Abstract
Odontogenic tumors are a group of tumors that originate from the tissues associated with tooth development and are classified into benign or malignant based on their behavior and characteristics. Tumor markers are substances that can be found in the blood, urine, or tissues of individuals with cancer. They are the substances produced either by tumor cells itself or by the body in response to tumor growth, can sometimes be used in the diagnosis, prognosis, and monitoring of various types of tumors. However, the use of tumor markers in odontogenic tumors is not as common as it is in other types of cancers, and their utility in this context is limited. Tumor markers are not the main tools for diagnosing cancer; instead, they serve as supplementary laboratory tests to aid in the diagnosis. Researchers continue to investigate potential biomarkers to improve our understanding of these tumors and their behavior. With this concept in mind, the objective of this study is to elucidate the key diagnostic markers essential for diagnosing odontogenic tumors.
Keywords: Tumor markers, Odontogenic tumors, Immunohistochemistry, Molecular markers
Introduction
Odontogenic tumors are the lesions that originate from the epithelial, ectomesenchymal, or a combination of both elements within the tooth development apparatus or its remnants. Consequently, their histological characteristics can resemble various stages of tooth development [1]. This group of lesions encompasses a diverse range of abnormalities, including benign and malignant tumors with the potential for metastasis, as well as hamartomas. These tumors are categorized into three groups based on their possible origin: those that are composed of epithelial tissue, tumors that contains both epithelial and mesenchymal tissue, and those produced by mesenchymal tissues [2].
Odontogenic tumors are infrequent, with an estimated incidence of less than 0.5 cases per 100,000 people per year. These tumors primarily occur in the jaw region, especially around areas with teeth bearing segments, but in some cases, they may manifest as localized swellings in the gum tissue, which are referred to as peripheral odontogenic tumors. These tumors are indeed infrequent, and in some cases exceptionally rare, can present substantial challenges in terms of diagnosis and treatment [3]. In one study, it was observed that odontogenic tumors accounted for just 1.8% of cases where oral biopsies were conducted. However, in studies conducted in Africa and Asia, a higher proportion was reported, with odontogenic tumors making up as much as 8.9% of all oral tumors [4].
The clinical signs and symptoms of odontogenic tumors can vary depending on the size of the lesion. Small lesions are unlikely to be diagnosed during a routine oral examination and are more likely to be discovered at an early stage through routine radiographic examinations. Odontogenic tumors typically exhibit slow growth and are often associated with unerupted teeth, swelling of the alveolar process, tooth loosening, and alterations in occlusion. These clinical manifestations can serve as important indicators for diagnosing these tumors [5].
A tumor marker is a substance (for example: protein) produced by neoplastic cells or other cells of the body in response to certain benign or malignant neoplasm. In a clinical context, these are molecules that can be identified in plasma and bodily fluids. Tumor markers are quantifiable biochemical substances linked to the presence of a tumor [6]. When these markers originate from the tumor cells themselves are referred to as tumor-derived markers and when these form from the body's response to the presence of tumor cells they are known as tumor-associated markers. These markers are usually substances that are released into the bloodstream and can therefore be measured in blood, cells, tissues, or various body fluids through quantitative or qualitative methods using chemical, immunological, or molecular biological techniques [7, 8]. However, it is important to note that they are not the primary methods for diagnosing cancer; instead, they serve as laboratory tests that provide additional support for the diagnosis [6].
According to the National Cancer Institute (NCI), a biomarker is defined as a biological molecule that can be detected in blood, other bodily fluids, or tissues, and it serves as an indicator of a normal or abnormal biological process, or the presence of a particular condition or disease, such as cancer [9]. These markers can be situated intracellularly within cells, on the cell surface, or they may be released into the extracellular space, which includes the bloodstream and other bodily fluids. The detection and measurement of these markers are valuable in determining the presence of neoplastic conditions [10].
Diagnostic markers are specific biological indicators or measurable characteristics that are used to identify the presence or absence of a particular disease, condition, or physiological state in an individual. Specific diagnostic markers play a crucial role in accurately diagnosing particular types of odontogenic tumors, contributing to an enhanced understanding of their pathogenesis and molecular genetic characteristics [11].
Properties of an ideal tumor marker
It is important to note that while tumor markers can be valuable tools in cancer diagnosis and management, no single marker possesses all these ideal properties as summarized in Table 1. Typically, a combination of markers and other diagnostic tools, such as imaging and clinical assessment, is used to achieve the most accurate and comprehensive evaluation of a patient's tumoral status. The choice of a tumor marker and its application depends on the specific type of neoplasm, clinical context, and available diagnostic tools [12]. The illustration in Fig. 1 delineates the diverse categories of odontogenic tumor markers categorized according to their biological functions.
Table 1.
Properties of an ideal tumor marker
| Property | Description |
|---|---|
| Sensitivity | High ability to detect early-stage cancer |
| Specificity | Accuracy in identifying the targeted cancer |
| Clinical utility | Provides valuable information for diagnosis, prognosis, treatment, and monitoring |
| Measurability | Quantifiable using reliable laboratory methods |
| Stability | Stable levels over time, minimizing variability |
| Non-Invasiveness | Obtained through minimally invasive means (e.g., blood sample) |
| Early Detection | Capable of detecting cancer at an early, treatable stage |
| Availability | Affordable and widely accessible testing technology |
| Validation | Rigorously validated in diverse patient populations |
| Responsiveness to treatment | Reflects changes in tumor burden or disease status accurately |
| Predictive value | Provides information on treatment response or disease recurrence |
| Ethical and privacy considerations | Complies with ethical and privacy standards |
Fig. 1.
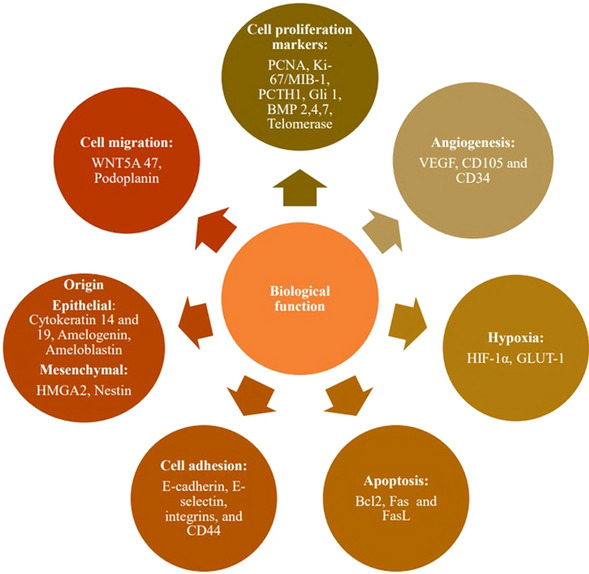
Odontogenic tumor based on its biological function
Molecular basis of tumor marker
The molecular mechanisms outlined in Table 2 underline the production and presence of tumor markers in cancer and are often used in the development of diagnostic tests and targeted therapies [13]. Genetic alterations within tumor cells can impact the gene expression patterns in the tumor cells themselves and the neighboring tissues, either through direct or indirect mechanisms. These genetic changes can manifest in various ways, including viral genome integration or genetic abnormalities, and they form the fundamental biological foundation for the development of tumor markers [14].
Table 2.
Molecular basis of tumor marker
| Molecular basis | Description |
|---|---|
| Genetic mutations | Tumor markers can result from specific gene mutations or genetic alterations within cancer cells. These mutations may lead to the production of abnormal proteins or altered gene expression patterns. Examples include BRAF mutations in melanoma and EGFR mutations in lung cancer |
| Overexpression | Some tumour markers are produced in larger quantities by cancer cells compared to normal cells. This can result from gene amplification or increased transcription and translation. Examples include HER2/neu in breast cancer and CA-125 in ovarian cancer |
| Altered protein processing | Changes in post-translational modification or protein processing can lead to the production of unique protein variants or isoforms that serve as tumour markers. For instance, the prostate-specific antigen (PSA) in prostate cancer can be found in different forms |
| Oncogene activation | Activation of oncogenes, which are genes that promote cell growth and division, can lead to the production of proteins that serve as tumour markers. The HER2 oncogene activation is an example seen in breast cancer |
| Tissue-specific expression | Some tumour markers are normal proteins produced in specific tissues but become elevated in the bloodstream when the tissue is affected by cancer. For example, elevated levels of AFP are associated with liver cancer, as AFP is produced by the liver |
| Epigenetic changes | Epigenetic modifications, such as DNA methylation and histone modifications, can silence tumour suppressor genes or activate oncogenes, contributing to tumour marker production. Examples include DNA hypermethylation in various cancer types—GSP1, DAP in lung cancer; p15, p16 in liver cancer |
Advantages and disadvantages of tumour marker
It is vital to understand that the utility and limitations of tumour markers as outlined in Table 3 vary depending on the specific marker, the type and stage of a tumor, and the clinical context. Tumor markers are most effective when used in conjunction with other diagnostic and clinical assessments [10].
