-19, IL-20, and PI3 (Fig. 1). Beta-defensin was identified separately as being elevated in psoriasis. IL-17A, IL-19, beta-defensin, and PI3 were selected to evaluate the correlation of the expression of these biomarkers in serum with psoriasis disease activity and clinical outcome.
Fig. 1.
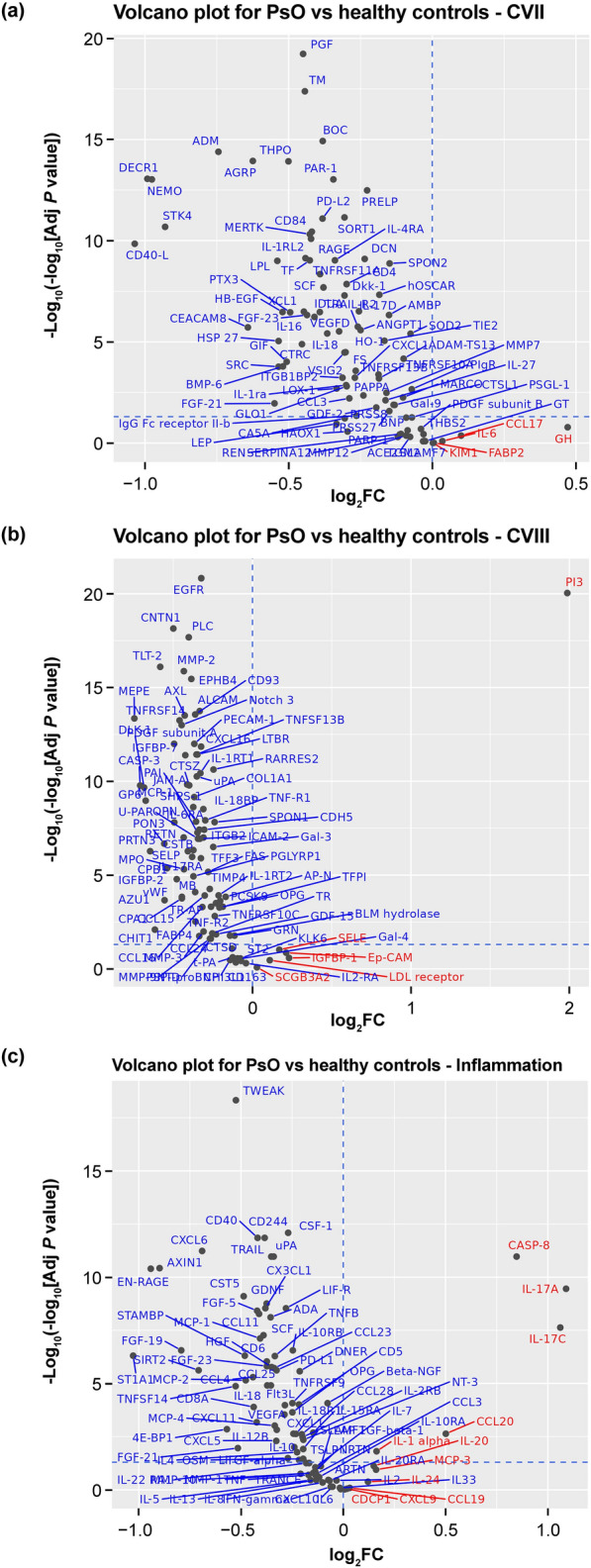
Proteins differentially expressed at baseline in serum from patients with psoriasis compared with healthy controls. Serum biomarker expression at baseline was compared in patients with psoriasis and in healthy controls using Olink proteomics technology. The Olink proteomics analysis evaluated 276 serum biomarkers in three cardiovascular disease and inflammation panels (Olink Cardiovascular II, Olink Cardiovascular III, and Olink Inflammation). a Cardiovascular II: there were five upregulated proteins [(log2FC > 0): GH, CCL17, IL-6, FABP2, KIM1; none were significant] and 87 down-regulated proteins [(log2FC < 0); 71 were significant (adjusted P < 0.05)] in patients with psoriasis compared with healthy controls. b Cardiovascular III: there were six up-regulated proteins (Ep-CAM, IGFBP-1, LDL receptor, PI3, SCGB3A2, SELE; PI3 was significant) and 86 down-regulated proteins (77 were significant). c Inflammation: there were 11 upregulated proteins (IL-17A, IL-17C, CASP-8, CCL20, IL-1α, MCP-3, IL-20, IL-24, CCL19, CXCL9, and CDCP1; five were significant) and 81 down-regulated proteins (60 were significant). Upregulated proteins are shown in red and down-regulated proteins are shown in blue. AMPs antimicrobial peptides, CCL chemokine ligand, IFN interferon, IL interleukin, JAK Janus kinase, mDC myeloid dendritic cell, pDC plasmacytoid dendritic cell, PI3 peptidase inhibitor 3, PMNs polymorphonuclear leukocytes, PsO patients with psoriasis, Th T helper, TNF tumor necrosis factor
