Abstract
Introduction
Patients with psoriasis (PSO) and psoriatic arthritis (PsA) may frequently switch biologic therapies over the course of treatment because of symptom variability and individual responses. Real-world studies analyzing patient characteristics and clinical factors associated with biologic switching are limited.
Methods
This longitudinal cohort study used real-world data from the CorEvitas Psoriasis Registry to evaluate the relationship between associated disease factors and biologic switching among patients with PSO and PsA in the United States (US) and Canada following initiation of a biologic. Patients were evaluated between April 2015–August 2022. Combinations of disease severity (as measured by Psoriasis Area Severity Index [PASI]) and Dermatology Life Quality Index (DLQI) as a measure of health-related quality of life (HRQoL) were assessed, and the association with time to switching was calculated using Cox proportional hazards regression modeling.
Results
Among 2580 patient-initiations (instances of patients initiating a biologic), 504 (19.5%) switched biologics within 30 months of initiation. Switching was more frequent when either PASI > 10 or DLQI > 5 compared with PASI ≤ 10 or DLQI ≤ 5 at follow-up. Patients with higher skin involvement (PASI > 10) and impact on HRQoL (DLQI > 5) were 14 times more likely to switch (hazard ratio = 14.2, 95% confidence interval: 10.7, 18.9) than those with lower skin involvement (PASI ≤ 10) and HRQoL (DLQI ≤ 5).
Conclusions
Patients with PSO and PsA treated in a real-world dermatology setting with substantial disease factors following biologic initiation were more likely to switch therapies. Those with PASI > 10 and DLQI > 5 switched more frequently than those with PASI ≤ 10 and DLQI ≤ 5.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01258-1.
Keywords: Psoriatic arthritis, CorEvitas Psoriasis Registry, Biologics, Real-world evidence, Therapy switch, Psoriasis treatment patterns, PASI, DLQI
Plain Language Summary
Many patients with psoriasis may also have a related condition called psoriatic arthritis. Biologic medications work by helping to reduce inflammation and are commonly used to treat the symptoms of psoriasis and psoriatic arthritis. Patients might not all respond the same way to treatment and may need to change their medications over time. It is important we understand the reasons for switching medications to help patients better manage their symptoms.
This study used information from a database on patients with both psoriasis and psoriatic arthritis. The database includes information on patients’ medical history, including when they start and change their medication. We looked at data from patients who switched medications and patients who did not switch medications and examined differences in both how serious a doctor found their disease and the patients’ own opinions of their overall health.
We found that patients were more likely to change their biologic medication if they had more difficult psoriasis and psoriatic arthritis symptoms that caused worse skin problems, joint pain, and effects on their overall health compared with patients who had not changed their medication. These results suggest that it is important to consider both how serious a doctor finds their disease and patients’ opinions of how much their symptoms affect their overall health. Understanding the reasons why patients switch medications will help to develop better ways of managing psoriasis and psoriatic arthritis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-024-01258-1.
Key Summary Points
| Patients with psoriasis (PSO) and psoriatic arthritis (PsA) may need to switch biologic therapies throughout the course of treatment for various reasons. |
| Factors that influence biologic switching among patients with PSO and PsA remain unclear. |
| The objectives of this study were to evaluate the association of jointly measured psoriatic disease severity (e.g., Psoriasis Area Severity Index [PASI]) and patient-centered outcomes (e.g., Dermatology Life Quality Index [DLQI]) with biologic switch status among patients with PSO and PsA and to describe patient characteristics at initiation among those who switched or did not switch biologic therapies. |
| In this analysis of PSO patients with PsA treated in a real-world dermatology setting, measures related to disease factors were more severe among patients who switched biologic therapies. |
| Patient-centered factors should be considered as potential targets for clinical interventions intended to optimize treatment strategies for patients with PSO and PsA. |
Introduction
Psoriasis (PSO) is a common, immune-mediated inflammatory disease of the skin, which is associated with red, itchy, and often painful plaques [1]. Psoriatic arthritis (PsA) is a spondyloarthropathy that causes inflammation and swelling of the joints and is diagnosed in up to 40% of patients with PSO [2–4]. Symptoms associated with a dual PSO and PsA diagnosis can be disabling and have significant negative effects on quality of life (QoL) [5].
Several effective systemic biologic therapies are available to treat PSO and PsA, but patients may often switch systemic biologic therapies over the course of treatment because of heterogeneity across symptom profiles and individual responses to treatment [2, 6–11]. Estimates from claims-based studies suggest that approximately 20% of patients with PSO and approximately 20% of patients with PsA switch treatments within 1 year of biologic initiation [12, 13]. For some patients, therapy switches may lead to poorer outcomes and increased healthcare costs [6, 10, 11, 14]. The relationship between health-related QoL (HRQoL) and disease severity measures as drivers of biologic therapy switching among patients with PSO or PsA is unclear [9, 15, 16].
The total disease burden of patients with PSO may not be captured by objective disease activity measurements alone (e.g., Psoriasis Area Severity Index; PASI) [17]. In addition to an objective evaluation of disease activity by a physician, the patient’s own evaluation of the overall effects on their QoL by assessing the Dermatology Life Quality Index (DLQI) is important for accurate disease assessment at the time of treatment initiation [18]. However, data suggest that the decision to initiate treatment in PSO is more strongly associated with PASI. As a result, the significance of a patient-reported outcome like the DLQI is likely undervalued [19].
Additional research is needed to identify PSO and PsA patient profiles as well as clinical factors that may be related to biologic treatment switching. Here, the association of disease factors, as suggested by estimates of both PASI and DLQI on observed biologic therapy switch occurrences, was assessed in real-world settings. More specifically, the primary objective of this analysis was to evaluate the association of psoriatic disease severity (e.g., PASI) and patient-centered outcomes (e.g., DLQI or joint pain) with systemic biologic therapy switch status after treatment initiation among patients with PSO and concomitant PsA in the CorEvitas Psoriasis Registry.
Methods
Patient Population and Study Design
The CorEvitas Psoriasis Registry is an independent, prospective, multicenter, observational United States (US) and Canada registry that was launched in April 2015 in collaboration with the National Psoriasis Foundation [20]. The US registry sites account for > 90% of the total number of patient initiations, whereas the Canadian sites account for < 10%. Registry data are collected from patients and providers at routine medical visits, spaced approximately 6 months apart, or at the time of a qualifying change in therapy, whichever occurs first. At each registry visit, clinicians report therapy start and discontinuation dates for any systemic biologic or non-biologic therapy used to treat PSO. Changes in therapy occurring at registry visits are recorded as being made on the date of the registry visit. Changes in medication occurring in between registry visits are recorded at the subsequent registry visit.
This study used a longitudinal cohort design and relied on the use of real-world PSO patient data from the CorEvitas Psoriasis Registry. Patient characteristics and associated disease factors were described among patients with PSO and PsA who either switched or did not switch biologic therapy following initiation of a biologic for PSO that was eligible for registry enrollment (Supplementary Table 1). Data for this analysis were collected during registry visits with a dermatologist from patients who initiated a biologic therapy on or after the date the registry was launched (April 1, 2015) until August 1, 2022, using the data available as of August 10, 2022 (Supplementary Figure 1).
Initiations, which were defined as the first use of any new biologic, were included in this study if patients had a history of plaque PSO, had PsA at the time of new biologic therapy initiation, and started a new biologic therapy at or after registry enrollment and up to 42 days following a registry visit (Supplementary Figure 2). Individual patients were allowed to contribute multiple initiations if they used multiple biologic therapies following registry enrollment. Additionally, a single initiation could contribute multiple follow-up visits within the study period if a patient was persistent on their initial systemic biologic therapy. Having PsA at the time of biologic initiation was defined as either physician-indicated (any rheumatologist- or dermatologist-confirmed diagnosis of PsA) or any history of a Psoriasis Epidemiology Screening Tool (PEST) score ≥ 3 [21], recorded in the CorEvitas Psoriasis Registry on or prior to the treatment initiation date. Each initiation was followed until a discontinuation/switch of the initial biologic therapy occurred, the last registry follow-up, or 913 days (30 months) had been reached after initiation, whichever occurred first. The number of biologic initiations and follow-up registry visits were recorded as intervals of 6–30 months, 12–30 months, 18–30 months, and 24–30 months. All registry visits past 30 months, including all biologic switches past 30 months, were not included in the analysis.