Table 3.
Advantages and disadvantages of tumour marker
| Advantages | Disadvantages |
|---|---|
| Early Detection: Tumor markers can help detect cancer at an early stage when treatment is more effective | Limited Specificity: Some markers may be elevated in non-cancerous conditions, leading to false-positive results |
| Monitoring Treatment Response: They can be used to assess the effectiveness of cancer treatment and make necessary adjustments | Limited Sensitivity: Not all cancers produce detectable markers, leading to false-negative results in some cases |
| Prognostic Information: Tumor markers can provide insights into the likely course and outcome of the disease | Variability: Marker levels can fluctuate due to factors unrelated to cancer, making interpretation challenging |
| Non-Invasive: Many marker tests require only blood samples or other minimally invasive procedures | Lack of Specificity for Cancer Type: Some markers are not specific to a particular cancer type, making it difficult to pinpoint the exact cancer source |
| Risk Assessment: They can aid in assessing a person's risk of developing certain cancers based on genetic or familial factors | Cost: Some marker tests can be expensive, limiting access to certain populations |
| Screening Tool: Tumor markers can be used in cancer screening programs to identify individuals at higher risk | False Positives and Negatives: False-positive results can lead to unnecessary anxiety and medical procedures, while false negatives may delay diagnosis |
| Personalized Treatment: Marker information can guide the selection of targeted therapies and personalized treatment plans | Lack of Standardization: Standardization of marker tests and reference ranges can vary, impacting consistency and comparability between labs |
| Research and Drug Development: Tumor markers play a crucial role in cancer research and drug development | Ethical and Psychological Impact: Positive marker results may have significant psychological and emotional effects on patients |
Tumour markers in odontogenic tumours
Ameloblastin
Ameloblastin (AMBN), also referred to as amelin or sheathlin, is a glycoprotein specific to teeth and is the most abundant enamel matrix protein aside from amelogenin. It is highly expressed during the secretory stage of enamel formation but decreases in expression during the maturation stage. While Ameloblastin is also found in dentin matrix and Hertwig's epithelial root sheath cells, its exact role in dentin and cementum formation has not been fully understood. Notably, AMBN is primarily located near the cell surface rather than deep within the enamel matrix layer [15].
Perdigao and colleagues research findings indicate that mutations in the AMBN gene are linked to the development of certain epithelial odontogenic tumours, including Ameloblastoma, Adenomatoid Odontogenic Tumour (AOT), Squamous Odontogenic Tumour (SOT), and Calcifying Epithelial Odontogenic Tumour (CEOT). Their findings suggest that mutations in the AMBN gene play a role in the tumorigenesis of these tumours, particularly those that arise from epithelial tissues without involvement of odontogenic ectomesenchyme, highlighting the genetic foundations of these specific types of tumors [16].
Amelogenin
Amelogenin is one of the key enamels—matrix proteins that plays a crucial role in the formation of dental enamel, which is the hard, outermost layer of teeth. It is primarily produced by secretory ameloblast cells during the secretory phase, which is essential for organizing and mineralizing developing enamel. The human amelogenin gene is found on both the X and Y chromosomes, specifically on Xp22.1–p22.3 and Yp11.2. Approximately 90% of amelogenin transcripts are produced from the X chromosome, while the remaining 10% originate from the Y chromosome [17].
Amelogenin diverse roles in enamel formation, genetics, forensics, and its potential applications in dental and regenerative medicine highlight its significance in various scientific and clinical fields as shown in Table 4.
Table 4.
Key points about the role of Amelogenin
| Role of amelogenin | Description |
|---|---|
| Enamel Formation | Amelogenin is a crucial protein in the formation of dental enamel, contributing to enamel's structure and mineralization |
| Enamel Matrix Organization | It helps organize and guide the growth of hydroxyapatite crystals, the mineral component of enamel, providing a structural framework for enamel development |
| Amelogenesis | Participates in amelogenesis, the process of enamel formation, by regulating enamel thickness, hardness, and overall integrity |
| Genetic expression | Encoded by the amelogenin gene, it is responsible for producing the amelogenin protein, which is present in the enamel-forming ameloblast cells |
| Sex determination | In forensic science, differences between X and Y chromosome versions of the amelogenin gene are used for gender identification |
| Dental research | Important for studying dental development, enamel defects, and conditions like enamel hypoplasia, which result from amelogenin gene mutations |
| Dental restorations | Amelogenin-based materials have been used in restorative dentistry to mimic natural enamel and enhance the appearance and strength of teeth |
| Regenerative medicine | Amelogenin and enamel matrix proteins are explored for potential applications in regenerative medicine, including tooth tissue regeneration |
Mori and colleagues conducted a study where they found that amelogenin expression showed positive results in various types of lesions, including Ameloblastoma, Adenomatoid Odontogenic Tumor (AOT), Calcifying Epithelial Odontogenic Tumor (CEOT), Ameloblastic Fibroma (AF), Malignant Ameloblastoma, and Ameloblastic Carcinoma. Notably, the highest intensity of amelogenin expression was observed in the reduced ameloblasts within odontoma [18]. Consequently, the use of amelogenin as a marker proves to be a valuable tool for distinguishing and categorizing various types of epithelial lesions that may arise in the oral and maxillofacial regions. This marker aids in the differentiation of these lesions, enhancing the accuracy of diagnoses and facilitating appropriate clinical management [3, 19]. In a study by Kumamoto et al., samples of squamous cell carcinoma, mucoepidermoid carcinoma, adenoid cystic carcinoma, and basal cell carcinoma did not exhibit expression of amelogenin [19]
Basement membrane proteins
Basement membrane proteins are a group of specialized proteins that play a crucial role in the formation and maintenance of the basement membrane, a thin, sheet-like structure that separates and supports epithelial and endothelial tissues from underlying connective tissues. Basement membrane proteins collectively provide structural support, facilitate cell adhesion and migration, regulate the passage of molecules, and contribute to various biological functions within tissues as shown in Table 5 [20].
Table 5.
Key biological functions of basement membrane proteins
| Basement membrane protein | Function |
|---|---|
| Laminins | Critical for cell adhesion, signaling, and tissue organization |
| Type IV Collagen | Provides structural support and stability to the membrane |
| Nidogens (Entactin’s) | Glycoproteins bridging laminins and collagens, stabilizing the basement membrane's structure |
| Perlecan | Heparan sulfate proteoglycan maintaining basement membrane porosity and involved in cell signaling, growth factor regulation, and tissue development |
| Heparan Sulfate Proteoglycans (HSPGs) | Involved in cell adhesion, signaling, growth factor regulation, and extracellular matrix assembly |
| Tissue Inhibitors of Metalloproteinases (TIMPs) | Proteins that inhibit matrix metalloproteinases (MMPs) to maintain the balance between matrix synthesis and degradation |
| Fibronectin | Adhesion glycoprotein that facilitates cell adhesion and migration within the basement membrane zone and extracellular matrix |
The study conducted by Poomsawat and colleagues revealed the expression of several basement membrane proteins, including laminins 1 and 5, collagen type IV, and fibronectin, in different types of odontogenic tumors such as ameloblastomas, and adenomatoid odontogenic tumors (AOTs). Interestingly, they found that laminin 1 expression was specifically observed in odontogenic epithelium but not in mucosal epithelium., highlighting the diagnostic significance of laminin 1 in characterizing and differentiating various odontogenic tumors and related oral lesions [21].
Bone morphogenic protein
Bone morphogenic proteins (BMPs) are a group of multifunctional growth factors belonging to a subset of the transforming growth factor-beta (TGF-β) superfamily of proteins that play pivotal roles in various biological processes, particularly in bone and cartilage formation, promote tissue regeneration and repair, and play roles in embryonic development as summarized in Table 6 [20]. Bone morphogenic proteins are critical regulators of tissue development and regeneration, making them essential players in both normal physiology and clinical medicine.
Table 6.
Key roles of BMP in biological processes
| Function | Description |
|---|---|
| Osteogenesis | Stimulate differentiation of mesenchymal stem cells into osteoblasts and contribute to bone formation |
| Chondrogenesis | Promote differentiation of mesenchymal stem cells into chondroblasts and assist in cartilage development |
| Tissue Repair and Regeneration | Used in clinical settings to enhance bone healing, especially in spinal fusion, oral and maxillofacial, and orthopedic surgeries |
| Clinical Use | BMP-2 and BMP-7 have been approved for specific medical procedures; used as a therapeutic option in orthopedics and dentistry |
| Signaling Pathways | Activate intracellular signaling pathways, particularly the Smad pathway, to regulate gene expression and cell differentiation |
| Developmental Biology | Critical for embryonic development, including dorsal–ventral patterning, limb development, and organogenesis |
| Cancer | BMPs may act as tumor suppressors or contribute to cancer progression |
| Diversity | BMP-2 and BMP-7, for example, are well-known for their osteogenic properties, while BMP-4 is involved in early embryonic development [21] |
In a study conducted by Gao YH and colleagues, it was observed that cementoblastoma, odontogenic fibroma, and odontoma exhibited positive expression of BMP. Conversely, ameloblastoma, adenomatoid odontogenic tumor (AOT), and calcifying epithelial odontogenic tumor (CEOT) did not express BMP. This suggests that BMP likely plays a significant role in the formation of calcified dental tissues and the development of odontogenic tumors that contain such tissues [23].