Serum Biomarker Expression and Pharmacodynamic Responses to Deucravacitinib Treatment
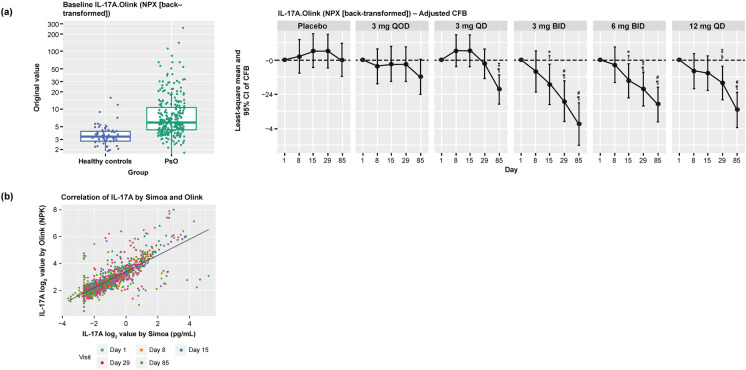
Levels of IL-17A, IL-17C, IL-19, IL-20, beta-defensin, and PI3 at baseline were elevated in serum from patients with psoriasis compared with healthy controls (Figs. 2 and 3). Deucravacitinib treatment reduced IL-17A, IL-17C, IL-19, IL-20, beta-defensin, and PI3 expression in a dose- and time-dependent manner, with significant reductions from baseline seen with deucravacitinib doses ≥ 3 mg BID (P ≤ 0.05; Fig. 2). Deucravacitinib treatment reduced biomarker expression from baseline toward levels observed in healthy controls (data not shown). A significant correlation was observed between IL-17A expression in the blood over time as determined using the single molecule array (Simoa®) ELISA and Olink proteomics technology (Pearson’s correlation coefficient [r] = 0.79, P < 0.0001; Fig. 3). Similarly, IL-17A data measured by Olink supported dose- and time-dependent inhibition by deucravacitinib treatment (Fig. 3).
Fig. 2.
Serum biomarker expression at baseline in patients with psoriasis and in healthy controls and in patients with psoriasis receiving deucravacitinib treatment over 12 weeks. a IL-17A and IL-17C. b IL-19 and IL-20. c beta-defensin and PI3. *P < 0.05 versus Day 1. †P < 0.05 versus placebo; ‡P < 0.01 versus Day 1; §P < 0.01 versus placebo; #P < 0.001 versus Day 1; ¶P < 0.001 versus placebo; obtained from mixed-effects model. BID twice daily, DEUC deucravacitinib, IL interleukin, LOD limit of detection, NPX normalized protein expression, PI3 peptidase inhibitor 3, PsO patients with psoriasis, QC quality control, QD every day, QOD every other day, SE standard error
Fig. 3.