All data for a particular initiation were excluded if a patient did not have an eligible registry visit within 42 days prior to starting a new biologic, had uncertainty in their start date that prevented determination of the date of biologic initiation, had any missing baseline measures (e.g., age, sex, race, ethnicity, duration of PSO, biologic experience, baseline PASI/DLQI category, body mass index [BMI], employment status, and number of concomitant diseases, as well as biologic, non-biologic, topical, and phototherapy history), did not have any follow-up registry visits in the subsequent 913 days (30 months) after biologic initiation, or had all individual follow-up visits excluded. Individual follow-up visits were excluded from the analysis if a therapy switch occurred prior to a subsequent registry visit (outside of the follow-up window), PASI or DLQI were missing at any registry follow-up visit, or there were indeterminate dates of initiations or discontinuations that prevented identification of a biologic switch event within the window around a registry follow-up visit. No imputation or other handling of missing data was performed.
This study was performed in accordance with the Declaration of Helsinki [22] and the Guidelines for Good Pharmacoepidemiology Practice [23]. All participating investigators were required to obtain full board approval for conducting noninterventional research involving human subjects with a limited dataset. Sponsor approval and continuing review were obtained through a central Institutional Review Board (IRB) (Advarra, protocol number Pro00051221). For academic investigative sites that did not receive authorization to use the central IRB, full board approval was obtained from their respective governing IRBs, and documentation of approval was submitted to CorEvitas, LLC, before the site’s participation and initiation of any study procedures. All patients in the registry were required to provide written informed consent and authorization before participating in the CorEvitas Psoriasis Registry.
Study Measures
Exposure Measures
The primary exposure for this study was recorded at each registry visit following systemic biologic therapy initiation and defined as a combination of DLQI and PASI as a four-level categorical variable; these values served as measures of HRQoL and skin clearance, respectively, as previously described [17]. PASI and DLQI were categorized into all pairwise combinations of PASI ≤ 10, PASI > 10, DLQI ≤ 5, and DLQI > 5. The PASI combines the extent of body surface involvement in four anatomical regions (head, trunk, arms, and legs) [24]. For each region, the percent area of skin involved was estimated from 0 (0%) to 6 (90–100%), and severity was estimated by clinical signs of erythema, induration, and scaling, with a score ranging from 0 (none) to 4 (very severe). Each area was scored separately, with scores then combined as the sum of severity parameters for each region × area score × weighing factor (head [0.1], upper limbs [0.2], trunk [0.3], lower limbs [0.4]) for the final PASI. Overall scores ranged from 0 (no psoriasis) to 72 (the most severe disease). The DLQI is a 10-item, participant self-administered dermatology-specific questionnaire that evaluates HRQoL across six domains including symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment. DLQI item response categories were scored 0 (not relevant) to 3 (very relevant) with a total score range of 0 to 30; higher scores indicated poorer HRQoL [25].
The secondary analysis considered joint pain as another measure of HRQoL. Patients were classified as having moderate/severe joint pain if the score on the Visual Analog Scale (VAS) Joint Pain measure was ≥ 40, as previously described [26]. This binary measure replaced DLQI as the time-varying measure of HRQoL, and methods used for the primary analysis were repeated using the PASI/VAS Joint Pain interaction. As VAS Joint Pain was only recorded for patients with a physician indication of PsA, the secondary analysis was restricted to only patients with physician-verified PsA.
Outcome Measures
The primary outcome for this study was the time from initiation to a switch in systemic biologic therapy. A biologic switch was defined as the (observed or planned) discontinuation of the initial systemic biologic therapy and a subsequent start or prescription of a different biologic ≤ 45 days after the discontinuation. At a given registry follow-up visit, a provider was able to report that a new biologic was prescribed but not yet started. This was defined as a planned switch, in which case the time from initiation to biologic switch was defined as initiation to the date that the prescription occurred. Biologic initiations that resulted in a treatment switch were summarized by the type of switch, which was categorized as starting a biologic therapy with the same mechanism of action (MOA), with a different MOA, or from/to a drug approved/unapproved by the Food and Drug Administration (FDA) or Health Canada for the treatment of PsA. Only biologic therapies deemed eligible for inclusion in the registry as previously described were considered as eligible biologic switches [20]. Either discontinuation of the initial biologic or start of the new biologic therapy must have occurred within 42 days of a registry visit to be considered for inclusion in this analysis. A patient not having a biologic switch at a registry follow-up visit (i.e., a non-switcher) could have remained on the initial therapy or discontinued the initial biologic without starting a new therapy. In these specific cases, the time-to-event outcome was defined as the date of the last registry visit and date of the biologic therapy discontinuation, respectively.
Covariates
All covariates were recorded at each biologic initiation and used to summarize the sample or considered as potential confounders. Concomitant disease, medical, and medication history considered the occurrence or previous use at any point in the patient’s history. Demographic and socioeconomic characteristics included age (years), sex (male, female), race (white, black, Asian, Hispanic, other/unknown), type of health insurance (private, non-private), education (high school or less, any college), and employment status (full time, not full time). Lifestyle characteristics included smoking status (never, any smoking history), alcohol use (< 1 drink per day, ≥ 1 drink per day), body weight (kg, continuous), BMI (underweight/normal: < 25 kg/m2, overweight: 25– < 30 kg/m2, Class 1 obesity: 30– < 35 kg/m2, ≥ Class 2 obesity: ≥ 35 kg/m2). History of any previous concomitant diseases was recorded as the number of concomitant diseases (none: 0, any: 1, ≥ 2) and considered the sum of prior (any history of) physician-reported concomitant diseases captured at the time of biologic initiation including congestive heart failure, peripheral vascular disease, cerebrovascular disease (stroke or transient ischemic attack), chronic obstructive pulmonary disease, peptic ulcer disease, diabetes mellitus, lymphoma, and solid tumor cancer (excluding non-melanoma skin cancer). Psoriatic disease severity measures included PSO duration (years), history of non-plaque morphology (inverse/intertriginous, guttate, erythrodermic, pustular-localized, pustular-generalized), history of PSO involving a high impact area (scalp, nail, palmoplantar, genital), PsA duration (years; restricted to only patients with physician-indicated PsA), PEST (score; patients scoring ≥ 3 are recommended for a rheumatologist referral) [27], body surface area ([BSA]; percentage) [28], PASI (0 [no body surface involvement] to 72 [most severe disease]) [24], Investigator Global Assessment ([IGA]; 5-point severity scale [0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, and 4 = severe]) [29]. Patient-reported symptom burden measures included the skin pain, itch/pruritis, and fatigue 100-point VAS (score; 0 [no pain/itch/fatigue] to 100 [most severe pain/itch/fatigue]) [30], 100-point VAS Joint Pain (score; 0 [no pain] to 100 [most severe pain]) [26], Patient Global Assessment (PGA) of PSO (100-point VAS; scored 0 [very well] to 100 [very poor]) [31], EuroQoL 5-dimensional, 3-level ([EQ-5D-3L]; scored 0 [death] to 1 [full health]) [32], EQ-VAS (score; 0 [worst imaginable health state] to 100 [best imaginable health state]) [33], DLQI (0 [no impairment on life quality] to 30 [maximum impairment on life quality]) [34], and combination of PASI/DLQI (categorized into groups of patients with PASI ≤ 10, PASI > 10, DLQI ≤ 5, and DLQI > 5) [17]. The PASI/DLQI response at follow-up was considered a time-varying covariate. PSO treatment characteristics included current biologic therapy MOA (TNFi, IL-12/23i, IL-17i, IL-23i), number of prior biologic therapies used at initiation (0, 1, 2, 3 +), number of prior non-biologic therapies used at initiation (0, 1, 2+), number of prior topical therapies used at initiation (0, 1, 2, 3+), and number of prior phototherapies used at initiation (0, 1, 2+).
Statistical Analysis
Means (standard deviations [SDs]) and frequencies (percentages) were reported for each patient characteristic at biologic therapy initiation. A summary of treatment switches/non-switches over follow-up was provided separately for each of the PASI/DLQI response levels. Frequencies (percentages) of treatment switches/non-switches were reported across all eligible registry visits. The frequency and rate of switching from all follow-up visits were reported as well. The median (interquartile range [IQR]) was reported in addition to the mean (SD) and/or frequencies (percentages) for baseline covariates and time until switch.