In the study conducted by Kumamoto and colleagues, they observed BMP expression in tooth germs and epithelial odontogenic tumors. This observation suggests that these regulatory proteins, which are involved in developmental processes, may have a role in influencing the cytodifferentiation of both normal and neoplastic odontogenic epithelium through interactions between epithelial and mesenchymal cells. In essence, BMPs may contribute to the processes that guide the development and differentiation of dental tissues, including those involved in odontogenic tumors [24].
Calretinin
Calretinin is a calcium-binding protein that plays important roles in various cellular processes, particularly in calcium signalling pathways within cells, which are crucial for various cellular processes, including neurotransmission and muscle contraction. In the nervous system, calretinin is commonly found in specific populations of neurons, including some interneurons in the central nervous system (CNS) and neurons in the peripheral nervous system (PNS). It is involved in modulating neuronal excitability and synaptic transmission [25]. It is also utilized as a valuable marker in some cancer types, including mesothelioma, where it can help differentiate between malignant and benign tissues [26].
In a study conducted by Alaeddini and colleagues, they observed that calretinin immunoreactivity was only positive in cases of ameloblastoma when compared to other odontogenic tumors such as calcifying epithelial odontogenic tumor, adenomatoid odontogenic tumor, ameloblastic fibroma, and odontogenic myxoma. They proposed that calretinin might be involved in the transition of dental lamina remnants into ameloblastoma, implying its potential relevance in the development and aggressive nature of this neoplasm. This observation highlights the potential significance of calretinin as a biomarker in distinguishing ameloblastoma from other similar lesions [27].
Chitra Anandani and colleagues suggested that calretinin could serve as a valuable immunohistochemical marker for identifying neoplastic ameloblastic epithelium. They observed that calretinin positivity was exclusively found in cases of ameloblastomas. Therefore, it can be utilized as an important diagnostic tool when distinguishing ameloblastomas from other dental lesions. The expression of calretinin in these neoplastic lesions may mirror the processes involved in normal tooth development, adding to its significance in ameloblastic tumors. Consequently, calretinin can be considered a specific immunohistochemical marker for neoplastic ameloblastic epithelium, demonstrating its presence exclusively in solid and unicystic ameloblastomas and not in any other types of odontogenic cysts or tumors. This highlights its diagnostic utility in the differential diagnosis of ameloblastomas [28].
Cytokeratin
Cytokeratins (CK) are a diverse group of intermediate filament proteins in epithelial cells, contributing to cell structure and function. Their tissue-specific expression and changes in expression patterns are valuable in diagnostics and cancer research. They also have emerging roles in cellular signalling and tissue repair processes. Cytokeratins are classified into two types: acidic and basic as shown in Table 7 [29]. They are numbered based on their molecular weight, with acidic cytokeratins having lower molecular weights and basic cytokeratins having higher molecular weights.
Table 7.
Classification of cytokeratin
| Types of cytokeratin | Examples |
|---|---|
| Type I or Low molecular weight C/Acidic CK | CK10, CK12, CK13, CK14, CK16, CK17, CK18, CK19, and CK20 |
| Type II or High molecular weight/asic or neutral CK | CK1, CK2, CK3, CK4, CK5, CK6, CK7, CK8, and CK9 |
The expression of cytokeratins in odontogenic tumors as summarized in Table 8 can vary, and the presence of specific cytokeratins may aid in the diagnosis and classification of these tumors [30–32]. The expression patterns of cytokeratins can provide valuable information for pathologists and clinicians in understanding the nature of the tumor and distinguishing it from other lesions.
Table 8.
Expression of cytokeratin’s in odontogenic tumors
| Type of cytokeratin | Odontogenic tumors | Role/expression |
|---|---|---|
| Cytokeratin 14 (CK14) | Ameloblastoma, | Odontogenic epithelium |
| Cytokeratin 19 (CK19) | Ameloblastoma, calcifying epithelial odontogenic tumor (CEOT) | Odontogenic epithelium components |
| Cytokeratin 5/6 (CK5/6) | Ameloblastoma | Odontogenic epithelium. May be useful in distinguishing ameloblastoma from other tumors |
| Cytokeratin 18 (CK18) | Ameloblastoma | Odontogenic epithelium |
| Cytokeratin 8/18 (CK8/18) | Ameloblastoma | Commonly found in simple epithelia, including some odontogenic tissues |
CD147
CD147, also known as Basigin (BSG) or EMMPRIN, M6 antigen, neurothelin (Extracellular Matrix Metalloproteinase-Inducer), is a type I transmembrane glycoprotein composed of two immunoglobulin-like domains (extracellular domain) and a short cytoplasmic tail and belongs to the immunoglobulin superfamily. It is primarily localized on the cell surface, where it functions as a receptor and cell adhesion molecule. It is widely distributed in various tissues and cell types, including leukocytes, red blood cells, endothelial cells, fibroblasts, and some epithelial cells. CD147 is well-known for its role in cancer progression. It promotes tumor invasion and metastasis by interacting with extracellular matrix proteins and facilitating the secretion of matrix metalloproteinases (MMPs), which degrade the extracellular matrix [33].
CD147 has been identified in ameloblastoma, or their precursor cells (dental lamina, remnants of dental lamina). Cairns et al. reported that CD147 expression was restricted to the cell membranes of columnar ameloblast-like cells and localized to the outer edges of epithelial cell clusters and the epithelial chords seen in Solid Multicystic Ameloblastoma. Its expression in ameloblastoma suggests a potential involvement in the local invasive behaviour of this tumor. CD147 may contribute to the breakdown of the extracellular matrix, facilitating tumor cell invasion into surrounding tissues [34]. CD147 has been implicated in the pathogenesis of various odontogenic tumors beyond ameloblastomas, including calcifying cystic odontogenic tumors and adenomatoid odontogenic tumors. Its role in these tumors may involve stimulating the production of matrix metalloproteinases (MMPs), which are associated with tumor invasiveness and aggressiveness [3].
Claudin
Claudins are a family of integral membrane proteins that play a crucial role in forming tight junctions between cells in various tissues. Tight junctions are specialized structures that seal the intercellular space between adjacent epithelial or endothelial cells, regulating the passage of ions, solutes, and macromolecules across cell layers [35].
The specific types of claudins recognized in odontogenic tumors can vary. The presence of these claudins in ameloblastoma, in particular, suggests their involvement in cell adhesion, tight junction formation, and potentially tumor behaviour as shown in Table 9 [36].
Table 9.
Types of Claudins expressed in odontogenic tumors
| Claudin type | Odontogenic tumors | Role/expression |
|---|---|---|
| Claudin-1 (CLDN1) | Ameloblastoma | Expression in ameloblastoma tissues. May contribute to tight junction formation in this tumor type |
| Claudin-3 (CLDN3) | Ameloblastoma | Expression identified in ameloblastoma, potentially playing a role in cell–cell adhesion and tumor behavior |
| Claudin-4 (CLDN4) | Ameloblastoma | Expression identified in ameloblastoma, suggesting a role in cell adhesion and intercellular junctions within the tumor |
| Claudin-7 (CLDN7) | Ameloblastoma | Expression observed in ameloblastoma tissues, potentially contributing to tight junction formation and barrier function |
| Claudin-18 (CLDN18) | Ameloblastoma | Expression observed in ameloblastoma tissues, with potential implications for tumor behavior and intercellular interactions |
Bello et al. noted a strong immunoreactivity of claudins 1, 4, and 7, particularly in stellate reticulum-like cells within ameloblastomas, suggesting their role in promoting cell–cell adhesion. Conversely, the expression of these claudins is typically weaker or moderate in ameloblastic carcinomas, possibly indicating a correlation with the aggressive behavior characteristic of these carcinomas. Abnormality in the permeability of tight junctions allows diffusion of the factors which has role in promoting tumor growth. Decreased expression of claudin is associated with dismantle of tight junction that can promote tumor invasion and has also been observed in oral squamous cell carcinoma [36]. Other odontogenic tumors, like adenomatoid odontogenic tumors (AOT), ameloblastic fibroma and ameloblastic carcinoma also exhibit expression of claudins.