IL-17A expression at baseline in patients with psoriasis and in healthy controls and in patients with psoriasis receiving deucravacitinib treatment over 12 weeks. a IL-17A. b Correlation of IL-17A between Simoa and Olink. *P < 0.05 versus Day 1; †P < 0.05 versus placebo; ‡P < 0.01 versus Day 1; §P < 0.01 versus placebo; #P < 0.001 versus Day 1; ¶P < 0.001 versus placebo; obtained from mixed-effects model. BID twice daily, IL interleukin, LOD limit of detection, NPX normalized protein expression, QD every day, QOD every other day, Simoa single molecule array
Serum Biomarker Expression and Psoriasis Disease Activity
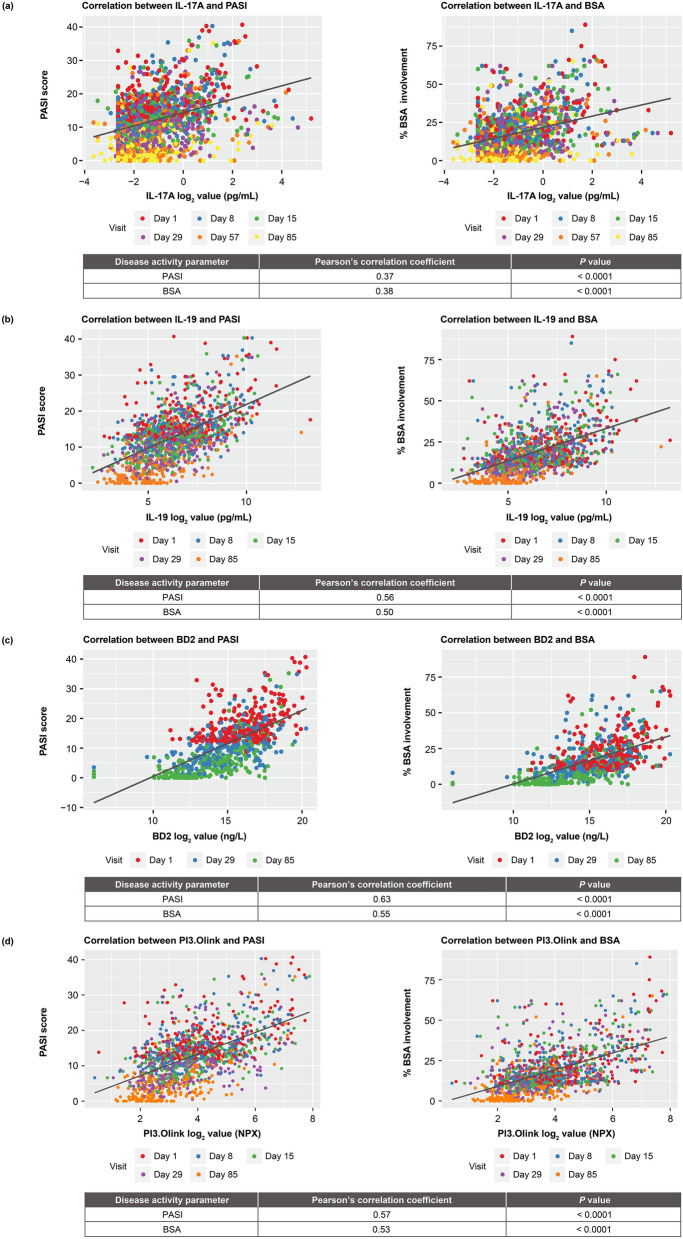
Correlations were assessed between IL-17A, IL-19, beta-defensin, and PI3 levels in serum and the disease activity measures of PASI and BSA involvement. Levels of these serum biomarkers significantly correlated with PASI and BSA involvement, respectively, at baseline and during treatment (IL-17A, r = 0.37, P < 0.0001, and r = 0.38, P < 0.0001; IL-19, r = 0.56, P < 0.0001, and r = 0.50, P < 0.0001; beta-defensin, r = 0.63, P < 0.0001, and r = 0.55, P < 0.0001; and PI3, r = 0.57, P < 0.0001, and r = 0.53, P < 0.0001; Fig. 4; Table 1).
Fig. 4.
Correlation between serum biomarker expression and PASI and BSA involvement at baseline and during treatment. a IL-17A. b IL-19. c Beta-defensin. d PI3. BSA body surface area, IL interleukin, PASI Psoriasis Area and Severity Index, PI3 peptidase inhibitor 3
Table 1.