Hazard ratios (HRs; 95% confidence intervals [CIs]) assessing the relative risk between the PASI/DLQI groups were reported as a measure of association among disease factors, baseline covariates, and biologic switch over time following biologic initiation. Proportional hazards regression models were used at the time of new biologic initiation to estimate unadjusted HRs between the PASI/DLQI levels and biologic switch. The outcome of this model was the time where the follow-up visit occurred with the biologic therapy switch or non-switch as previously defined. Patients who did not switch were considered right-censored at each follow-up visit in the statistical analysis because of this being the time at which PASI/DLQI was recorded.
Adjusted proportional hazards regression models were used to obtain HRs adjusted for characteristics observed at initiation. In addition to the PASI/DLQI response at follow-up, age, sex, race, ethnicity, duration of PSO, biologic experience, baseline PASI/DLQI category, BMI, employment status, and number of concomitant diseases, as well as biologic, non-biologic, topical, and phototherapy history (separately), all measured at each biologic initiation, were included as covariates. The PASI/DLQI indicators were included as time-varying covariates measured at follow-up registry visits. A study period indicator (time varying at follow-up) was used to account for temporal differences that may have affected switch rates. Time was divided into three periods covering April 15, 2015–June 13, 2017 (period 1: from the beginning of the registry to the approval of the first IL-23), June 14, 2017–February 28, 2020 (period 2: from the approval of the first IL-23 to the start of the Coronavirus disease 2019 [COVID-19] pandemic), and March 1, 2020–August 4, 2022 (period 3: from the start of the COVID-19 pandemic to the last day of data collection prior to the proposed data cut).
Unadjusted and adjusted HRs (95% CIs) were reported comparing the DLQI ≤ 5 and DLQI > 5 groups separately for patients with PASI ≤ 10 and PASI > 10. Conversely, HRs (95% CIs) between the PASI ≤ 10 and PASI > 10 groups were compared separately for patients with DLQI ≤ 5 and DLQI > 5. Unadjusted and adjusted HRs were also reported for each baseline measure considered.
Results
Baseline Demographics, Lifestyle, and Disease Characteristics
The final analytic sample contained 5497 follow-up registry visits originating from 2580 patient initiations and 2198 individual patients (Table 1). Of the patient initiations, 576, 967, 1416, and 2123 had their last registry follow-up at least 24 months, 18 months, 12 months, and 6 months after initiation, respectively. This corresponded to 4506, 2650, 1448, and 602 registry visits in these respective timeframes.
Table 1.
Baseline demographic and lifestyle characteristics of patients in the CorEvitas Psoriasis Registry with eligible biologic initiations
| Total patients included (N = 2580) | |
|---|---|
| Demographic characteristicsa | |
| Age (years) | |
| Mean (SD) | 52.0 (13.2) |
| Median (IQRb) | 53.0 (43.0, 61.0) |
| Sex, n (%) | |
| Male | 1234 (47.8) |
| Female | 1346 (52.2) |
| Race, n (%) | |
| white | 2017 (78.2) |
| Black | 79 (3.1) |
| Asianc | 186 (7.2) |
| Hispanic | 194 (7.5) |
| Other/unknown | 104 (4.0) |
| Health insurance (n = 2393), n (%) | |
| Private (vs. non-private) | 1721 (71.9) |
| Education (n = 2573), n (%) | |
| Any college (vs. no college) | 1725 (67.0) |
| Employment, n (%) | |
| Not full-time | 1130 (43.8) |
| Full-time | 1450 (56.2) |
| Lifestyle characteristics | |
| Smoking status (n = 2559), n (%) | |
| Never smoked | 1181 (46.2) |
| Former/current smoker | 1378 (53.8) |
| Alcohol use (n = 2425), n (%) | |
| ≤ 1 drink per day | 1512 (62.4) |
| > 1 drink per day | 913 (37.6) |
| BMI, n (%) | |
| Underweight/normal (< 25 kg/m2) | 379 (14.7) |
| Overweight (25– < 30 kg/m2) | 745 (28.9) |
| Class 1 obesity (30– < 35 kg/m2) | 635 (24.6) |
| ≥ Class 2 obesity (≥ 35.0 kg/m2) | 821 (31.8) |
| History of concomitant diseasesd, n (%) | |
| 0 | 1877 (72.8) |
| 1 | 581 (22.5) |
| ≥ 2 | 122 (4.7) |
BMI body mass index, COPD chronic obstructive pulmonary disease, IQR interquartile range, SD standard deviation, TIA transient ischemic attack
aSample sizes may differ because of missing data and denominators are specified only when missing data were present
bInterquartile ranges (IQRs) are presented as the first and third quartiles
cThe specific ethnicity or nationality cannot be specified as the patient demographic data come from a registry source (CorEvitas Psoriasis Registry), which does not provide this level of detailed information
dThe total number of the following conditions: congestive heart failure, peripheral vascular disease, cerebrovascular disease (captured as stroke or TIA), COPD, peptic ulcer disease, diabetes mellitus, lymphoma, and solid tumor cancer (excluding non-melanoma skin cancer)
The mean (SD) patient age at initiation was 52.0 (13.2) years with 52.2% (n = 1346) being female. Approximately one-third of patient initiations (31.8%; n = 821) had at least Class 2 obesity (BMI ≥ 35.0 kg/m2), 53.8% (n = 1378) were current or former smokers, and roughly one-quarter (27.2%; n = 703) had a history of ≥ 1 concomitant disease. Patient initiations had PSO for a mean (SD) duration of 17.0 (13.7) years (Table 2). Regarding PsA (n = 2103), the mean (SD) duration was 7.8 (9.1) years, and 63.6% (n = 1219) had clinically meaningful joint pain at baseline (VAS Joint Pain ≥ 40).
Table 2.