High-mobility group A protein 2 (HMGA2)
High Mobility Group A Protein 2 (HMGA2) is a protein encoded by the HMGA2 gene in humans. It belongs to the high mobility group (HMG) protein family, which consists of non-histone chromosomal proteins involved in DNA binding, chromatin structure, and gene regulation, cell proliferation and tumorigenesis. Sato et al. proposed that alterations in the HMGA2 gene, such as rearrangements, and the increased expression of HMGA2 protein could potentially be linked to the development of odontogenic tumors, including odontogenic myxoma and odontogenic myxofibroma. Therefore, they suggest that these HMGA2 protein and gene characteristics are associated with odontogenic mesenchymal tumors [37].
Integrins
Integrins are a family of cell adhesion receptors that play a pivotal role in cell–cell and cell-extracellular matrix (ECM) interactions. These transmembrane proteins are crucial for various cellular processes, including cell adhesion, signalling, migration, and tissue organization.
As per the findings of Andrade ES et al., α5β1 integrin expression is notably higher in ameloblastomas compared to other odontogenic tumors such as AOT. This heightened expression implies a probable involvement of α5β1 integrin in the invasion mechanism of tumors. By binding to fibronectin, it potentially augments the secretion and presence of metalloproteinases, which are known to facilitate tumor invasion. Conversely, α3β1 integrin focal expression might induce disorganization of the basement membrane in certain areas of ameloblastomas, contributing to their invasive nature [38].
Increased expression of specific integrins, such as α5β1, indicates an enhanced interaction between tumor cells and the extracellular matrix (ECM). This upregulation may facilitate tumor invasiveness by strengthening cell adhesion, migration, and the release of matrix-degrading enzymes like metalloproteinases. Such elevation could signify a more aggressive tumor phenotype, predisposing to local invasion and metastasis. On the contrary, reduced levels of integrins observed in ameloblastomas, notably α3β1, might induce changes in cell—ECM interactions, resulting in impaired adhesion. This reduction could disturb the structural integrity of the basement membrane, potentially enabling tumor cells to detach and migrate towards adjacent tissues.
The absence of integrin α3β1 detection in tooth germs indicates its potential utility as a marker for neoplastic transformation in odontogenic tissues. Additionally, considering the heightened expression of integrins α2β1 and α5β1 in ameloblastomas, targeting these integrins may emerge as a significant therapeutic approach to manage or mitigate the local invasiveness of this tumor [38].
Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are a family of enzymes that play a crucial role in tissue remodelling and degradation of the extracellular matrix (ECM). These enzymes are involved in various physiological processes like embryogenesis, angiogenesis, immune response and are also associated with several pathological conditions such as cancer, arthritis, atherosclerosis and neurological disorders.
MMPs particularly MMP-2 (gelatinase A) and MMP-9 (gelatinase B), are crucial in facilitating the invasion and metastasis of odontogenic tumors such as ameloblastoma and ameloblastic carcinoma, as they participate in breaking down the extracellular matrix (ECM). Their role in breaking down components of the extracellular matrix (ECM), such as collagen and gelatin, may facilitate tumor cell invasion. The presence and activity of various MMPs, including MMP-1, MMP-2, MMP-7, MMP-9, and MMP-26, within ameloblastomas (AB) showed significant correlations with several clinicopathologic features of these tumors. These characteristics encompassed aspects like the rate of growth, invasive behavior, and the potential for metastasis. This suggests that the expression of these MMPs may be associated with the aggressive behavior and clinical characteristics of ameloblastomas [39, 40]. Other odontogenic tumors, like odontogenic myxoma, adenomatoid odontogenic tumors (AOT), and ameloblastic fibroma, also exhibit expression of MMPs.
Nestin
Nestin is an intermediate filament protein that is commonly associated with stem cells, particularly neural stem cells, and is involved in various aspects of cellular processes, including cytoskeletal organization, cell division, and differentiation. It has been found in other tissues and organs, including muscle, pancreas, and blood vessels.
The expression of nestin in odontogenic tumors is associated with the ectomesenchymal tissue's origin from the neural crest. However, a study conducted by Fujita et al. revealed that nearly all cases of ameloblastomas and malignant ameloblastomas did not exhibit nestin expression. In contrast, in mixed tumors like ameloblastic fibroma (AF) and Ameloblastic fibro—sarcoma, the odontogenic ectomesenchyme displayed robust nestin expression, particularly in the vicinity of the neoplastic follicular odontogenic epithelium [41]. Hence, it can be inferred that nestin may serve as a valuable marker for tumors originating from odontogenic ectomesenchymal tissues.
Podoplanin
Podoplanin, also known as PDPN, is a transmembrane glycoprotein that plays important roles in lymphangiogenesis, cancer metastasis, platelet aggregation, and tissue regeneration. Podoplanin can also enhance cancer cell invasiveness and migration. As reported by Gonzalez-Alva et al., strong expression of podoplanin was observed in the peripheral columnar cells of ameloblastomas, with a relatively weaker expression noted in the central stellate reticulum-like cells of these tumors [42]. Ganvir et al. observed robust expression of Podoplanin at the invasive front, particularly in the peripheral odontogenic epithelial cells of the majority of tumors and dental follicles. In cases of ameloblastomas, the membranous expression of Podoplanin was more intense in instances displaying aggressive behavior compared to non-aggressive ameloblastomas. The presence of podoplanin at the invasive front, particularly in peripheral cells, of odontogenic tumors suggests its association with neoplastic odontogenic tissues. This molecule is believed to potentially contribute to the progression and local invasion of odontogenic tumors which may be linked to its ability to induce cytoskeletal reorganization within the cells [43].
SOX 2 and OCT 4
SOX2 (SRY-Box Transcription Factor 2) and OCT (Octamer-Binding Transcription Factor) are transcription factors that play crucial roles in the regulation of gene expression and cell differentiation, stem cell maintenance, pluripotency, and development. Both are highly expressed in embryonic stem cells, neural stem cells, germ cells and various adult stem cell populations. In the odontogenic tumors, stem cell populations within these tumors may express SOX2, potentially contributing to their growth and maintenance. SOX2, in collaboration with the transcription factors OCT4 (Octamer binding protein 4) and Nanog, plays a crucial role in maintaining the stemness properties of pluripotent stem cells, including their ability to self-renew and differentiate into various cell types. Furthermore, when expressed in unusual locations, SOX2, together with OCT4 and KLF4, can reprogram differentiated cells into induced pluripotent stem cells (iPSCs) [44].
Syndecan
Syndecans are a family of transmembrane heparan sulfate proteoglycans (HSPGs) that play essential roles in various cellular processes. These cell surface molecules are involved in cell–cell communication, adhesion, and signaling. Bologna-Molina et al. observed that solid ameloblastoma (SA) exhibited lower levels of syndecan-1 (SDC1) expression compared to unicystic ameloblastoma (UA). This reduced syndecan-1 expression in SA suggests that SA may exhibit a more aggressive biological behavior in comparison to UA [45]. Syndecan-1 appears to play a role in the development of ameloblastoma, ameloblastic fibroma (AF), and adenomatoid odontogenic tumor (AOT). According to the findings of Al-Otaibi et al., the presence of syndecan-1 (SD1) expression in stromal cells and the extracellular matrix (ECM) is an important factor contributing to the development of intra-osseous ameloblastomas and their ability to locally invade surrounding tissues [46].
Syndecans have been detected in various odontogenic tumors like ameloblastic carcinoma, adenomatoid odontogenic tumors (AOTs), and ameloblastic fibromas. Research indicates distinct syndecan expression patterns in these tumors compared to normal tissues, suggesting their probable role in tumor progression and development. Particularly, the expression of syndecan-1 (SDC1) varies among different odontogenic tumors, with some tumors showing reduced SDC1 levels. This variation in expression might mirror differences in tumor aggressiveness and behavior. Overall, syndecan expression in odontogenic tumors highlights its potential as a diagnostic marker and its involvement in tumor pathogenesis [46].
Tenascin
Tenascin is a family of extracellular matrix (ECM) glycoproteins that play significant roles in various biological processes, including tissue development, wound healing, and immune responses. There are four major members of the tenascin family: tenascin-C, tenascin-R, tenascin-X, and tenascin-W. Tenascins can influence cell behavior and tissue structure through their interactions with other ECM components, cell surface receptors, and signaling pathways. Nagai et al. conducted a study to investigate the expression of tenascin in different dental tissues, including ameloblastomas, ameloblastic fibromas, ameloblastic carcinomas, and tooth germs. Their findings revealed varying levels of tenascin expression: Ameloblastic fibromas showed high tenascin expression in both the basement membrane and stromal areas, Follicular ameloblastomas displayed tenascin positivity primarily in the basement membrane zone, displaying an uneven linear pattern and Ameloblastic carcinomas exhibited an irregular and robust response to tenascin in both stromal and basement membrane regions. To sum up, the presence of tenascin can indicate interactions between epithelial and mesenchymal components in tooth development, and its expression varies among different dental tissues. Additionally, it serves as a valuable marker for distinguishing odontogenic tumors such as CEOT & odontomas that form calcifying masses from those that do not [47].