Correlation between serum biomarker expression and PASI and BSA involvement at baseline and during treatment
| Pearson’s Correlation Coefficient | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PASIa | BSAa | |||||||||||
| Day 1 | Day 8 | Day 15 | Day 29 | Day 57 | Day 85 | Day 1 | Day 8 | Day 15 | Day 29 | Day 57 | Day 85 | |
| IL-17A | 0.38 | 0.42 | 0.41 | 0.25 | 0.25 | 0.25 | 0.39 | 0.36 | 0.38 | 0.29 | 0.31 | 0.29 |
| IL-19 | 0.38 | 0.47 | 0.54 | 0.53 | – | 0.63 | 0.39 | 0.42 | 0.44 | 0.46 | – | 0.58 |
| Beta-defensin | 0.42 | – | – | 0.52 | – | 0.64 | 0.40 | – | – | 0.44 | – | 0.60 |
| PI3 | 0.46 | 0.52 | 0.53 | 0.49 | – | 0.62 | 0.47 | 0.45 | 0.48 | 0.47 | – | 0.58 |
aCorrelations between serum biomarker expression and PASI and BSA involvement were statistically significant (P < 0.05) at each time point
BSA body surface area, IL interleukin; PASI Psoriasis Area and Severity Index, PI3 peptidase inhibitor 3
Serum Biomarker Expression and Clinical Outcome
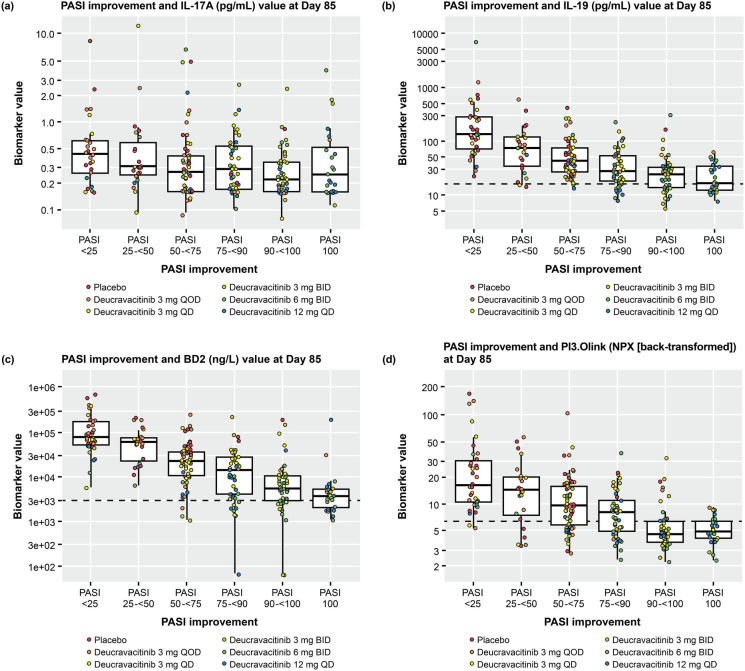
The relationship between IL-17A, IL-19, beta-defensin, and PI3 serum levels versus PASI improvement at Week 12 was also assessed. The majority of patients who received deucravacitinib ≥ 3 mg BID achieved the primary endpoint of ≥ 75% reduction from baseline in PASI (PASI 75; Fig. 5). Lower expression of IL-17A, IL-19, beta-defensin, and PI3 trended with greater PASI improvement at Week 12.
Fig. 5.
Correlation between serum biomarker expression and PASI at Week 12. a IL-17A. b IL-19. c beta-defensin. d PI3. Dashed line indicates the upper limit of the 95% confidence interval for healthy controls: IL-19, 16.05; BD2, 2799.83; PI3, 6.22. BID twice daily, IL interleukin, NPX normalized protein expression, PASI Psoriasis Area and Severity Index, PASI 25/50/75/90/100 ≥ 25/50/75/90/100% reduction from baseline in PASI, PI3 peptidase inhibitor 3, QD every day, QOD every other day
Discussion
This exploratory biomarker analysis demonstrated that biomarkers derived from inflamed psoriatic lesions can be detected in the blood of patients with psoriasis. These biomarkers reflect the level of disease activity and changes resulting from pharmacological intervention with drugs. Relevant pathways identified include the IL-23/Th17 pathway and pathways related to keratinocyte hyperproliferation. The biomarkers IL-17A, IL-17C, IL-19, IL-20, beta-defensin, and PI3 were upregulated in serum from patients with psoriasis compared with healthy controls. Deucravacitinib treatment improved the disease while concomitantly reducing serum biomarker expression in a dose- and time-dependent manner in patients with moderate to severe plaque psoriasis in a phase 2 trial. The majority of patients in the higher deucravacitinib dose groups achieved PASI 75. Biomarker expression was significantly associated with two measures of disease activity at baseline, PASI and BSA involvement. In addition, the data indicated a positive association between lower biomarker expression and greater improvements in PASI and BSA scores at Week 12, suggesting that these biomarkers can serve as surrogate markers of disease activity and response to treatment.