Baseline disease and treatment characteristics of patients in the CorEvitas Psoriasis Registry with eligible biologic initiations
| Total patients included (N = 2580) | |
|---|---|
| Psoriatic disease severitya | |
| PSO duration (years) | |
| Mean (SD) | 17.0 (13.7) |
| Median (IQRb) | 14.0 (6.0, 26.0) |
| History of non-plaque morphologyc, n (%) | 459 (17.8) |
| Inverse/intertriginous | 294 (11.4) |
| Guttate | 154 (6.0) |
| Erythrodermic | 57 (2.2) |
| Pustular-localized | 37 (1.4) |
| Pustular-generalized | 15 (0.6) |
| History of difficult to treat areac, n (%) | 1311 (50.8) |
| Scalp | 1093 (42.4) |
| Nail | 594 (23.0) |
| Palmoplantar | 332 (12.9) |
| Genital (n = 2262) | 273 (12.1) |
| PsA diagnosisc, d, n (%) | |
| Physician-indicated | 2103 (81.5) |
| History of PEST ≥ 3 | 1872 (72.6) |
| PsA durationc, d (years; n = 2103) | |
| Mean (SD) | 7.8 (9.1) |
| Median (IQRb) | 5.0 (1.0, 11.0) |
| PEST (N = 2547) | |
| Mean (SD) | 2.9 (1.3) |
| Median (IQRb) | 3.0 (2.0, 4.0) |
| BSA (% involvement; n = 2577) | |
| Mean (SD) | 13.6 (15.9) |
| Median (IQRb) | 9.0 (4.0, 17.0) |
| BSA (n = 2577), n (%) | |
| Mild disease (< 3%) | 467 (18.1) |
| Moderate disease (≥ 3–10%) | 1139 (44.2) |
| Severe disease (> 10%) | 971 (37.7) |
| PASI | |
| Mean (SD) | 7.7 (7.4) |
| Median (IQRb) | 5.5 (2.6, 10.8) |
| PASI > 10, n (%) | 726 (28.1) |
| IGA (N = 2576) | |
| Mean (SD) | 2.8 (1.0) |
| Median (IQRb) | 3.0 (2.0, 3.0) |
| Patient-reported disease factorsa | |
| Skin paine (VAS-100) (n = 2574) | |
| Mean (SD) | 37.1 (32.9) |
| Median (IQRb) | 30.0 (5.0, 65.0) |
| Itche (VAS-100) (n = 2575) | |
| Mean (SD) | 52.7 (33.5) |
| Median (IQRb) | 58.0 (20.0, 80.0) |
| Fatiguee (VAS-100) (n = 2573) | |
| Mean (SD) | 44.0 (29.7) |
| Median (IQRb) | 47.0 (15.0, 70.0) |
| Joint paind, e (VAS-100) (n = 1918) | |
| Mean (SD) | 50.1 (30.8) |
| Median (IQRb) | 50.0 (23.0, 77.0) |
| Joint pain (VAS-100) ≥ 40, n (%) | 1219 (63.6) |
| PGAe of PSO (VAS-100) (n = 2575) | |
| Mean (SD) | 50.1 (28.5) |
| Median (IQRb) | 50.0 (25.0, 75.0) |
| EQ-5D-3L utility score (n = 2525) | |
| Mean (SD) | 0.8 (0.2) |
| Median (IQRb) | 0.8 (0.7, 0.8) |
| EQ-VASe (n = 2572) | |
| Mean (SD) | 67.1 (20.4) |
| Median (IQRb) | 70.0 (50.0, 80.0) |
| DLQI | |
| Mean (SD) | 8.1 (6.3) |
| Median (IQRb) | 7.0 (3.0, 12.0) |
| Baseline treatment characteristicsa | |
| Biologic therapy MOA, n (%) | |
| TNFi | 418 (16.2) |
| IL-12/23i | 167 (6.5) |
| IL-17i | 1157 (44.8) |
| IL-23i | 838 (32.5) |
| Number of prior biologic therapies usedf, n (%) | |
| 0 (e.g., biologic naïve) | 709 (27.5) |
| 1 | 627 (24.3) |
| 2 | 505 (19.6) |
| 3+ | 739 (28.6) |
| Number of prior non-biologic therapies usedf, n (%) | |
| 0 (e.g., non-biologic naïve) | 932 (36.1) |
| 1 | 1087 (42.1) |
| 2+ | 561 (21.7) |
| Number of prior topical therapies usedf, n (%) | |
| 0 (e.g., topical therapy naïve) | 87 (3.4) |
| 1 | 967 (37.5) |
| 2 | 731 (28.3) |
| 3+ | 795 (30.8) |
| Number of prior phototherapies usedf, n (%) | |
| 0 (e.g., phototherapy naïve) | 1933 (74.9) |
| 1 | 564 (21.9) |
| 2+ | 83 (3.2) |
BSA body surface area, DLQI Dermatology Life Quality Index, EQ-5D-3L EuroQoL 5-dimensional, 3-level, EQ-VAS EuroQoL VAS, i inhibitor, IGA Investigator Global Assessment, IL interleukin, IQR interquartile range, MOA mechanism of action, PASI Psoriasis Area and Severity Index, PEST Psoriasis Epidemiology Screening Tool, PGA Patient Global Assessment, PsA psoriatic arthritis, PSO psoriasis, QoL quality of life, SD standard deviation, TNF tumor necrosis factor, VAS visual analog scale
aSample sizes may differ because of missing data, and denominators are specified only when missing data were present
bIQRs are presented as the first and third quartiles; cindicators are not mutually exclusive and may not sum to the total
dAssessment was restricted to patients with a rheumatologist- or dermatologist-confirmed diagnosis of PsA at initiation
eScores range from 0 to 100 with higher scores indicating worse disease state, symptom burden, or QoL at the time of starting biologic therapy
fNumbers refer to the quantity of prior therapies (within the respective subcategory) used by each patient at initiation
The mean (SD) BSA percent involvement at baseline was 13.6 (15.9; n = 2577). Among these initiations, 44.2% (n = 1139) noted moderate disease (BSA = 3–10%), while 37.7% (n = 971) noted starting with severe PSO according to BSA involvement (> 10%). The mean (SD) PASI was 7.7 (7.4), and mean (SD) DLQI was 8.1 (6.3) at baseline among patient initiations in this cohort. Based on PASI, 28.1% (n = 726) were considered to have severe disease (PASI > 10).
Biologic Therapy Switch Patterns and Associated Characteristics
Among the patient initiations evaluated, 19.5% (n = 504) switched biologic therapies (Table 3). The median (IQR) time from initiation to biologic switch was 6.5 (4.6, 12.4) months. Within the patient initiations that switched, about three-quarters (74.4%; n = 375) changed biologic classes and 64.5% (n = 325) switched to a biologic therapy approved for the treatment of PsA. Discontinuations due to ineffectiveness or safety issues (n = 426) were reported as the most common reasons for switching biologics (Table 4). Among the discontinuations related to ineffectiveness or safety issues, failure to maintain initial response and inadequate initial response constituted almost 70% (n = 298) of the reasons for discontinuing the initial biologic therapy.
Table 3.
Biologic switch patterns among patient initiations
| Summary (N = 2580), n (%) | Time until switch (months), median (IQRa) | |
|---|---|---|
| Result of biologic initiation | ||
| Persistent at last visitb | 1973 (76.5) | – |
| Discontinuation without switchc | 103 (4.0) | – |
| Switch to different biologic | 504 (19.5) | 6.5 (4.6, 12.4) |
| MOA type after switchb | ||
| Switch to a biologic therapy within the same/initial MOA | 129 (25.6) | 8.0 (5.2, 16.4) |
| Switch to a biologic therapy with a different MOA | 375 (74.4) | 6.4 (4.5, 11.6) |
| Approval status of biologic therapiesd, e | ||
| Biologic approved for PsA → biologic approved for PsA | 248 (49.2) | 6.2 (4.4, 11.0) |
| Biologic approved for PsA → biologic not approved for PsA | 115 (22.8) | 6.4 (4.6, 11.8) |
| Biologic not approved for PsA → biologic approved for PsA | 77 (15.3) | 6.3 (4.8, 11.3) |
| Biologic not approved for PsA → biologic not approved for PsA | 64 (12.7) | 14.6 (8.1, 20.4) |
FDA Food and Drug Administration, IQR interquartile range, MOA mechanism of action, PsA psoriatic arthritis
aIQRs are presented as the first and third quartiles
bIndicates persistent use of the initial biologic therapy without switching at the last registry visit (non-switch)
cPatient stopped biologic therapy without switching to a new biologic (non-switch)
dAmong all patients that switched
eA biologic therapy was considered approved for PsA based on the FDA or Health Canada approval status at the time of initiation or switch
Table 4.
Reasons for discontinuation and median time until switch among patients with PSO and PsA who switched biologic therapy
| Switchers (N = 504), n (%) | Time from biologic initiation to switcha, median (IQRb) | |
|---|---|---|
| Reasons for biologic therapy switch | ||
| Effectiveness or safety (n = 426) | ||
| Failure to maintain initial response | 171 (40.1%) | 8.7 (5.7, 14.4) |
| Inadequate initial response | 127 (29.8%) | 5.6 (3.4, 6.7) |
| Alternative MOA | 45 (10.6%) | 6.4 (5.2, 11.7) |
| Active disease | 44 (10.3%) | 11.0 (5.5, 17.8) |
| Side effect (minor and serious) | 35 (8.2%) | 3.8 (2.7, 7.0) |
| Improve tolerability | 3 (0.7%) | 6.7 (–, –) |
| Improve compliance | 1 (0.2%) | 1.8 (–, –) |
| Other reason not related to effectiveness or safety (n = 78) | ||
| Other reasonc | 35 (44.9%) | 6.7 (3.5, 16.6) |
| Patient preference | 21 (26.9%) | 6.8 (5.0, 17.7) |
| Missing reason | 17 (21.8%) | – |
| Fear of future side effect | 3 (3.8%) | 8.3 (–, –) |
| Temporary interruption | 1 (1.3%) | 6.6 (–, –) |
| Drug administration | 1 (1.3%) | 12.8 (–, –) |
COVID-19 Coronavirus disease 2019, IQR interquartile range, MOA mechanism of action
aTime from initiation was measured in months. These quantities are suppressed when sample sizes for each category were < 5
bIQRs are presented as the first and third quartiles
cOther reasons included: denied by insurance, co-pay/patient cost, patient doing well, pregnancy, breastfeeding, COVID-19 concerns, or other
Among patients with PSO with dermatologist-verified PsA who initiated biologic therapies and were included in this analysis, characteristics and baseline measures that were associated with biologic switching included younger age (compared with a 10-year increase from the baseline age), female sex, and white race, while a longer duration of PSO was associated with less frequent switching (Supplementary Table 2). Prior to the initiation included in this study, an approximately equal percentage of patient initiations had never used a biologic therapy (27.5%; n = 709) as those who had used ≥ 3 (28.6%; n = 739) (Table 2). Patients who had previously used a biologic therapy or phototherapy were 42% (HR = 1.42, 95% CI: 1.15, 1.76) and 60% (HR = 1.60, 95% CI: 1.28, 2.00) more likely to switch, respectively, while patients who had previously used a non-biologic therapy were 27% (HR = 0.73, 95% CI: 0.60, 0.90) less likely to switch (Supplementary Table 2).