Wingless type 1 glycoprotein (Wnt 1)
Wingless type 1 glycoprotein, often referred to as Wnt-1, is a member of the Wnt family of signaling proteins. Wnt proteins are secreted glycoproteins that play crucial roles in various developmental processes, including embryogenesis, tissue regeneration, odontogenesis, regulation of cell fate and aberrant Wnt signaling can promote tumor growth, invasion, and metastasis. According to the findings reported by Chuah et al., a significant majority of primary conventional ameloblastomas, specifically 76.9%, exhibited robust Wnt1 immunoreactivity. In contrast, a lower percentage, specifically 42.9%, of primary unicystic ameloblastomas demonstrated strong Wnt1 immunoreactivity. These differences in Wnt1 expression patterns suggest potential variations in the molecular characteristics and behavior of these two types of ameloblastomas [48]. As reported by Siar CH et al., changes in the expressions of Wnt signaling molecules, specifically Wnts 1, 2, 5a, and 10a, have been observed in ameloblastomas. Among these, Wnt 1 appears to be a critical signaling molecule that may play a central role in the tumorigenesis of ameloblastomas. These findings suggest that aberrations in the Wnt signaling pathway contribute to the development of ameloblastomas by impacting cell proliferation and influencing the oncogenesis and cytodifferentiation of odontogenic epithelium [49].
Ki—67
Ki-67 is a 319—385 KDa protein marker that is commonly used as a proliferation marker in various tumors, including odontogenic tumors and indicates the proliferation or growth fraction of cells within a tumor. Ki-67 is expressed during the active phases of the cell cycle (G1, S, G2, and mitosis) but is absent in the resting phase (G0). In the context of odontogenic tumors, Ki-67 expression can provide valuable information about the tumor’s growth potential and aggressiveness. High Ki-67 labelling index (percentage of positively stained cells) may suggest a more rapidly proliferating and potentially more aggressive tumor [50].
Carreón-Burciaga et al. showed that Ki-67 expression is elevated in ameloblastic carcinomas (AC) compared to ameloblastomas (AM). This heightened expression was found to be associated with the aggressiveness of AC tumors, including features such as invasion and the presence of metastatic neoplasms [51]. Although it is beneficial, this immunomarker does not provide a clear distinction between benign and malignant tumors.
Glypican
Glypican (GPCs) is a family of cell surface heparan sulfate proteoglycans (HSPGs) that play crucial roles in various cellular processes, including cell signaling, growth, and differentiation. These proteoglycans are anchored to the cell membrane by a glycosylphosphatidylinositol (GPI) anchor and consist of a core protein linked to heparan sulfate chains. The GPC (Glypican) gene family comprises six members (GPC1-6), and Glypican 3 is recognized for its interactions with various signaling pathways. These pathways encompass those integral to embryonic development, tissue morphogenesis, and the control of cell growth. Glypicans can influence signaling pathways such as Wnt, Hedgehog (Hh), and FGF, which are essential for normal development but can also be dysregulated in cancer such as hepatocellular carcinoma, malignant melanoma, neuroblastoma, and colon cancer [52, 53].
According to Mendes et al. and Chaturvedi et al., the studies concluded that GPC3 plays a role in odontogenic tumors by differentiating between aggressive—ameloblastomas & unicystic ameloblastoma and nonaggressive odontogenic tumors—AOT. The expression of molecules associated with the Sonic Hedgehog (SHH) signaling pathway, including SHH, PTC, smoothened, and GLI1, has been identified in various odontogenic tumors. This suggests that the SHH signaling pathway contributes to epithelial–mesenchymal interactions and cell proliferation in the growth of odontogenic tumors, as well as during tooth development [52, 53]. Bologna-Molina et al. revealed the existence of GPC-1, another member of the GPC family, in various types of ameloblastomas. However, they did not observe any distinctions between solid and unicystic cases [54].
The exact significance of glypicans in diagnosing or predicting the prognosis of odontogenic tumors remains unclear. However, studies propose that glypicans, specifically glypican-3, might participate in diverse cellular signaling pathways linked to tumor development and advancement. Elevated levels of GPC3 correlate with the aggressive clinical nature of odontogenic tumors and an increased likelihood of recurrence. Analyzing the expression patterns of glypicans in odontogenic tumors could potentially offer understanding into their biological characteristics and clinical implications. Therefore, this marker shows potential for assessing the prognosis of odontogenic tumors [52, 53].
PITX 2
PITX2 (Pituitary homeobox 2) is a gene that codes for a transcription factor belonging to the homeobox family. Its expression is regulated by the Wnt cell signaling pathway, and it plays a vital role in embryonic development, as well as in processes such as cell proliferation and migration. It is involved in the regulation of various developmental processes, including the development of the pituitary gland, eyes, and other facial structures. Pitx2, identified as the initial transcriptional marker for tooth development, governs the embryonic establishment and arrangement of epithelial signaling centres throughout incisor development [55]. During the process of odontogenesis, PITX2 exhibits selective expression in the initial stages of morphogenesis within the oral ectoderm and epithelial cells. Disruptions in PITX2 expression during embryonic development can result in developmental irregularities, including conditions like enamel hypoplasia and anodontia [56].
García-Muñoz and colleagues propose a hypothesis suggesting that PITX2 serves as a significant transcription factor in odontogenic tumors, particularly in Ameloblastic carcinoma (AC) and to a lesser extent in unicystic ameloblastoma (UA). In Ameloblastic Carcinoma and to a lesser extent in benign ameloblastomas, akin to its roles in pituitary or colorectal cancer, PITX2 could potentially function as an activator of downstream oncogenes in the course of tumor progression. This association may be linked to tumor behavior, including factors such as aggressiveness, recurrence, and metastasis [56].
Wilms tumor (WT—1) gene
WT1 (Wilms tumor 1) is a gene that encodes a transcription factor involved in normal kidney development and function. Mutations or dysregulation of the WT1 gene have been implicated in the development of Wilms tumor, a Pediatric kidney cancer.
In the research conducted by Mukhopadhyay et al., their conclusion emphasized that the upregulation or overexpression of WT-1 is linked to tumorigenesis, proliferation, and localized aggressiveness in Ameloblastoma. The study also demonstrated that WT-1, typically a cytoplasmic marker, exhibited positivity in both the nucleus and nucleolus across various cell types, particularly in ameloblast-like cells [57]. In research conducted by Bologna-Molina et al., it was observed that none of the tooth germs exhibited WT-1 expression, while over half of the Ameloblastoma tissue samples displayed an overexpression of WT-1. These results indicate a potential oncogenic role for WT-1 in these lesions [58]. Research in the field of odontogenic tumors is ongoing, and molecular markers like WT1 may be explored in the future to understand their potential involvement in tumorigenesis or as diagnostic and prognostic markers.
Perilipin
Perilipin is a protein that plays a crucial role in lipid metabolism, particularly in the regulation of lipid storage in adipocytes (fat cells). It is primarily associated with the surface of lipid droplets and is involved in controlling lipolysis (the breakdown of stored fat into fatty acids) and lipogenesis (the synthesis of fat). In the context of odontogenic tumors and embryonic odontogenic tissues, previous research has indicated that the heightened expression of glucose transporter 1 (GLUT1), reflecting increased glycolytic capacity, and fatty acid synthase (FASN), indicative of lipogenic activity. Additionally, the presence of cyclooxygenase-2 expression in certain odontogenic tumors has led to the hypothesis that these tumors, notably ameloblastoma (AM) and ameloblastic carcinoma (AC) tumors, may contain cytoplasmic lipid droplets [59].
In the study conducted by Sánchez-Romero et al., it was demonstrated that Adipophilin exhibits significant activity in human tooth development. The immunoexpression of perilipin 1 and adipophilin in the samples of ameloblastoma (AM) and ameloblastic carcinoma (AC) suggests the presence of lipid droplets, providing further evidence of metabolic alterations in these tumors. Additionally, both proteins were more highly expressed in the AC samples (malignant tumor) than in the AM (benign tumor) samples [59]. In a study conducted by Sangamithra et al., it was concluded that the diffuse cytoplasmic positivity of adipophilin (perilipin 2) in ameloblastoma indicates the production and accumulation of lipid droplets, providing new evidence of metabolic alterations that may be involved in tumor progression [60].
Connexin
Connexin 43 (Cx43) are a family of proteins that form gap junctions, which are specialized intercellular channels allowing direct communication between neighboring cells. Gap junctions play a crucial role in the regulation of various cellular processes, including cell signaling, proliferation, and differentiation and also enable the exchange of ions, small molecules, and signaling molecules, contributing to coordinated cellular functions within tissues [61].
