Abstract
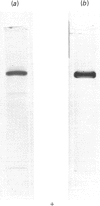
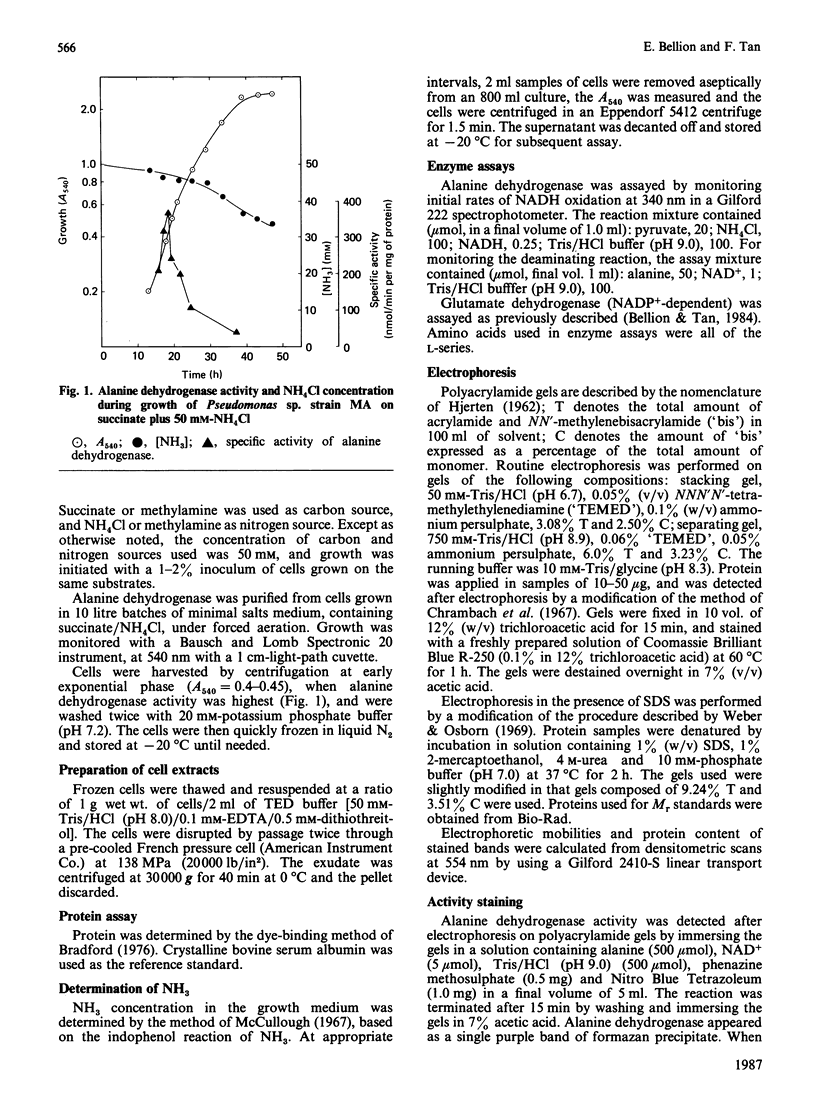
A study was made of the NAD+-dependent alanine dehydrogenase (EC 1.4.1.1) elaborated by the methylotrophic bacterium Pseudomonas sp. strain MA when growing on succinate and NH4Cl. This enzyme was purified 400-fold and was found to be highly specific for NH3 and NAD+; however, hydroxypyruvate and bromopyruvate, but not alpha-oxoglutarate or glyoxylate, could replace pyruvate to a limited extent. The Mr of the native enzyme was shown to be 217,000, and electrophoresis in SDS/polyacrylamide gels revealed a minimum Mr of 53,000, suggesting a four-subunit structure. The enzyme, which has a pH optimum of 9.0, operated almost exclusively in the aminating direction in vitro. It was induced by NH3 or by alanine, and was repressed by growth on methylamine or glutamate. It is suggested that this enzyme has two roles in this organism, namely in NH3 assimilation and in alanine catabolism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonowitz Y., Friedrich C. G. Alanine dehydrogenase of the beta-lactam antibiotic producer Streptomyces clavuligerus. Arch Microbiol. 1980 Mar;125(1-2):137–142. doi: 10.1007/BF00403210. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion E., Hersh L. B. Methylamine metabolism in a pseudomonas species. Arch Biochem Biophys. 1972 Nov;153(1):368–374. doi: 10.1016/0003-9861(72)90457-2. [DOI] [PubMed] [Google Scholar]
- Bellion E., Tan F. NADP-dependent glutamate dehydrogenase from a facultative methylotroph, Pseudomonas sp. strain AM1. J Bacteriol. 1984 Feb;157(2):435–439. doi: 10.1128/jb.157.2.435-439.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellion E., Woodson J. Two distinct isocitrate lyases from a pseudomonas species. J Bacteriol. 1975 May;122(2):557–564. doi: 10.1128/jb.122.2.557-564.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brenchley J. E., Baker C. A., Patil L. G. Regulation of the ammonia assimilatory enzymes in Salmonella typhimurium. J Bacteriol. 1975 Oct;124(1):182–189. doi: 10.1128/jb.124.1.182-189.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrambach A., Reisfeld R. A., Wyckoff M., Zaccari J. A procedure for rapid and sensitive staining of protein fractionated by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Jul;20(1):150–154. doi: 10.1016/0003-2697(67)90272-2. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREESE E., OOSTERWYK J. THE INDUCTION OF ALANINE DEHYDROGENASE. Biochemistry. 1963 Nov-Dec;2:1212–1216. doi: 10.1021/bi00906a006. [DOI] [PubMed] [Google Scholar]
- HJERTEN S. "Molecular sieve" chromatography on polyacrylamide gels, prepared according to a simplified method. Arch Biochem Biophys. 1962 Sep;Suppl 1:147–151. [PubMed] [Google Scholar]
- Hersh L. B., Stark M. J., Worthen S., Fiero M. K. N-methylglutamate dehydrogenase: kinetic studies on the solubilized enzyme. Arch Biochem Biophys. 1972 May;150(1):219–226. doi: 10.1016/0003-9861(72)90029-x. [DOI] [PubMed] [Google Scholar]