Association of Disease Factors and Joint Pain with Biologic Switching
Biologic switching was more common among follow-up visits with higher compared with lower PASI, DLQI, or VAS Joint Pain threshold levels (Figs. 1 and 2). Switching occurred more frequently when there was high skin involvement (PASI > 10) or increased patient-reported symptom burden (DLQI > 5 or VAS Joint Pain ≥ 40) at follow-up (Fig. 1 and Table 5). Among patient initiations with PASI ≤ 10 and DLQI ≤ 5, only 5.0% (n = 217) were switchers compared with roughly 21–49% of those with either PASI > 10 (n = 99) or DLQI > 5 (n = 263). Conversely, switches were less likely to occur when both skin involvement (PASI ≤ 10) and patient-reported symptom burden were low (DLQI ≤ 5 or VAS Joint Pain < 40). After initiation, 79.6% of patients were noted as having both DLQI ≤ 5 and PASI ≤ 10 (n = 4374 of follow-up registry visits).
Fig. 1.
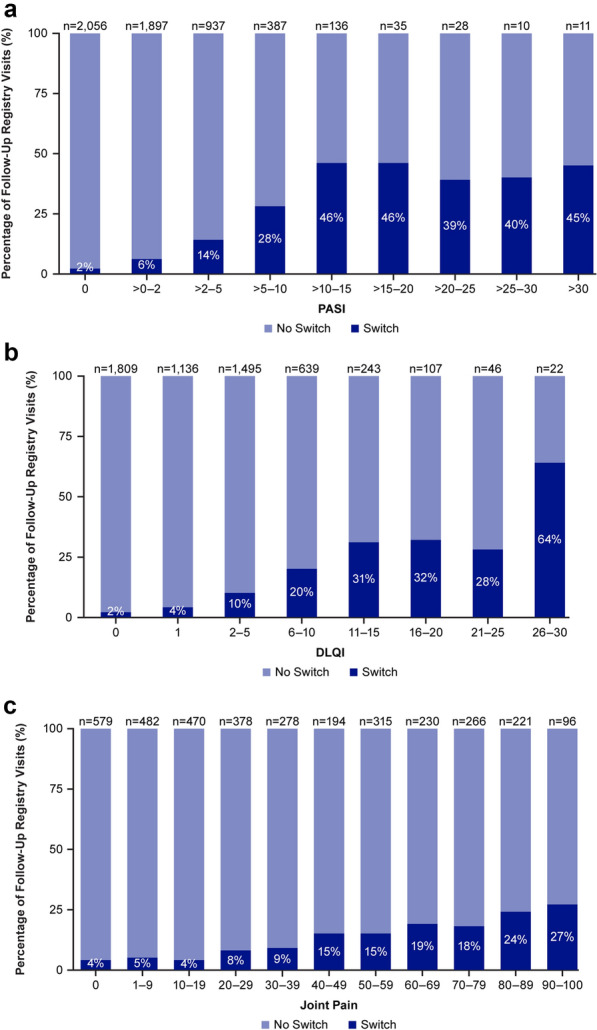
Rate of biologic switching across all follow-up visits by A PASI, B DLQI, or C VAS joint pain thresholds. N number of follow-up registry visits, DLQI Dermatology Life Quality Index, PASI Psoriasis Area Severity Index, VAS visual analog scale
Fig. 2.
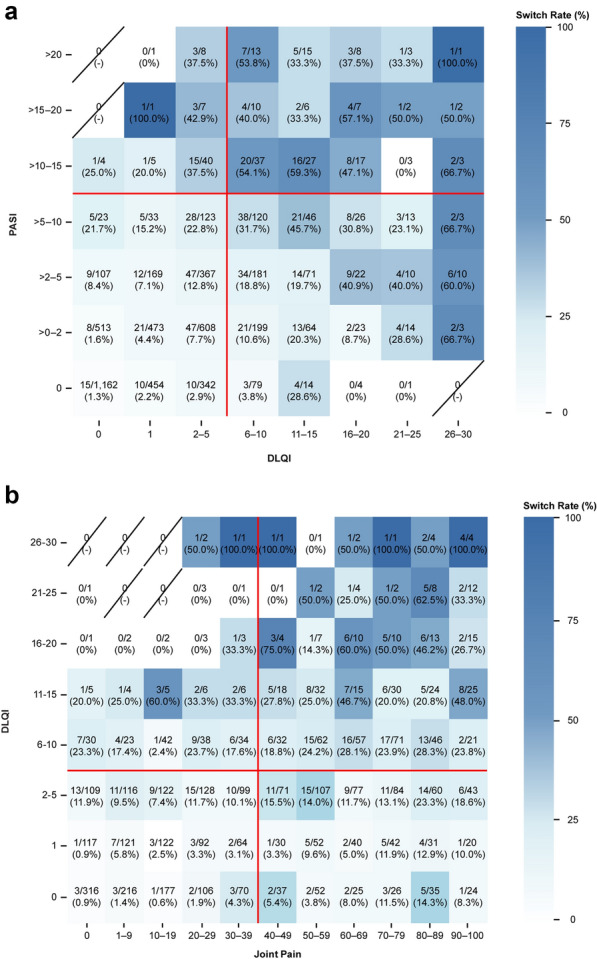
Relationship of A PASI and DLQI or B DLQI and VAS joint pain with biologic switch across all follow-up visitsa. aSwitch rates are represented in each cell as n/N (%), with n = number of biologic therapy switches and N = sample sizes (the number of registry follow-up visits for each PASI/DLQI or DLQI/VAS joint pain level). Red lines correspond to PASI and DLQI thresholds (i.e., PASI > 10 or DLQI > 5) or VAS joint pain and DLQI thresholds (i.e., VAS Joint Pain ≥ 40 or DLQI > 5) used in the primary analysis. DLQI Dermatology Life Quality Index, PASI Psoriasis Area Severity Index, VAS visual analog scale
Table 5.
Psoriatic disease factors and joint pain levels across all follow-up visits among switchers and non-switchers
| Switchers (n = 504) | Non-switchers (n = 4993) | Total (N = 5497) | |
|---|---|---|---|
| PASI/DLQI categories, n (%)b | |||
| PASI ≤ 10 and DLQI ≤ 5 | 217 (5.0) | 4157 (95.0) | 4374 |
| PASI > 10 and DLQI ≤ 5 | 24 (36.4) | 42 (63.6) | 66 |
| PASI ≤ 10 and DLQI > 5 | 188 (20.8) | 715 (79.2) | 903 |
| PASI > 10 and DLQI > 5 | 75 (48.7) | 79 (51.3) | 154 |
| Switchersb (n = 389) | Non-switchersb (n = 3188) | Total (N = 3577) | |
|---|---|---|---|
| PASI/VAS joint painc categories, n (%)b | |||
| PASI ≤ 10 and VAS joint pain < 40 | 104 (4.9) | 2032 (95.1) | 2136 |
| PASI > 10 and VAS joint pain < 40 | 21 (41.2) | 30 (58.8) | 51 |
| PASI ≤ 10 and VAS joint pain ≥ 40 | 208 (16.2) | 1079 (83.8) | 1287 |
| PASI > 10 and VAS joint pain ≥ 40 | 56 (54.4) | 47 (45.6) | 103 |
DLQI Dermatology Life Quality Index, PASI Psoriasis Area and Severity Index, PsA psoriatic arthritis, VAS visual analog scale
aAll eligible follow-up visits were used and the PASI/DLQI recategorized based on these values at follow-up
bPercentage per category indicated
cPatients with a dermatologist diagnosis of PsA at initiation; scores range from 0 to 100 with higher scores indicating worse symptom burden
Additionally, greater joint pain was associated with higher switch rates, irrespective of PASI. Among patient initiations with PASI ≤ 10, switch rates were 4.9% (n = 104) for follow-up visits where no clinically meaningful joint pain was reported and 16.2% (n = 208) where joint pain was reported (Table 5). Switching occurred more frequently after initiation when either PASI/DLQI or DLQI/VAS Joint Pain scores were high (Fig. 2).