In odontogenic tumors, the expression of Connexin 43 has been studied to understand its role in the pathogenesis of these tumors. It is involved in various cellular processes, including cell growth, differentiation, and apoptosis. In the research conducted by Yamada et al., findings indicate that Connexin 43 (Cx43) plays a role in regulating intercellular communication via gap junction activity. This regulation occurs through the modulation of transforming growth factor-beta 1 (TGF-β1)-mediated extracellular signal-regulated kinase (ERK) signaling pathways, contributing to the process of enamel formation. Additionally, their study demonstrated high expression of Cx43 in pre-secretory ameloblasts, differentiated ameloblasts, and odontoblasts, as evidenced by single-cell RNA-sequence analysis and immunostaining [62]. As per the research conducted by Silveira et al., the study's results suggest that Connexin 43 (Cx43) is a protein with significant expression in the mesenchymal layer of embryonic and odontogenic tissues. The findings indicate that Cx43 may play a role in mineralization events, given its expression pattern in the epithelial and mesenchymal layers of odontogenic tissues [63].
Gene mutation
Gene mutations serve as crucial markers for diagnosing neoplastic lesions. The B-Raf proto-oncogene (BRAF) plays a regulatory role in the mitogen-activated protein kinase (MAPK) signaling pathway. This pathway is integral to various cellular processes, including tumor cell proliferation, differentiation, apoptosis, angiogenesis, invasion, and metastasis, and has been identified in numerous neoplasms [64]. The BRAF V600E gene mutation has been detected in mandibular ameloblastomas, while mutations in the Smoothened (SMO) protein are implicated in maxillary ameloblastomas. The SMO gene plays a role in regulating the Hedgehog pathway, which operates independently of the MAPK pathway [65]. In the study conducted by Lapthanasupkul et al., 72.5% of conventional ameloblastoma cases tested positive for the BRAF V600E mutation, with a higher positivity rate of 95.5% observed in unicystic ameloblastoma cases. These findings suggest the potential for BRAF-targeted therapies in the future management of ameloblastomas with these mutations [66].
The KRAS gene (Ki-ras2 Kirsten rat sarcoma viral oncogene homolog) encodes a protein known as KRAS, which plays a critical role in regulating cell division. Mutations in the KRAS gene render it oncogenic, leading to the development of various cancers [67]. Studies indicate that KRAS gene mutations can be a pathogenic factor in the development of adenomatoid odontogenic tumors [66]. The human homologue of the PTC gene, known as PTCH, plays a crucial role in regulating programmed cell proliferation, ensuring it remains under control. Consequently, any deletion or mutation in the PTCH gene can disrupt this regulation, resulting in uncontrolled cell proliferation. Research by Rosenstein et al. and Samuel Ebele Udeabor et al. suggests the expression of PTCH in ameloblastoma [68]. The catenin beta-1 protein, encoded by the CTNNB1 gene, is associated with various cellular processes, including cell adhesion and signaling. Mutations in the CTNNB1 gene have been linked to the development of calcifying cystic odontogenic tumors [69].
Conclusion
Odontogenic tumors are a diverse group of lesions arising from the dental apparatus or its remnants, encompassing a range of benign and malignant neoplasms. The diagnosis and management of these tumors can be challenging due to their rarity and histological diversity. Although the definitive diagnosis of odontogenic tumors is histomorphological, the search for potential markers in odontogenic tumors is essential for the differential pathological diagnosis with other lesions and these markers contribute to a more comprehensive assessment of the disease. Tumor markers have evolved into indispensable assets in the realm of odontogenic tumors. They enhance the diagnostic process, aid in risk assessment, provide valuable prognostic information, and contribute to efficient disease monitoring. As technology continues to advance, the discovery of novel biomarkers promises to further elevate our understanding and management of these complex lesions. Table 10 provides a summary of some important markers used for the diagnosis of odontogenic tumors.
Table 10.
Key tumour markers in odontogenic tumors
| Marker | Expressed in Odontogenic tumor | Significance |
|---|---|---|
| Ameloblastin (AMBN) | Ameloblastoma, Adenomatoid odontogenic tumor, Squamous odontogenic tumour, Calcifying epithelial odontogenic tumour |
Differentiates odontogenic lesions |
| Amelogenin | Ameloblastoma, Adenomatoid Odontogenic Tumor, Calcifying Epithelial Odontogenic Tumor, Ameloblastic Fibroma, Malignant Ameloblastoma, Ameloblastic Carcinoma | Expressed in odontogenic epithelial components of odontogenic tumor |
| Basement membrane proteins | Ameloblastomas and Adenomatoid odontogenic tumors | Odontogenic epithelial marker |
| BMP (Bone Morphogenetic Protein) | Odontogenic fibroma, Odontoma |
Implicated in odontogenic tumor development |
| Calretinin | Ameloblastoma | Differentiates ameloblastoma from other tumors |
| Cytokeratins | Ameloblastoma, Calcifying epithelial odontogenic tumor | To differentiate odontogenic epithelial tumor from other tumors |
| CD147 | Ameloblastoma, Adenomatoid odontogenic tumor | Tumor invasiveness and aggressiveness |
| Claudin | Ameloblastoma, adenomatoid odontogenic tumors, ameloblastic fibroma, ameloblastic carcinoma | Aggressive behavior of tumor |
| High-mobility group A protein 2 (HMGA2) | Odontogenic myxoma, Odontogenic myxofibroma | Indicates odontogenic mesenchymal tumors |
| Integrins | Ameloblastomas | Invasive behavior |
| Matrix metalloproteinases (MMPs) | Ameloblastomas, odontogenic myxoma, adenomatoid odontogenic tumors, ameloblastic fibroma | Invasive behaviour |
| Podoplanin | Ameloblastoma |
Linked to local invasion and cytoskeletal changes |
| Syndecan-1 (SDC1) | Ameloblastoma, Ameloblastic fibroma, Adenomatoid odontogenic tumor |
May indicate aggressive behavior in some cases |
| Nestin | Ameloblastic fibroma, Ameloblastic fibrosarcoma |
Useful marker for odontogenic epithelium with odontogenic ectomesenchymal tumors |
| SOX 2 and OCT 4 | SOX 2 expressed in Ameloblastic fibroma, Ameloblastic carcinoma | Differentiate among odontogenic tumors |
| Tenascin | Ameloblastoma, Ameloblastic fibroma, Ameloblastic carcinoma, Adenomatoid odontogenic tumor | Expressed in tumors forming calcified masses |
| Wnt Signaling Molecules (e.g., Wnt1) | Ameloblastoma |
Implicates Wnt pathway in tumor development |
| Ki-67 | Ameloblastic carcinomas | Increased cell proliferation |
| Glypican | Ameloblastomas, Ameloblastic carcinoma, Ameloblastic fibroma | aggressive clinical nature of odontogenic tumors and increased likelihood of recurrence |
| PITX 2 | Ameloblastomas, Ameloblastic carcinoma | aggressiveness, recurrence, and metastasis |
| Wilms Tumor (WT—1) Gene | Ameloblastomas | Aggressiveness of tumor |
| Perilipin | Ameloblastoma, Ameloblastic carcinoma | Indicative of tumor progression |
| Connexin 43 | Ameloblastoma, Ameloblastic fibroma | Odontogenic tissues |
Author contribution
1. VR, SC wrote the main manuscript text 2. DD and NM prepared Tables 1, 2, 3, 4, 5, 6, 7, 8, 9 and 10 and Fig. 1. All authors reviewed the manuscript.
Funding
The authors did not receive support from any organization for the submitted work.
Data availability
The data is available within the article.
Declarations
Ethics approval and consent to participate
For this type of study formal consent is not required.
Consent for publication
For this type of study consent for publication is not required.
Informed consent
For this type of study informed consent is not required.
