Abstract
Inflammatory bowel disease (IBD) significantly diminishes an individual’s quality of life and increases the risk of colorectal cancer. Recent clinical and experimental findings suggest that infection with parasitic helminths may suppress the development of certain inflammatory conditions. The objective of this study was to evaluate the immunoregulatory effects of Dicrocoelium eggs on experimentally induced colitis in C57BL/6 mice using dextran sulfate sodium (DSS). C57BL/6 mice received 3.5% DSS orally for 7 days to induce colitis, during which they were treated intraperitoneally with Dicrocoelium eggs. The severity of colitis was assessed through parameters such as body weight, stool consistency or bleeding, disease activity index (DAI), colon lengths, macroscopic scores, histopathological findings, colon gene expression levels, and serum cytokine levels. Our results indicated that Dicrocoelium eggs administration significantly reduced the severity of colitis and disease activity. Histopathological scores improved, correlating with downregulation of IFN-γ and upregulation of IL-4 expression. This findings suggest the therapeutic potential of Dicrocoelium eggs in treating colitis. Immunotherapy involving Dicrocoelium eggs primarily induces a Th2 response and modulates IFN-γ, contributing to reduced inflammation in colitis. Thus, this approach could be a promising therapeutic strategy for alleviating inflammation in IBD.
Keywords: Dicrocoelium dendriticum, Colitis, DSS (Dextran Sulfate Sodium)
Subject terms: Immunotherapy, Parasitology
Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic immunological disorder of the digestive tract1. In recent decade, these disease have emerged as a global public health concern, with increasing morbidity and prevalence among both adults and children2,3. The rising prevalence of IBD is likely to negatively impact quality of life and increase the risk to colorectal cancer4. So far, the exact cause of IBD remains unclear, but current theories suggests that a combination of genetic and environmental factors, linked to dysregulation of the mucosal immune system and excessive expression of inflammatory cytokines, is associated with the development and progression of IBD5.
IBD is mediated by CD4+ Th1 and Th17 immune responses6. There is strong evidence showing that IFN-γ-producing Th1 cells contribute to the pathogenicity of IBD7. Numerous studies indicate that an imbalance of CD4+ T cell subsets, including Th1/Th2, Th17/Treg cells, participates in IBD pathogenesis8. Currently, there is no definitive cure for IBD, and conventional treatments focus on reducing chronic inflammation and shortening the duration of acute inflammation episode. Although various anti-inflammatory drugs, antibiotics, and biologics are available, many patients do not respond to these treatments and/or experience long-term side effects8–10.
Therefore, there is a need for effective therapeutic strategies with fewer side effects in the treatment of IBD. According to the “hygiene hypothesis”, there is an inverse correlation between exposure to helminth parasites and the incidence of inflammatory disease. This, coupled with the known immunoregulatory capabilities of helminths, has sparked interest in therapeutic helminth interventions11. Helminths, or parasitic worms, act as the immune regulators by skewing the immune system towards Th2 and Treg responses, inhibiting pro-inflammatory Th1 and Th17 responses, and thereby reducing inflammation12. IL-4 (Th2) actively suppresses Th1 pro-inflammatory cells and reduces the production of TNF-α and IFN-γ13.
A growing body of research in murine models supports the protective role of helminth infections and their products in modulating immune responses and blocking ongoing inflammation8. Dextran sulphate sodium (DSS) is widely used for inducing colitis, as the inflammation it causes develops rapidly and closely resembles human IBD14. Various worm species, such as Trichuris suis ova or larvae4, Syphacia obvelata5, Trichuris trichiura ova15, Schistosoma japonicum eggs16, Necator americanus17, Hymenolepis diminuta18 and Heligmosomoides polygyrus bakeri19, have been shown to reverse or prevent experimental colitis. More research is needed to identify available and low-risk worms for helminth therapy20.
Iran has a high incidence and prevalence of both UC and CD in the Middle East21. Furthermore, Dicrocoelium dendriticum is a zoonotic trematode reported among domestic animals worldwide, particularly in Europe, North Africa, North America, and Asia, including Iran22,23. This study provides novel insights into the protective role of Dicrocoelium dendriticum eggs and their underlying mechanisms in treating DSS-induced colitis, focusing on the balance between Th1 and Th2 responses. These findings offer experimental evidence for further exploration of Dicrocoelium dendriticum eggs as a potential candidate for IBD treatment.