Association of Disease Factors and Baseline Covariates with Biologic Switching
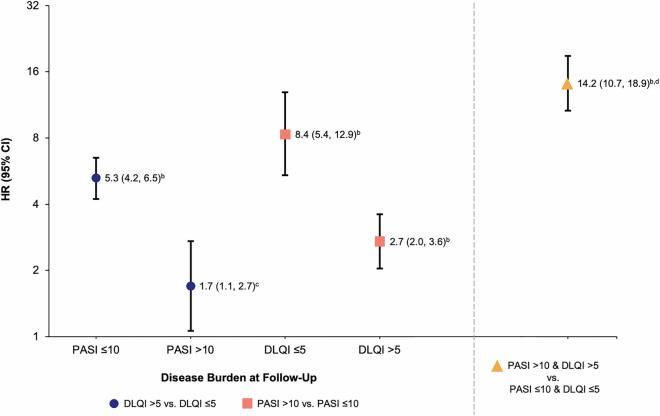
Overall, biologic therapy switching was more frequently observed when either DLQI > 5 or PASI > 10 compared with DLQI ≤ 5 or PASI ≤ 10, respectively, in multivariable adjusted regression models (Fig. 3). Among patients with lower skin involvement at follow-up (PASI ≤ 10), those with higher impact of PSO on HRQoL (DLQI > 5) were 5.3 times more likely to switch than patients with a lower impact of PSO on HRQoL (DLQI ≤ 5). Similarly, among patients with lower impact of PSO on HRQoL (DLQI ≤ 5), those with more severe skin involvement (PASI > 10) were 8.4 times more likely to switch than patients with less severe skin involvement (PASI ≤ 10). Patients with higher skin involvement and higher impact on HRQoL (PASI > 10 and DLQI > 5) were 14.2 times more likely to switch than those with lower skin involvement and lower impact on HRQoL (PASI ≤ 10 and DLQI ≤ 5).
Fig. 3.
Adjusted HRs for switching across PASI and DLQI levels at follow-upa. aModel also included age, sex, race, ethnicity, duration of PSO, biologic experience, baseline PASI/DLQI category, BMI, employment status, and number of concomitant diseases as well as biologic, non-biologic, topical, and phototherapy history (separately); bp < 0.001; cp = 0.027; dOf the four possible PASI/DLQI combinations, the point shown by the orange triangle is a separate value comparing the worst combination (high PASI/high DLQI) with the best (low PASI/low DLQI) combination; those with high PASI/low DLQI and low PASI/high DLQI were not considered in this comparison. BMI body mass index, CI confidence interval, DLQI Dermatology Life Quality Index, HR hazard ratio, PASI Psoriasis Area Severity Index, PSO psoriasis
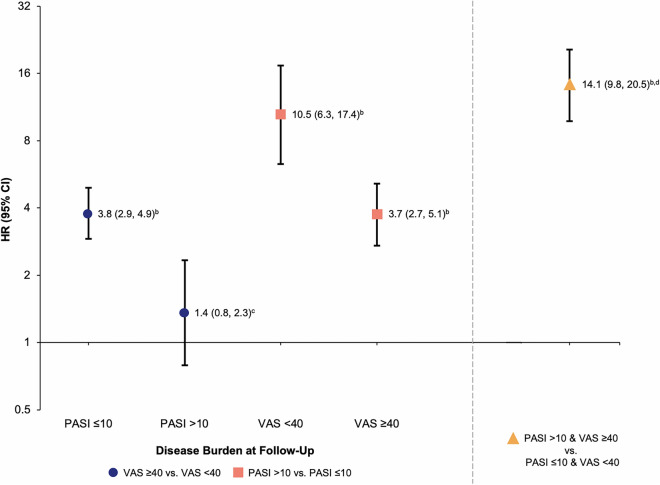
Similar results were observed when substituting PsA-specific measures (e.g., VAS Joint Pain) for PSO-related HRQoL (e.g., DLQI) among patients with dermatologist-diagnosed PsA (Fig. 4). Among patients with dermatologist-diagnosed PsA, switching was more likely when either VAS Joint Pain ≥ 40 or PASI > 10 compared with VAS Joint Pain < 40 or PASI ≤ 10 at follow-up, respectively. For patients with lower skin involvement (PASI ≤ 10) at follow-up, those with higher joint pain (VAS Joint Pain ≥ 40) were 3.8 times more likely to switch than patients with lower joint pain (VAS Joint Pain < 40). Similarly, among patients with lower joint pain at follow-up (VAS Joint Pain < 40), those with higher skin involvement (PASI > 10) were 10.5 times more likely to switch than patients with lower skin involvement (PASI ≤ 10). Switching occurred 14.1 times more at follow-up visits where both higher skin involvement and joint pain were observed (PASI > 10 and VAS Joint Pain ≥ 40) compared with those with lower skin clearance and joint pain (PASI ≤ 10 and VAS Joint Pain < 40). Findings among patients with dermatologist-confirmed PsA did not materially differ from findings among all included patients with concomitant PSO and PsA.
Fig. 4.
Adjusted HRs for switching across PASI and VAS joint pain levels at follow-upa. aModel also included age, sex, race, ethnicity, duration of PSO, biologic experience, baseline PASI/DLQI category, BMI, employment status, and number of concomitant diseases as well as biologic, non-biologic, topical, and phototherapy history (separately); bp < 0.001; cp = 0.276; dOf the four possible PASI/VAS combinations, the point shown by the orange triangle is a separate value comparing the worst combination (high PASI/high VAS) with the best (low PASI/low VAS) combination; those with high PASI/low VAS and low PASI/high VAS were not considered in this comparison. BMI body mass index, CI confidence interval, DLQI Dermatology Life Quality Index, HR hazard ratio, PASI Psoriasis Area Severity Index, PSO psoriasis, VAS visual analog scale
Discussion
In this analysis of patients with PSO and PsA treated in real-world clinical settings, those with greater skin involvement (i.e., PASI) or higher impact on HRQoL (i.e., DLQI or VAS Joint Pain) were significantly more likely to switch biologic therapies within 30 months of initiation. Patient initiations with PASI > 10 and DLQI > 5 were more than 14 times as likely to switch biologics than those with PASI ≤ 10 and DLQI ≤ 5. Similarly, among patients with dermatologist-diagnosed PsA, higher skin involvement or joint pain after initiation was associated with an increased likelihood of switching. Patients with PASI > 10 and VAS Joint Pain ≥ 40 were 14 times more likely to switch than those with PASI ≤ 10 and VAS Joint Pain < 40. Importantly, these associations persisted following adjustment for study covariates of interest at baseline (e.g., patient demographics, clinical characteristics, baseline disease activity, treatment history). This suggests that patients with more significant disease factors due to impaired HRQoL were more inclined to switch biologic therapies regardless of the severity of skin involvement.
The overall findings suggest that there are multiple factors that contribute to therapeutic switch patterns. In addition to objective measures of disease severity (i.e., PASI), factors related to HRQoL may be key drivers of switches in biologic therapies. Our results also demonstrate that patients may switch biologic therapies despite showing low disease activity, which may further highlight the significance of more subjectively focused, patient-centered outcomes that contribute to disease factors, such as joint pain and DLQI. Correspondingly, as the most common reasons for switching biologics were found to be related to ineffectiveness, it will be valuable to develop therapeutics that address PSO and PsA disease features that encompass both disease activity (as evaluated by a physician) as well as symptoms (as assessed by a patient).
An important consideration is that the data used for this analysis were collected from patients with PSO and concomitant PsA during clinical registry visits with a dermatologist and not a rheumatologist. Differences in care delivery based on practitioner type are not well understood and may be associated with therapeutic switching patterns [35]. Consequently, pertinent information normally collected by a rheumatologist and specifically tailored for the treatment of PsA may be unavailable. The extent to which biologic switching behaviors vary between patients with PSO and PsA managed by dermatologists versus rheumatologists has not been well researched, and additional studies are needed to address whether results may differ based on provider specialty.