IL-19 and beta-defensin expression were also strongly correlated with each other (r = 0.81) and highly significant (P < 0.0001), suggesting that both biomarkers are involved in the same pathway of psoriasis pathogenesis. Additionally, serum beta-defensin levels (mean, 146.4 ng/mL) were orders of magnitude higher than IL-19 levels (mean, 289.7 pg/mL) at baseline, which is consistent with the role of IL-19, along with other cytokines, in inducing expression of beta-defensin, a terminal effector of the IL-23/Th17 inflammatory pathway [8, 12]. These results support the suggestion that downstream biomarkers that are biologically linked to psoriatic disease activity and have low expression in healthy subjects provide the best opportunity to evaluate the effect of therapeutic agents in psoriasis.
IL-17A expression was significantly reduced by deucravacitinib treatment, especially at higher doses and at later time points. Notably, the ability to discern changes in IL-17A expression or associations with clinical parameters is limited by the lower limits of quantification inherent in IL-17A assays. Additionally, change from baseline was smaller for IL-17A compared with IL-19 and beta-defensin. Therefore, given the low levels of IL-17 detected in serum from patients with psoriasis, assessment of downstream biomarkers such as beta-defensin that are highly expressed may be more useful in identifying markers of psoriatic disease activity and response to treatment.
This study is one of the few conducted to define the pharmacodynamic relationship between a given reduction in IL-17A expression and the expression of skin biomarkers regulated by IL-17A (e.g., IL-19 or beta-defensin), as well as to provide insights regarding the magnitude of reduction in IL-17A expression necessary to utilize it as a biomarker of clinical improvement. Previous studies of biologic agents have not addressed this issue comprehensively, as most doses studied resulted either in complete inhibition or very large reductions in IL-17A expression [25–27]. Here, the range of deucravacitinib doses studied and the evaluation of their pharmacodynamic effects provide greater insights into what might be considered pathogenic versus nonpathogenic levels of IL-17A. This study did not assess the effect of TYK2 inhibition on other IL-17 isoforms, such as IL-17F. This will be an important aspect of future research because various T-cell subsets generate IL-17A, IL-17A/F, and IL-17F [28], raising the question of whether all T-cell subsets are equally sensitive to TYK2 blockade.
The results of this serum biomarker analysis are consistent with those of the previous gene expression analysis, which demonstrated that deucravacitinib treatment was associated with suppression of the IL-23/Th17 pathway and keratinocyte activation, as well as reduction in type-I IFN responses, in the skin of patients with moderate to severe plaque psoriasis [23]. Results are similar to those reported during treatment with the IL-23 antagonist risankizumab and the dual IL-12/IL-23 antagonist ustekinumab, where decreases were seen early in the levels of several IL-23/Th17 pathway genes, including IL-17A, IL-17C, IL-17F, IL-22, IL-23A, and beta-defensin, and genes associated with keratinocytes, epidermal cells, and monocytes [29]. Suppression of IL-17A and IL-17F levels was significantly greater in patients switched from ustekinumab to the IL-23 antagonist guselkumab, as was neutralization of molecular scar and psoriasis-related gene markers in the skin [30]. Similarly, the IL-17A antagonist ixekizumab demonstrated dose-dependent reductions in IL-17A, IL-17F, IL-22, IL-23, and IFN-γ in psoriatic lesions, accompanied by reductions in keratinocyte proliferation, hyperplasia, epidermal thickness, and dermal infiltration by T cells and dendritic cells [31]. No direct comparative analysis of the various inhibitors has been performed.
Study limitations included the relatively short duration of this analysis and the potential variability in biomarker expression levels. Larger studies may provide additional insights about pharmacodynamic responses to deucravacitinib treatment in patients with plaque psoriasis.
Conclusion
In conclusion, biomarkers associated with IL-23/Th17 pathway expression and keratinocyte activation, which are elevated in the serum of patients with psoriasis, correlate with disease activity and response to deucravacitinib treatment. Clinical correlation with the phase 3 deucravacitinib clinical trials in moderate to severe plaque psoriasis [POETYK PSO-1 (NCT03611751) and PSO-2 (NCT03624127)] [32, 33] may provide valuable additional insights on efficacy of deucravacitinib for the treatment of plaque psoriasis.
Acknowledgments
Medical Writing and Editorial Assistance
Writing and editorial assistance was provided by Jieming Fang, MD, and Ann Marie Fitzmaurice, PhD, of Peloton Advantage, LLC, an OPEN Health company, funded by Bristol Myers Squibb.