- Johansson B. C., Gest H. Inorganic nitrogen assimilation by the photosynthetic bacterium Rhodopseudomonas capsulata. J Bacteriol. 1976 Nov;128(2):683–688. doi: 10.1128/jb.128.2.683-688.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large P. J., Quayle J. R. Microbial growth on C(1) compounds. 5. Enzyme activities in extracts of Pseudomonas AM1. Biochem J. 1963 May;87(2):386–396. doi: 10.1042/bj0870386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCORMICK N. G., HALVORSON H. O. PURIFICATION AND PROPERTIES OF L-ALANINE DEHYDROGENASE FROM VEGETATIVE CELLS OF BACILLUS CEREUS. J Bacteriol. 1964 Jan;87:68–74. doi: 10.1128/jb.87.1.68-74.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCowen S. M., Phibbs P. V., Jr Regulation of alanine dehydrogenase in Bacillus (licheniformis). J Bacteriol. 1974 May;118(2):590–597. doi: 10.1128/jb.118.2.590-597.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough H. The determination of ammonia in whole blood by a direct colorimetric method. Clin Chim Acta. 1967 Aug;17(2):297–304. doi: 10.1016/0009-8981(67)90133-7. [DOI] [PubMed] [Google Scholar]
- Meers J. L., Pedersen L. K. Nitrogen assimilation by bacillus licheniformis organisms growning in chemostat cultures. J Gen Microbiol. 1972 Apr;70(2):277–286. doi: 10.1099/00221287-70-2-277. [DOI] [PubMed] [Google Scholar]
- Meers J. L., Tempest D. W., Brown C. M. 'Glutamine(amide):2-oxoglutarate amino transferase oxido-reductase (NADP); an enzyme involved in the synthesis of glutamate by some bacteria. J Gen Microbiol. 1970 Dec;64(2):187–194. doi: 10.1099/00221287-64-2-187. [DOI] [PubMed] [Google Scholar]
- Meloche H. P., Glusker J. P. Aldolase catalysis: single base-mediated proton activation. Science. 1973 Jul 27;181(4097):350–352. doi: 10.1126/science.181.4097.350. [DOI] [PubMed] [Google Scholar]
- Pollock R. J., Hersh L. B. N-methylglutamate synthetase. Purification and properties of the enzyme. J Biol Chem. 1971 Aug 10;246(15):4737–4743. [PubMed] [Google Scholar]
- Rowell P., Stewart W. D. Alanine dehydrogenase of the N2-fixing blue-green alga, Anabaena cylindrica. Arch Microbiol. 1975 Sep;107(2):115–124. doi: 10.1007/BF00446830. [DOI] [PubMed] [Google Scholar]
- Salem A. R., Hacking A. J., Quayle J. R. Cleavage of malyl-Coenzyme A into acetyl-Coenzyme A and glyoxylate by Pseudomonas AM1 and other C1-unit-utilizing bacteria. Biochem J. 1973 Sep;136(1):89–96. doi: 10.1042/bj1360089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Tsai L., Stadtman E. R. The enzymatic synthesis of N-methylglutamic acid. J Biol Chem. 1966 Feb 25;241(4):935–945. [PubMed] [Google Scholar]
- Tyler B. Regulation of the assimilation of nitrogen compounds. Annu Rev Biochem. 1978;47:1127–1162. doi: 10.1146/annurev.bi.47.070178.005403. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YOSHIDA A., FREESE E. ENZYMATIC PROPERTIES OF ALANINE DEHYDROGENASE OF BACILLUS SUBTILIS. Biochim Biophys Acta. 1965 Feb 22;96:248–262. [PubMed] [Google Scholar]
- YOSHIDA A., FREESE E. PURIFICATION AND CHEMICAL CHARACTERIZATION OF ALANINE DEHYDROGENASE OF BACILLUS SUBTILIS. Biochim Biophys Acta. 1964 Oct 23;92:33–43. doi: 10.1016/0926-6569(64)90266-4. [DOI] [PubMed] [Google Scholar]