Strengths and Limitations
The longitudinal nature and length of follow-up, extending to up to 913 days (30 months) after initiation, were strengths of this study. The study results are generalizable to adult patients with PSO and concomitant PsA encountered in typical clinical practice in the US and Canada who have been treated with a systemic biologic therapy for up to 30 months. An additional strength was the degree to which reasons for switching were captured in this analysis, since documented reasons are often limited or missing in reports evaluating biologic switching patterns among PSO and patients with PsA [10, 12, 36]. Finally, the total number of patients excluded for missing covariate information at baseline or PASI/DLQI at follow-up was small (< 5%) as the study measures were defined to minimize or account for missing data, and any effects of bias were expected to be minimal because of the completeness of the registry data.
This study was subject to several limitations. Though over half of the patient-initiations included in the analysis had both a dermatologist diagnosis of PsA and a history of a PEST score ≥ 3 (42.1%; n = 1395), and 27.4% (n = 708) had a dermatologist diagnosis only; a smaller percentage (18.5%; n = 477) had a history of PEST score ≥ 3 only. Therefore, this fraction may have included cases of suspected PsA. Additionally, potential bias from excluding treatment failures related to discontinuations or changes in therapy occurring outside of the 42-day (6-week) window following a discontinuation (e.g., selection bias) may be present. Reasons for patients not switching biologic therapies were not captured, thereby limiting conclusions that can be drawn regarding non-switchers with high disease activity. Given these data were collected as individual follow-up visits and patients were allowed to contribute multiple initiations as well as potentially multiple follow-up visits from each initiation, this may have impacted the degree of remaining disease activity reported.
Conclusions
In this study of patients with concomitant PSO and PsA treated with a biologic therapy in real-world dermatology practices for up to 30 months, those with greater disease severity and associated impacts on HRQoL were more likely to switch biologics after initiation compared with those who had lower disease severity and impact on HRQoL. This association persisted following adjustment for study covariates of interest. These data suggest that patient-centered factors impacting HRQoL may be important drivers of medication switch behaviors. As such, these patient-centered factors should be viewed as potential targets for clinical interventions designed to optimize treatment strategies for patients with PSO and PsA.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all of the investigators, their clinical staff, and the patients who participate in the CorEvitas Psoriasis Registry. The CorEvitas Psoriasis Registry was developed in collaboration with the National Psoriasis Foundation.
Medical Writing/Editorial Assistance
The authors acknowledge Quinn Ho, PhD, and Patrick Reilly, BS, of Costello Medical, Boston, MA, USA, for medical writing and editorial assistance based on the authors’ input and direction. Support for third-party medical writing was funded by UCB Pharma.
Author Contributions
Access to study data was limited to CorEvitas and CorEvitas statisticians completed all analyses; all authors contributed to the interpretation of the results. Substantial contributions to study conception and design: Adam P. Sima, Marie Gurrola; substantial contributions to analysis and interpretation of the data: Philip J. Mease, Andrew Blauvelt, Adam P. Sima, Silky W. Beaty, Robert Low, Braulio Gomez, Marie Gurrola, Mark G. Lebwohl; drafting the article or revising it critically for important intellectual content: Philip J. Mease, Andrew Blauvelt, Adam P. Sima, Silky W. Beaty, Robert Low, Braulio Gomez, Marie Gurrola, Mark G. Lebwohl; final approval of the version of the article to be published: Philip J. Mease, Andrew Blauvelt, Adam P. Sima, Silky W. Beaty, Robert Low, Braulio Gomez, Marie Gurrola, Mark G. Lebwohl.
Funding
This study was sponsored by CorEvitas, LLC, and the analysis was funded by UCB Pharma. Access to study data was limited to CorEvitas, and CorEvitas statisticians completed all of the analyses; all authors contributed to the interpretation of the results. CorEvitas has been supported through contracted subscriptions in the last 2 years by AbbVie, Amgen, Inc., Arena, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Chugai, Eli Lilly and Company, Genentech, Gilead Sciences, Inc., GlaxoSmithKline, Janssen Pharmaceuticals, Inc., LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Inc., Regeneron Pharmaceuticals, Inc., Sanofi, Sun Pharmaceutical Industries Ltd., and UCB Pharma. This manuscript's publication, including the journal’s Rapid Service and Open Access Fees, is funded by UCB Pharma.
Data Availability
Data are available from CorEvitas, LLC, through a commercial subscription agreement and are not publicly available. No additional data are available from the authors.
Declarations
Conflict of Interest
Philip J. Mease: Has received research grants from AbbVie, Acelyrin, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Gilead, Janssen, Novartis, Pfizer, Sun Pharma, and UCB Pharma; consulting fees from AbbVie, Acelyrin, Aclaris, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly and Company, Galapagos, Gilead, GSK, Janssen, Moonlake Pharma, Novartis, Pfizer, Sun Pharma, Takeda, UCB Pharma, and Ventyx; speakers bureau fees from AbbVie, Amgen, Eli Lilly and Company, Janssen, Novartis, Pfizer, and UCB Pharma; Andrew Blauvelt: Has served as a speaker (received honoraria) for Eli Lilly and Company and UCB Pharma, served as a scientific adviser (received honoraria) for AbbVie, Abcentra, Aclaris, Affibody, Aligos, Almirall, Alumis, Amgen, Anaptysbio, Apogee, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Celldex, CTI BioPharma, Dermavant Sciences, EcoR1, Eli Lilly and Company, Escient, Evelo, Evommune, Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, LEO Pharma, Lipidio, Merck, Microbion, Monte Rosa Therapeutics, Nektar, Novartis, Overtone Therapeutics, Paragon, Pfizer, Q32 Bio, Rani, Rapt, Regeneron, Sanofi, Spherix Global Insights, Sun Pharma, Takeda, TLL Pharmaceutical, TrialSpark, UCB Pharma, Union, Ventyx, Vibliome, and Xencor, and has acted as a clinical study investigator (institution has received clinical study funds) for AbbVie, Acelyrin, Allakos, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Concert, Dermavant Sciences, DermBiont, Eli Lilly and Company, Evelo, Evommune, Galderma, Incyte, Janssen, LEO Pharma, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, UCB Pharma, and Ventyx, and owns stock in Lipidio and Oruka; Adam P. Sima, Marie Gurrola: Employees of CorEvitas, LLC; Silky W. Beaty: Employee at the time of the study and current shareholder of UCB Pharma; Robert Low, Braulio Gomez: Employees and shareholders of UCB Pharma; Mark G. Lebwohl: Employee of Mount Sinai and receives research funds from: Abbvie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Cara Therapeutics, Dermavant Sciences, Eli Lilly and Company, Incyte, Inozyme, Janssen Research & Development, LLC, Novartis, Ortho Dermatologics, Regeneron, and UCB Pharma; consultant for Almirall, AltruBio Inc., AnaptysBio, Arcutis Inc., Arena Pharmaceuticals, Aristea Therapeutics, AstraZeneca, Avotres, BioMX, Boehringer Ingelheim, Brickell Biotech, Bristol Myers Squibb, Castle Biosciences, Celltrion, CorEvitas, LLC, Dermavant Sciences, EPI, Evommune Inc., Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Galderma, Genentech, Hexima Ltd, Incyte, LEO Pharma, Meiji Seika Pharma, Mindera, National Society of Cutaneous Medicine, New York College of Podiatric Medicine, Pfizer, Seanergy, Strata, SUN Pharma, Trevi, Verrica, and Vial. Mark G. Lebwohl is an Editorial Board member of Dermatology and Therapy. Mark G. Lebwohl was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
This study was performed in accordance with the Declaration of Helsinki [22] and the Guidelines for Good Pharmacoepidemiology Practice [23]. All participating investigators were required to obtain full board approval for conducting noninterventional research involving human subjects with a limited dataset. Sponsor approval and continuing review were obtained through a central Institutional Review Board (IRB), (Advarra, Protocol number Pro00051221). For academic investigative sites that did not receive authorization to use the central IRB, full board approval was obtained from their respective governing IRBs, and documentation of approval was submitted to CorEvitas, LLC, before the site’s participation and initiation of any study procedures. All patients in the registry were required to provide written informed consent and authorization before participating.