Author Contributions
Ian M. Catlett, Subhashis Banerjee, and James G. Krueger contributed to the study concept and design, served as study investigators, and were involved in developing the methodology. Ian M. Catlett, Lu Gao, Yanhua Hu, Subhashis Banerjee, and James G. Krueger conducted the formal analysis. Ian M. Catlett enrolled patients and contributed to supervision of the study. Ian M. Catlett, Lu Gao, Yanhua Hu, Subhashis Banerjee, and James G. Krueger contributed to the original draft preparation, review, and editing. Bristol Myers Squibb funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing and approval of the publication. All authors had access to relevant data and participated in the drafting, review and approval of this publication. No honoraria or payments were made for authorship.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Bristol Myers Squibb.
Data Availability
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Declarations
Conflict of Interest
Ian M. Catlett was an employee of and shareholder in Bristol Myers Squibb at the time of study conduct. Lu Gao and Yanhua Hu are employees of and shareholders in Bristol Myers Squibb. Subhashis Banerjee was an employee of and shareholder in Bristol Myers Squibb at the time of study conduct. James G. Krueger has received research grants from Bristol Myers Squibb during the conduct of the study, personal fees from AbbVie, Baxter, Biogen, Delenex, Kineta, Sanofi, Serono, and Xenoport, and research grants from Amgen, Dermira, Innovaderm, Janssen, Kadmon, Kyowa, Lilly, Merck, Novartis, Paraxel, and Pfizer outside the submitted work.
Ethical Approval
This biomarker study was performed in accordance with the ethical principles that have their origin in the Declaration of Helsinki. The protocol, amendments, and patient informed consent received appropriate approval by the institutional review board or independent ethics committee prior to the initiation of the trial at the study center. All patients provided written informed consent before any study-related procedures were performed.
Footnotes
Prior Presentation: Catlett IM, et al. Presented at the 4th Inflammatory Skin Disease Summit; 3–6 November 2021; New York, NY.
References
- 1.Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Prim. 2016;2:16082. [DOI] [PubMed] [Google Scholar]
- 2.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-alpha production. J Exp Med. 2005;202(1):135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bissonnette R, Papp K, Maari C, Yao Y, Robbie G, White WI, et al. A randomized, double-blind, placebo-controlled, phase I study of MEDI-545, an anti-interferon-alfa monoclonal antibody, in subjects with chronic psoriasis. J Am Acad Dermatol. 2010;62(3):427–36. [DOI] [PubMed] [Google Scholar]
- 4.Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann Rheum Dis. 2018;77(2):175–87. [DOI] [PubMed] [Google Scholar]
- 5.Burke JR, Cheng L, Gillooly KM, Strnad J, Zupa-Fernandez A, Catlett IM, et al. Autoimmune pathways in mice and humans are blocked by pharmacological stabilization of the TYK2 pseudokinase domain. Sci Transl Med. 2019;11(502):eaaw1736. [DOI] [PubMed] [Google Scholar]
- 6.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. [DOI] [PubMed] [Google Scholar]
- 7.Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. 2017;140(3):645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witte E, Kokolakis G, Witte K, Philipp S, Doecke WD, Babel N, et al. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J Invest Dermatol. 2014;134(11):2757–67. [DOI] [PubMed] [Google Scholar]
- 9.Chan TC, Hawkes JE, Krueger JG. Interleukin 23 in the skin: role in psoriasis pathogenesis and selective interleukin 23 blockade as treatment. Ther Adv Chronic Dis. 2018;9(5):111–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Commins S, Steinke JW, Borish L. The extended IL-10 superfamily: IL-10, IL-19, IL-20, IL-22, IL-24, IL-26, IL-28, and IL-29. J Allergy Clin Immunol. 2008;121(5):1108–11. [DOI] [PubMed] [Google Scholar]
- 11.Konrad RJ, Higgs RE, Rodgers GH, Ming W, Qian YW, Bivi N, et al. Assessment and clinical relevance of serum IL-19 levels in psoriasis and atopic dermatitis using a sensitive and specific novel immunoassay. Sci Rep. 2019;9(1):5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolbinger F, Loesche C, Valentin MA, Jiang X, Cheng Y, Jarvis P, et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139(3):923-32.e8. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Chen L, Peng W, Deng H, Ni H, Tong H, et al. Th17/Treg balance: the bloom and wane in the pathophysiology of sepsis. Front Immunol. 2024;15:1356869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Gang X, Yang S, Cui M, Sun L, Li Z, et al. The alterations in and the role of the Th17/Treg balance in metabolic diseases. Front Immunol. 2021;12: 678355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui H, Wang N, Li H, Bian Y, Wen W, Kong X, et al. The dynamic shifts of IL-10-producing Th17 and IL-17-producing Treg in health and disease: a crosstalk between ancient “Yin-Yang” theory and modern immunology. Cell Commun Signal. 2024;22(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sotyktu [package insert]. Princeton, NJ, USA: Bristol Myers Squibb, 2022.