Materials and methods
Mice
Thirty pathogen-free, 3 to 4-week-old male C57BL/6 mice were purchased from Pastor Institute of Tehran (Iran) and housed at 23 ± 2 °C, with a relative humidity of 50 ± 5% and a 12-hour light/dark cycle in a pathogen-free animal laboratory. They were provided with autoclaved food and water. Animal experiments were conducted in accordance with the ARRIVE guidelines and the Guidelines for the Care and Use of Laboratory Animals from the Mashhad University of Medical Sciences Ethics Committee. The study was approved by the Ethics Committee of Mashhad University of Medical Sciences (Approval ID: IR.MUMS.AEC.1401.030), and we confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Dicrocoelium spp. eggs preparation
A Dicrocoelium-infected sheep liver was obtained from an abattoir in Mashhad (Iran). Mature Dicrocoelium parasites were identified and separated based on their microscopic characteristics. The parasites were then washed with sterile PBS and cut into pieces with a scalpel to extract the eggs. After the eggs were deposition, they were washed with antibiotics (penicillin 500 U/mL and streptomycin 0.5 mg/mL; catalog number XC-A4122/100). To remove the antibiotics, the eggs were washed several times with sterile PBS. Finally the eggs were counted using a Neobauer slides, and 10,000 eggs were placed in each microtube and stored at − 80 °C.
Colitis model and experimental design
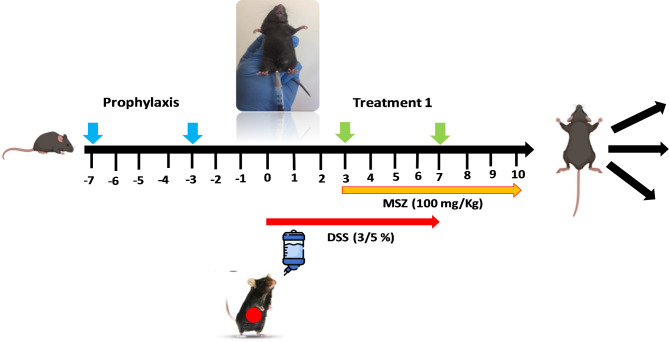
Thirty mice were randomly divided into five groups, with six mice in each group. The groups were labeled as follows: control (Normal), Colitis (DSS induction only, without therapeutic intervention), Prophylaxis (Dicrocoelium eggs + DSS), Treatment (DSS + Dicrocoelium eggs), and Standard Treatment (DSS + Mesalazine). In the control group, mice received normal water for 10 days, while in the other four groups, colitis-like symptoms were induced by adding 3.5% (W/V) DSS (dextran sulfate sodium, molecular weight 40-k-Da, CAS No. 9011-18-1) to the drinking water for 7days, followed by normal drinking water from days 7 to 10.
In the Prophylaxis group, 10,000 Dicrocoelium eggs were injected intraperitoneally (IP) on days 7 and 3 before DSS induction.
In the Standard Treatment (ST) group, treatment with the common therapeutic drug Mesalazine (100 mg/kg/day, administered via oral gavage) began 3 days after DSS induction, while the Colitis group remained untreated. A schematic diagram of the experimental colitis treatment protocol is shown in Fig. 1.
Fig. 1.
Schematic diagram of the study design.
From the time of DSS induction, mice were monitored daily for weight loss, rectal prolapse, stool consistency, and rectal bleeding, with data recorded accordingly. Inflammation was assessed using the disease activity index (DAI) for IBD, which includes the four variables described in Table 124.
Table 1.
Disease Activity Index (DAI) scoring system.
| Score | Rectal bleeding | Stool consistency | Rectal prolapse | Lose weight (%) |
|---|---|---|---|---|
| 0 | None | Normal | None | < 5 |
| 1 | Red | Soft | Sign of prolapse | 5–10 |
| 2 | Dark red | Very soft | Clear prolapse | 10–15 |
| 3 | Gross bleeding | Diarrhea | Extensive prolapse | > 15 |
On the 10th day, observing clinical signs, all mice were anesthetized by intraperitoneal injection of a 10% ketamine and 2% xylazine mixture into the right lower quadrant of the abdomen using an insulin syringe. The mice were then sacrificed via cervical dislocation. Following sacrifice, the colon and spleen were collected, weighed, and their lengths recorded. Colonic tissues were placed in 10% formalin or preserved in liquid nitrogen for further analysis.