Competing interests
The authors have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Guimarães LM, Coura BP, Gomez RS, Gomes CC. The molecular pathology of odontogenic tumors: expanding the spectrum of MAPK pathway driven tumors. Front Oral Health. 2021. 10.3389/froh.2021.740788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vered M, Wright JM. Update from the 5th edition of the World Health Organization classification of head and neck tumors: odontogenic and maxillofacial bone tumours. Head Neck Pathol. 2022;16(1):63–75. 10.1007/s12105-021-01404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Premalatha BR, Patil S, Rao RS, Reddy NP, Indu M. Odontogenic tumor markers—an overview. J Int Oral Health. 2013;5(2):59–69. [PMC free article] [PubMed] [Google Scholar]
- 4.Kokubun K, Yamamoto K, Nakajima K, Akashi Y, Chujo T, Takano M, et al. Frequency of odontogenic tumors: a single center study of 1089 cases in japan and literature review. Head Neck Pathol. 2022;16(2):494–502. 10.1007/s12105-021-01390-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruhin B, Lézot F, Bouattour A, Ghoul-Mazgar S, Berdal A, Descroix V. Chapter 7—facts and hypothesis on osteolytic lesions related to normal and tumoral epithelial dental cell differentiation. In: Heymann D, editor. Bone cancer. San Diego: Academic Press; 2010. p. 77–96. [Google Scholar]
- 6.Bhatt AN, Mathur R, Farooque A, Verma A, Dwarakanath BS. Cancer biomarkers—current perspectives. Indian J Med Res. 2010;132:129–49 (PMID: 20716813). [PubMed] [Google Scholar]
- 7.Sotiriou C, Lothaire P, Dequanter D, Cardoso F, Awada A. Molecular profiling of head and neck tumors. Curr Opin Oncol. 2004;16(3):211–4. 10.1097/00001622-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Chander Y, Subramanya H. Serological tumor markers—their role. Med J Armed Forces India. 2000;56(4):279–81. 10.1016/S0377-1237(17)30207-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henry NL, Hayes DF. Cancer biomarkers. Mol Oncol. 2012;6(2):140–6. 10.1016/j.molonc.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagpal M, Singh S, Singh P, Chauhan P, Zaidi MA. Tumor markers: a diagnostic tool. Natl J Maxillofacial Surg. 2016;7(1):17–20. 10.4103/0975-5950.196135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farshbaf A, Zare R, Mohajertehran F, Mohtasham N. New diagnostic molecular markers and biomarkers in odontogenic tumors. Mol Biol Rep. 2021;48(4):3617–28. 10.1007/s11033-021-06286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velpula N, Yathavakilla CN, Ramesh A. Tumor markers: a review. Int J Oral Health Dentistry. 2017;3(1):1–5. 10.18231/2395-499X. [Google Scholar]
- 13.Sharma S. Tumor markers in clinical practice: General principles and guidelines. Indian J Med Paediatric Oncol Off J Indian Soc Med Paediatric Oncol. 2009;30(1):1–8. 10.4103/0971-5851.56328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niederhuber JE, Armitage JO, Doroshow JH, Kastan MB, Tepper JE. Abeloff’s clinical oncology. 5th ed. Amsterdam: Elsevier Inc.; 2013. p. 2186. [Google Scholar]
- 15.Fukumoto S, Kiba T, Hall B, Iehara N, Nakamura T, Longenecker G, et al. Ameloblastin is a cell adhesion molecule required for maintaining the differentiation state of ameloblasts. J Cell Biol. 2004;167(5):973–83. 10.1083/jcb.200409077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perdigão PF, Carvalho VM, Dem L, Gomez RS. Mutation of ameloblastin gene in calcifying epithelial odontogenic tumor. Anticancer Res. 2009;29(8):3065–7. [PubMed] [Google Scholar]
- 17.Fincham A, Moradian-Oldak J. Simmer JJJosb. Struct Biol Dev Dental Enamel Matrix. 1999;126(3):270–99. 10.1006/jsbi.1999.4130. [DOI] [PubMed] [Google Scholar]
- 18.Mori M, Yamada K, Kasai T, Yamada T, Shimokawa H, Sasaki S. Immunohistochemical expression of amelogenins in odontogenic epithelial tumors and cysts. Virchows Archiv A Pathol Anatomy Histopathol. 1991;418(4):319–25. 10.1007/BF01600161. [DOI] [PubMed] [Google Scholar]
- 19.Kumamoto H, Yoshida M, Ooya K. Immunohistochemical detection of amelogenin and cytokeratin 19 in epithelial odontogenic tumors. Oral Dis. 2001;7(3):171–6. 10.1034/j.1601-0825.2001.70306.x. [PubMed] [Google Scholar]
- 20.Paulsson M. Basement membrane proteins: structure, assembly, and cellular interactions. Critical Rev Biochem Mol Biol. 1992;27(1–2):93–127. 10.3109/10409239209082560. [DOI] [PubMed] [Google Scholar]
- 21.Poomsawat S, Punyasingh J, Vejchapipat P. Expression of basement membrane components in odontogenic tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(5):666–75. 10.1016/j.tripleo.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 22.Aberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (Bmps) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dynam Off Publ Am Assoc Anatomists. 1997;210(4):383–96. [DOI] [PubMed] [Google Scholar]
- 23.Canalis E, Economides AN, Gazzerro E. Bone morphogenetic proteins, their antagonists, and the skeleton. Endocrine Rev. 2003;24(2):218–35. 10.1210/er.2002-0023. [DOI] [PubMed] [Google Scholar]
- 24.Kumamoto H, Ooya K. Expression of bone morphogenetic proteins and their associated molecules in ameloblastomas and adenomatoid odontogenic tumors. Oral Dis. 2006;12(2):163–70. 10.1111/j.1601-0825.2005.01177.x. [DOI] [PubMed] [Google Scholar]
- 25.Schwaller B. Calretinin: from a “simple” Ca2+ buffer to a multifunctional protein implicated in many biological processes. Front Neuroanat. 2014. 10.3389/fnana.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chhieng DC, Yee H, Schaefer D, Cangiarella JF, Jagirdar J, Chiriboga LA, et al. Calretinin staining pattern aids in the differentiation of mesothelioma from adenocarcinoma in serous effusions. Cancer. 2000;90(3):194–200. 10.1002/1097-0142(20000625)90:3%3c194::aid-cncr8%3e3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Alaeddini M, Etemad-Moghadam S, Baghaii FJH. Comparative expression of calretinin in selected odontogenic tumours: a possible relationship to histogenesis. Histopathology. 2008;52(3):299–304. 10.1111/j.1365-2559.2007.02948.x. [DOI] [PubMed] [Google Scholar]
- 28.Anandani C, Metgud R, Singh K. Calretinin as a diagnostic adjunct for ameloblastoma. Pathol Res Int. 2014;2014:308240. 10.1155/2014/308240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar A, Jagannathan N. Cytokeratin: a review on current concepts. Int J Orofac Biol. 2018;2:6–11. 10.4103/ijofb.ijofb_3_18. [Google Scholar]
- 30.Martínez-Mata G, Mosqueda-Taylor A, Carlos-Bregni R, de Almeida OP, Contreras-Vidaurre E, Vargas PA, et al. Odontogenic myxoma: clinico-pathological, immunohistochemical and ultrastructural findings of a multicentric series. Oral Oncol. 2008;44(6):601–7. 10.1016/j.oraloncology.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Crivelini MM, de Araújo VC, de Sousa SO, de Araújo NS. Cytokeratins in epithelia of odontogenic neoplasms. Oral Dis. 2003;9(1):1–6. 10.1034/j.1601-0825.2003.00861.x. [DOI] [PubMed] [Google Scholar]
- 32.Sudhakara M, Rudrayya SP, Vanaki SS, Bhullar RK, Shivakumar MS, Hosur M. Expression of CK14 and vimentin in adenomatoid odontogenic tumor and dentigerous cyst. J Oral Maxillofacial Pathol JOMFP. 2016;20(3):369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang K, Chen W, Zhang Z, Deng Y, Lian J-Q, Du P, et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther. 2020;5(1):283. 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang P, Chang S, Jiang X, Su J, Dong C, Liu X, Yuan Z, Zhang Z, Liao H. RNA interference targeting CD147 inhibits the proliferation, invasiveness, and metastatic activity of thyroid carcinoma cells by down-regulating glycolysis. Int J Clin Exp Pathol. 2015;8(1):309–18 (PMID: 25755717). [PMC free article] [PubMed] [Google Scholar]
- 35.Lal-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10(8):235. 10.1186/gb-2009-10-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bello IO, Vilen S-T, Niinimaa A, Kantola S, Soini Y, Salo T. Expression of claudins 1, 4, 5, and 7 and occludin, and relationship with prognosis in squamous cell carcinoma of the tongue. Hum Pathol. 2008;39(8):1212–20. 10.1016/j.humpath.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Sato K, Terai K, Ozaki M, Ueda Y, Katsuda S. Odontogenic myxofibroma with HMGA2 overexpression and HMGA2 rearrangement. Pathol Int. 2010;60(11):760–4. 10.1111/j.1440-1827.2010.02589.x. [DOI] [PubMed] [Google Scholar]