- 17.Sotyktu [package insert]. Seoul, South Korea: BMS Korea Pharmaceuticals Co., 2023.
- 18.Sotyktu [product information]. Mulgrave, VIC, Australia: Bristol Myers Squibb Australia Pty. Ltd., 2022.
- 19.Sotyktu [product monograph]. Montreal, QC, Canada: Bristol Myers Squibb Canada Co., 2022.
- 20.Sotyktu [European summary of product characteristics]. Dublin, Ireland: Bristol Myers Squibb EEIG, 2023.
- 21.Sotyktu [package insert]. Tokyo, Japan: Bristol Myers Squibb K.K., 2022.
- 22.Wrobleski ST, Moslin R, Lin S, Zhang Y, Spergel S, Kempson J, et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62(20):8973–95. [DOI] [PubMed] [Google Scholar]
- 23.Catlett IM, Hu Y, Gao L, Banerjee S, Gordon K, Krueger JG. Molecular and clinical effects of selective tyrosine kinase 2 inhibition with deucravacitinib in psoriasis. J Allergy Clin Immunol. 2022;149(6):2010-20.e8. [DOI] [PubMed] [Google Scholar]
- 24.Papp K, Gordon K, Thaci D, Morita A, Gooderham M, Foley P, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379(14):1313–21. [DOI] [PubMed] [Google Scholar]
- 25.Krueger JG, Wharton KA Jr, Schlitt T, Suprun M, Torene RI, Jiang X, et al. IL-17A inhibition by secukinumab induces early clinical, histopathologic, and molecular resolution of psoriasis. J Allergy Clin Immunol. 2019;144(3):750–63. [DOI] [PubMed] [Google Scholar]
- 26.Oliver R, Krueger JG, Glatt S, Vajjah P, Mistry C, Page M, et al. Bimekizumab for the treatment of moderate-to-severe plaque psoriasis: efficacy, safety, pharmacokinetics, pharmacodynamics and transcriptomics from a phase IIa, randomized, double-blind multicentre study. Br J Dermatol. 2022;186(4):652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sofen H, Smith S, Matheson RT, Leonardi CL, Calderon C, Brodmerkel C, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133(4):1032–40. [DOI] [PubMed] [Google Scholar]
- 28.Blauvelt A, Chiricozzi A. The immunologic role of IL-17 in psoriasis and psoriatic arthritis pathogenesis. Clin Rev Allergy Immunol. 2018;55(3):379–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visvanathan S, Baum P, Vinisko R, Schmid R, Flack M, Lalovic B, et al. Psoriatic skin molecular and histopathologic profiles after treatment with risankizumab versus ustekinumab. J Allergy Clin Immunol. 2019;143(6):2158–69. [DOI] [PubMed] [Google Scholar]
- 30.Campbell K, Li K, Yang F, Branigan P, Elloso MM, Benson J, et al. Guselkumab more effectively neutralizes psoriasis-associated histologic, transcriptomic, and clinical measures than ustekinumab. ImmunoHorizons. 2023;7(4):273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green L, Weinberg JM, Menter A, Soung J, Lain E, Jacobson A. Clinical and molecular effects of interleukin-17 pathway blockade in psoriasis. J Drugs Dermatol. 2020;19(2):138–43. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong AW, Gooderham M, Warren RB, Papp KA, Strober B, Thaçi D, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88(1):29–39. [DOI] [PubMed] [Google Scholar]
- 33.Strober B, Thaçi D, Sofen H, Kircik L, Gordon KB, Foley P, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, program for evaluation of TYK2 inhibitor psoriasis second phase 3 trial. J Am Acad Dermatol. 2023;88(1):40–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.