Macroscopic and histopathological analysis
Histological investigation was conducted out on the colon tissue of five groups of mice (Control, Colitis, Prophylaxis, Treatment, MSZ; n = 6 per group). Spleen weight (measured in gram) and colons length (measured in centimeters) were recorded. The entire colon was removed, separated from the cecum, and washed with cold phosphate-buffered saline (PBS) to remove blood and feces. A small portion of the distal colon were cut and immediately placed in 10% buffered formalin for histological analysis. Colon sections were stained using the hematoxylin and eosin technique by histopathologists. Tissue changes, including the severity of inflammation, mucosal damage, and loss of crypts, were scored under light microscopy based on the criteria described in Table 224.
Table 2.
Histopathological standards used for determine the severity colon tissue lesion.
| Score | 0 | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Inflammation | None | Mild | Moderate | Sever | |
| Mucosal damage | None | Mucus layer | Submucosa | Muscular and serosa | |
| Crypt loss | None | 1/3 | 2/3 | %100 + intactepithelium | 100% with epithelium lose |
| Pathological change range | None | 1–25% | 26–50% | 51–75% | 76–100% |
Quantitative real-time PCR
Total RNA was extracted from colon tissues using a mini kit (Parstous Biotechnology), and the quality and quantity of RNA were assessed by measuring the absorbance at 260 and 280 nm using a Nanodrop system. cDNA was synthesized from 1 µg of total RNA for each sample using the Easy cDNA synthesis kit (Yekta Tajhiz Azma, Iran). The mRNA levels of IFN-γ, IL-4, and GAPDH were analyzed by qPCR using SYBER Green PCR Master Mix without Rox (Ampliqon, Batch number: 22H2901) and specific primer pairs. GAPDH was used as a reference gene for normalization. All samples were run in duplicate, with each reaction containing 10 µL of 2x Master Mix, 0.5 µL of each specific primer (10 pmol/ µL), and 1 µL (5 ngr) of cDNA, for a total volume of 20 µL. The reaction conditions were: 15 min at 95 °C, followed by 40 cycles of 20 s at 95 °C and 1 min at 58 °C.
The primers sequence used in PCR reactions were as follows: IFN-γ (F, 5′-CCAAGTTT GAGGTCAACA-3′; R, 5′-CTGGCAGAATTATTCTTATTGG-3′), IL-4 (F, 5′-CTGGATTCATCGATAAGC-3′; R, 5′-GATGCTCTTTAGGCTTTC-3′) and GAPDH (F, 5′-CAA CGA CCC CTT CAT TGA CC-3′; R, 5′-CTT CCC ATT CTC GCC TTG A -3′). Melting curve analysis confirmed the specificity of the PCR products, showing consistent single melting peaks for all primer pairs used. Gene expression levels were normalized to GAPDH expression in the same group, and relative expression differences were calculated using the comparative Ct (2−∆∆Ct) method.
ELISA assessment
The tissue levels of IL-4 and IFN-γ were investigated in the colon tissue of mice. Tissue samples were homogenized in sterile PBS following the manufacturer’s instructions. After homogenization, protein concentration was determined using the BCA method. Samples with a concentration of 2500 µg/ml were diluted with PBS, and the tissue levels of IL-4 and IFN-γ were measured using ELISA kits (Zell Bio GmbH, Catalog No. RK00019 and RK00036) according to the provided instructions.
Statistical analysis
All statistical analyses were performed using a one-way ANOVA test with GraphPad Prism v8 software. Data are reported as means ± standard error of the mean (SEM). A p-value less than 0.05 (P < 0.05) was considered statistically significant.
Results
Dicrocoelium dendriticum egg were found to alleviate the clinical symptoms associated with ulcerative colitis in the mouse model
During the 7-days DSS induction period, with daily weight monitoring, distinct weight loss patterns were observed among the groups. The prophylaxis and treatment groups experienced significantly less weight loss compared to the colitis group. Additionally, mice in the prophylaxis and treatment groups exhibited a significantly lower percentage of body weight loss compared to the colitis group. Moreover, these groups showed a faster recovery in body weight than the colitis group. The apparent improvement in body weight in the colitis group from days 7 to 10 was attributed to the switch to normal drinking water instead of DSS (Fig. 2A).
Fig. 2.