- 38.de Souza Andrade ES, Miguel MC, de Freitas R, Pinto LP, de Souza LB. Immunoexpression of Integrins in ameloblastoma, adenomatoid odontogenic tumor, and human tooth germs. Int J Surg Pathol. 2008;16(3):277–85. [DOI] [PubMed] [Google Scholar]
- 39.Zhang B, Zhang J, Xu Z-Y, Xie H-L. Expression of RECK and matrix metalloproteinase-2 in ameloblastoma. BMC Cancer. 2009;9(1):427. 10.1186/1471-2407-9-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y-M, Zhong Q-B, Ye K-N, Wang H-Y, Ren Z-H. Expression of Matrix Metalloproteinases in Ameloblastomas and Ameloblastic Carcinoma: Systematic Review and Meta-analysis. Exp Res Hypothesis Med. 2019;4:19–28. 10.14218/ERHM.2019.00001. [Google Scholar]
- 41.Fujita S, Hideshima K, Ikeda T. Nestin expression in odontoblasts and odontogenic ectomesenchymal tissue of odontogenic tumours. J Clin Pathol. 2006;59(3):240–5. 10.1136/jcp.2004.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.González-Alva P, Tanaka A, Oku Y, Miyazaki Y, Okamoto E, Fujinami M, et al. Enhanced expression of podoplanin in ameloblastomas. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2010;39(1):103–9. 10.1111/j.1600-0714.2009.00818.x. [DOI] [PubMed] [Google Scholar]
- 43.Ganvir SM, Khobragade PG, Bamane SA, Kumavat R, Dalmia A. Role of podoplanin expression in deciding the invasive potential of ameloblastoma—a retrospective IHC study. J Oral Boil Craniofacial Res. 2016;6(3):187–93. 10.1016/j.jobcr.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swain N, Thakur M, Pathak J, Swain B. SOX2, OCT4 and NANOG: the core embryonic stem cell pluripotency regulators in oral carcinogenesis. J Oral Maxillofac Patho. 2020;24(2):368–73. 10.4103/jomfp.JOMFP_22_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bologna MR, Mosqueda TA, Lopez CE, Almeida OP, Carrasco DD, Garcia VF, et al. Syndecan-1 (CD138) and Ki-67 expression in different subtypes of ameloblastomas. Oral Oncol. 2008;44(8):805–11. 10.1016/j.oraloncology.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Al-Otaibi O, Khounganian R, Anil S, Rajendran R. Syndecan-1 (CD 138) surface expression marks cell type and differentiation in ameloblastoma, keratocystic odontogenic tumor, and dentigerous cyst. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2013;42(2):186–93. 10.1111/j.1600-0714.2012.01195.x. [DOI] [PubMed] [Google Scholar]
- 47.Nagai N, Yamachika E, Nishijima K, Inoue M, Shin HI, Suh MS, et al. Immunohistochemical demonstration of tenascin and fibronectin in odontogenic tumors and human fetal tooth germs. Eur J Cancer Part B Oral Oncol. 1994;30(3):191–5. 10.1016/0964-1955(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 48.Chuah KS, Siar CH, Nakano K, Nagatsuka H, Khoo SP, Han K, et al. Wingless- type protein 1 (Wnt 1) Expression in primary conventional and unicystic ameloblastomas and their recurrent tumors. J Hard Tissue Biol. 2009;18(2):63–70. 10.2485/jhtb.18.63. [Google Scholar]
- 49.Siar CH, Nagatsuka H, Han PP, Buery RR, Tsujigiwa H, Nakano K, Ng KH, Kawakami T. Differential expression of canonical and non-canonical Wnt ligands in ameloblastoma. J Oral Pathol Med. 2012;41(4):332–9. 10.1111/j.1600-0714.2011.01104.x. [DOI] [PubMed] [Google Scholar]
- 50.Jabbarzadeh M, Hamblin MR, Pournaghi-Azar F, Vakili Saatloo M, Kouhsoltani M, Vahed N. Ki-67 expression as a diagnostic biomarker in odontogenic cysts and tumors: a systematic review and meta-analysis. J Dent Res Dent Clin Dent Prospects. 2021;15(1):66–75. 10.34172/joddd.2021.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carreón-Burciaga RG, González-González R, Molina-Frechero N, Bologna-Molina R. Immunoexpression of Ki-67, MCM2, and MCM3 in ameloblastoma and ameloblastic carcinoma and their correlations with clinical and histopathological patterns. Dis Markers. 2015. 10.1155/2015/683087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaturvedi TP, Gupta K, Agrawal R, Naveen Kumar PG, Gupta J. Immunohistochemical expression of Ki-67 and Glypican-3 to distinguish aggressive from nonaggressive benign odontogenic tumors. J Cancer Res Ther. 2022;18(Supplement):S205–9. 10.4103/jcrt.JCRT_223_20. [DOI] [PubMed] [Google Scholar]
- 53.Mendes RB, Dias RB, Figueiredo AL, et al. Glypican-3 distinguishes aggressive from non-aggressive odontogenic tumors: a preliminary study. J Oral Pathol Med. 2017;46(4):297–300. 10.1111/jop.12501. [DOI] [PubMed] [Google Scholar]
- 54.Bologna-Molina R, Mosqueda-Taylor A, Molina-Frechero N. Differential expression of glypican-1 in ameloblastoma variants. Appl Immunohistochem Mol Morphol. 2015;23(2):153–60. 10.1097/PAI.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 55.Yu W, Sun Z, Sweat Y, Sweat M, Venugopalan SR, Eliason S, Cao H, Paine ML, Amendt BA. Pitx2-Sox2-Lef1 interactions specify progenitor oral/dental epithelial cell signaling centers. Development. 2020. 10.1242/dev.186023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.García-Muñoz A, Rodríguez MA, Licéaga-Escalera C, et al. Expression of the transcription factor PITX2 in ameloblastic carcinoma. Arch Oral Biol. 2015;60(6):799–803. 10.1016/j.archoralbio.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 57.Mukhopadhyay A, Panda A, Mishra P, et al. Comparative immunohistochemical analysis of WT-1, syndecan and snail in ameloblastoma and odontogenic keratocyst: a retrospective study. J Oral Maxillofac Pathol. 2023;27(2):295–301. 10.4103/jomfp.jomfp_301_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bologna-Molina R, Takeda Y, Kuga T, et al. Expression of Wilms’ tumor 1 (WT1) in ameloblastomas. J Oral Sci. 2016;58(3):407–13. 10.2334/josnusd.15-0546. [DOI] [PubMed] [Google Scholar]
- 59.Sánchez-Romero C, Carreón-Burciaga R, Gónzalez-Gónzalez R, et al. Perilipin 1 and adipophilin immunoexpression suggests the presence of lipid droplets in tooth germ, ameloblastoma, and ameloblastic carcinoma. J Oral Pathol Med. 2021;50(7):708–15. 10.1111/jop.13175. [DOI] [PubMed] [Google Scholar]
- 60.Sangamithra S, Gheena S, Ramani P. Adipophilin immunoexpression and its pathophysiology in human tooth germ and ameloblastoma. J Populat Therapeutics Clin Pharmacol. 2023;30(10):267–74. 10.47750/jptcp.2023.30.10.031. [Google Scholar]
- 61.Abdelaziz A, Essa M. Downregulation of connexin 43 is crucial for basal cell alignment in ameloblastoma and odontogenic keratocyst. Saudi Dental J. 2024. 10.1016/j.sdentj.2024.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamada A, Yoshizaki K, Ishikawa M, et al. Connexin 43-mediated gap junction communication regulates ameloblast differentiation via ERK1/2 phosphorylation. Front Physiol. 2021;12:748574. 10.3389/fphys.2021.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silveira FM, Molina-Frechero N, López-Verdín S, et al. Connexin 43 expression in tooth germ and benign odontogenic tumors. Oral Surg Oral Med Oral Pathol Oral Radiol. 2023;135(5):661–8. 10.1016/j.oooo.2023.01.010. [DOI] [PubMed] [Google Scholar]
- 64.Loo E, Khalili P, Beuhler K, Siddiqi I, Vasef MA. BRAF V600E mutation across multiple tumor types: correlation between DNA-based sequencing and mutation-specific immunohistochemistry. Appl Immunohistochem Mol Morphol. 2018;26(10):709–13. 10.1097/PAI.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 65.Mamat Yusof MN, Ch’ng ES, Rahman NRA. BRAF V600E mutation in ameloblastoma: a systematic review and meta-analysis. Cancers (Basel). 2022;14(22):5593. 10.3390/cancers14225593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silveira FM, Pereira-Prado V, Bologna-Molina R. Molecular bases of benign odontogenic tumors: a review of the literature in the context of the latest classification of the World Health Organization. Odontoestomatologia. 2022. 10.22592/ode2022n39e315. [Google Scholar]
- 67.Jancík S, Drábek J, Radzioch D, Hajdúch M. Clinical relevance of KRAS in human cancers. J Biomed Biotechnol. 2010;2010:150960. 10.1155/2010/150960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Srinagesh S, Sahana NS, Suganya G, Renuga S, Natarajan S, Kulkarni M. Expression of PTCH gene in ameloblastoma and odontogenic keratocyst: a comparative study. J Oral Maxillofac Pathol. 2023;27:427–8. 10.4103/jomfp.jomfp_198_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yukimori A, Oikawa Y, Morita KI, Nguyen CT, Harada H, Yamaguchi S, Kayamori K, Yamaguchi A, Ikeda T, Sakamoto K. Genetic basis of calcifying cystic odontogenic tumors. PLoS ONE. 2017;12(6): e0180224. 10.1371/journal.pone.0180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available within the article.