References
- 1.Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(Suppl 2):ii18-23. 10.1136/ard.2004.033217. (discussion ii24–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mease PJ, Armstrong AW. Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis. Drugs. 2014;74(4):423–41. 10.1007/s40265-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gladman DD, Antoni C, Mease PJ, et al. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–7. 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skornicki M, Prince P, Suruki R, et al. Clinical burden of concomitant joint disease in psoriasis: a US-linked claims and electronic health records database analysis. Adv Ther. 2021;38(5):2458–71. 10.1007/s12325-021-01698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merola JF, Ogdie A, Gottlieb AB, et al. Patient and physician perceptions of psoriatic disease in the United States: results from the UPLIFT survey. Dermatol Ther. 2023;13:1329–46. 10.1007/s13555-023-00929-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibofsky A, Skup M, Mittal M, et al. Effects of non-medical switching on outcomes among patients prescribed tumor necrosis factor inhibitors. Curr Med Res Opin. 2017;33(11):1945–53. 10.1080/03007995.2017.1375903. [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb A, Korman NJ, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 2. Psoriatic arthritis: overview and guidelines of care for treatment with an emphasis on the biologics. J Am Acad Dermatol. 2008;58(5):851–64. 10.1016/j.jaad.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 8.Raharja A, Mahil SK, Barker JN. Psoriasis: a brief overview. Clin Med. 2021;21(3):170–3. 10.7861/clinmed.2021-0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merola JF, Lockshin B, Mody EA. Switching biologics in the treatment of psoriatic arthritis. Semin Arthritis Rheum. 2017;47(1):29–37. 10.1016/j.semarthrit.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong AW, Patel M, Li C, et al. Real-world switching patterns and associated characteristics in patients with psoriasis treated with biologics in the United States. J Dermatol Treat. 2023;34(1):1–19. 10.1080/09546634.2023.2200870. [DOI] [PubMed] [Google Scholar]
- 11.Costa L, Perricone C, Chimenti MS, et al. Switching between biological treatments in psoriatic arthritis: a review of the evidence. Drugs R D. 2017;17:509–22. 10.1007/s40268-017-0215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh JA, Adejoro O, Chastek B, et al. Treatment patterns among patients with psoriatic arthritis treated with a biologic in the United States: descriptive analyses from an administrative claims database. J Manag Care Spec Pharm. 2018;24(7):623–31. 10.18553/jmcp.2018.24.7.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonafede M, Johnson BH, Fox KM, et al. Treatment patterns with etanercept and adalimumab for psoriatic diseases in a real-world setting. J Dermatol Treat. 2013;24(5):369–73. 10.3109/09546634.2012.755255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivieri I, Cortesi PA, De Portu S, et al. Long-term costs and outcomes in psoriatic arthritis patients not responding to conventional therapy treated with tumour necrosis factor inhibitors: the extension of the Psoriatic Arthritis Cost Evaluation (PACE) study. Clin Exp Rheumatol. 2016;34(1):68–75. [PubMed] [Google Scholar]
- 15.Wu JJ, Pelletier C, Ung B, et al. Treatment switch patterns and healthcare costs in biologic-naive patients with psoriatic arthritis. Adv Ther. 2020;37(5):2098–115. 10.1007/s12325-020-01262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song Y, Betts KA, Lu Y, et al. Economic burden of switching to different biologic therapies among tumor necrosis factor inhibitor-experienced patients with psoriatic arthritis. Rheumatol Ther. 2019;6(2):285–97. 10.1007/s40744-019-0158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imafuku S, Kanai Y, Murotani K, et al. Utility of the dermatology life quality index at initiation or switching of biologics in real-life Japanese patients with plaque psoriasis: Results from the ProLOGUE study. J Dermatol Sci. 2021;101(3):185–93. 10.1016/j.jdermsci.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Feldman SR, Regnier SA, Chirilov A, et al. Patient-reported outcomes are important elements of psoriasis treatment decision making: a discrete choice experiment survey of dermatologists in the United States. J Am Acad Dermatol. 2019;80(6):1650–7. 10.1016/j.jaad.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 19.Hägg D, Sundström A, Eriksson M, et al. Decision for biological treatment in real life is more strongly associated with the psoriasis area and severity index (PASI) than with the dermatology life quality index (DLQI). J Eur Acad Dermatol Venereol. 2015;29(3):452–6. 10.1111/jdv.12576. [DOI] [PubMed] [Google Scholar]
- 20.Strober B, Karki C, Mason M, et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: results from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2018;78(2):323–32. 10.1016/j.jaad.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Mease PJ, Palmer JB, Hur P, et al. Utilization of the validated Psoriasis Epidemiology Screening Tool to identify signs and symptoms of psoriatic arthritis among those with psoriasis: a cross-sectional analysis from the US-based Corrona Psoriasis Registry. J Eur Acad Dermatol Venereol. 2019;33(5):886–92. 10.1111/jdv.15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Med Assoc. 2013;310(20):2191–4. 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 23.International Society for Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol Drug Saf. 2016;25(1):2–10. 10.1002/pds.3891. [DOI] [PubMed] [Google Scholar]
- 24.Fredriksson T, Pettersson U. Severe psoriasis—oral therapy with a new retinoid. Dermatologica. 1978;157(4):238–44. 10.1159/000250839. [DOI] [PubMed] [Google Scholar]
- 25.Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303(1):1–10. 10.1007/s00403-010-1080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4(7):407–14. 10.1016/s1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 27.Ibrahim G, Buch M, Lawson C, et al. Evaluation of an existing screening tool for psoriatic arthritis in people with psoriasis and the development of a new instrument: the Psoriasis Epidemiology Screening Tool (PEST) questionnaire. Clin Exp Rheumatol. 2009;27(3):469–74. [PubMed] [Google Scholar]
- 28.Lin Y-L, Huang A, Yang C-Y, et al. Measurement of body surface area for psoriasis using U-net models. Comput Math Methods Med. 2022;2022:1–9. 10.1155/2022/7960151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langley RG, Feldman SR, Nyirady J, et al. The 5-point Investigator’s Global Assessment (IGA) Scale: a modified tool for evaluating plaque psoriasis severity in clinical trials. J Dermatol Treat. 2015;26(1):23–31. 10.3109/09546634.2013.865009. [DOI] [PubMed] [Google Scholar]
- 30.Flytström I, Stenberg B, Svensson A, et al. Patients’ visual analogue scale: a useful method for assessing psoriasis severity. Acta Derm Venereol. 2012;92(4):347–8. 10.2340/00015555-1237. [DOI] [PubMed] [Google Scholar]
- 31.Eder L, Thavaneswaran A, Chandran V, et al. Factors explaining the discrepancy between physician and patient global assessment of joint and skin disease activity in psoriatic arthritis patients. Arthritis Care Res. 2015;67(2):264–72. 10.1002/acr.22401. [DOI] [PubMed] [Google Scholar]
- 32.Swinburn P, Lloyd A, Boye K, et al. Development of a disease-specific version of the EQ-5D-5L for use in patients suffering from psoriasis: lessons learned from a feasibility study in the UK. Value Health. 2013;16(8):1156–62. 10.1016/j.jval.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Strober B, Greenberg JD, Karki C, et al. Impact of psoriasis severity on patient-reported clinical symptoms, health-related quality of life and work productivity among US patients: real-world data from the Corrona Psoriasis Registry. BMJ Open. 2019;9(4):1–9. 10.1136/bmjopen-2018-027535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis V, Finlay AY. 10 years experience of the dermatology life quality index (DLQI). J Investig Dermatol Symp Proc. 2004;9(2):169–80. 10.1111/j.1087-0024.2004.09113.x. [DOI] [PubMed] [Google Scholar]
- 35.Yarnall M. Key differences between US rheumatologists' and dermatologists' management of psoriatic arthritis. Arthritis Rheumatol; 2022: American College of Rheumatology.
- 36.Foster SA, Zhu B, Guo J, et al. Patient characteristics, health care resource utilization, and costs associated with treatment-regimen failure with biologics in the treatment of psoriasis. J Manag Care Spec Pharm. 2016;22(4):396–405. 10.18553/jmcp.2016.22.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from CorEvitas, LLC, through a commercial subscription agreement and are not publicly available. No additional data are available from the authors.