Immunization with Dicrocoelium dendriticum eggs attenuated clinical signs of DSS-induced colitis in C57BL/6 mice. (A) Weight change in percent from day 0 during experimental study, (B) The score of disease activity index and (C) The highest changes of Disease activity index, valuated in each groups. Data are presented as the mean ± SEM. (***P < 0.001, **P < 0.01, * P < 0.05 compared to colitis group, ## P < 0.01 # P < 0.05 compared to MSZ group).
Symptoms such as rectal bleeding, stool consistency, and rectal prolapse were assessed and scored across the groups. Results indicated a significant reduction in symptoms in the prophylaxis and treatment groups compared to the colitis group. Mesalazine treatment also reduced symptoms in the MSZ group, though no significant difference was observed compared to the colitis group. These finding highlight the effectiveness of Dicrocoelium eggs in alleviating clinical symptoms in DSS-induced colitis among the mouse groups (Fig. 2B, C).
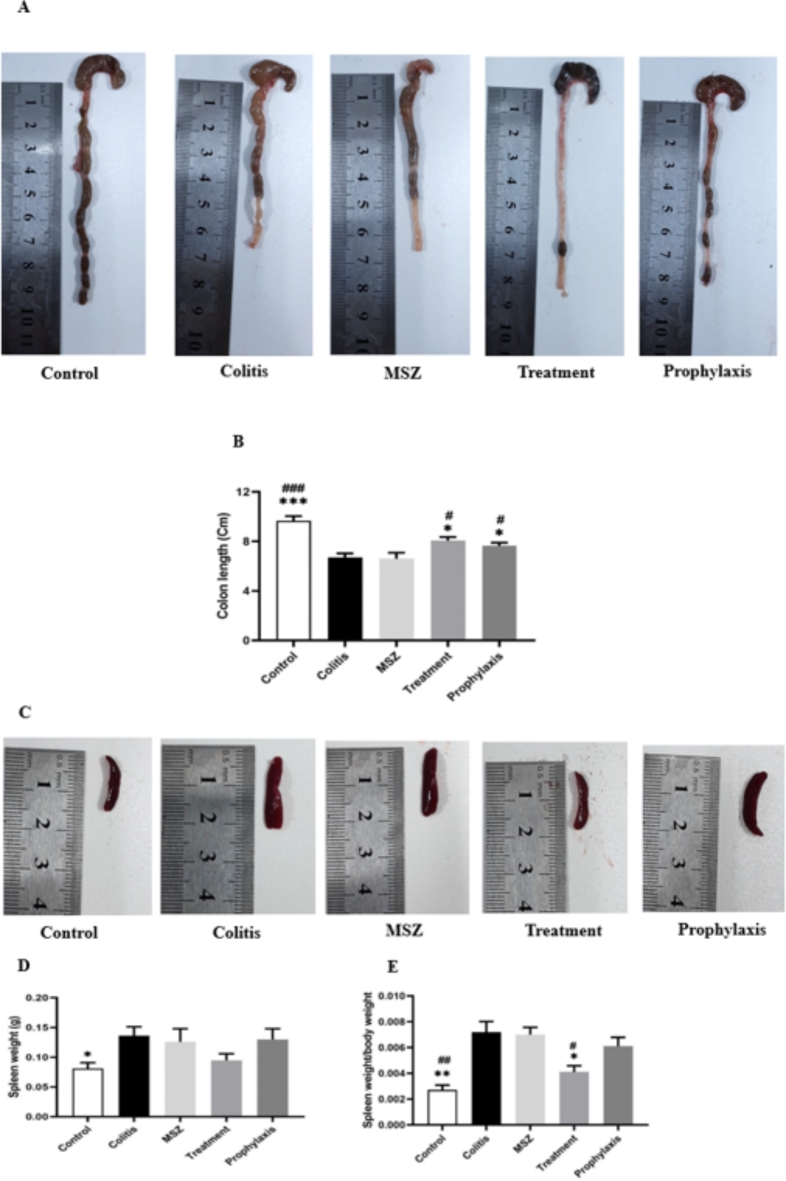
To evaluate the impact of Dicrocoelium eggs on colon size, the colon length was measured and compared across all five groups, considering the inverse relationship between colon length and disease severity. Result from Fig. 3A and B revealed a significant improvement in colon length in the treatment and prophylaxis groups compared to the colitis and MSZ groups.
Fig. 3.
Mice in each group were euthanized and dissected to remove colons and spleens. (A) Macroscopic view of colons. (B) Comparison of colon length in different groups. (C) Macroscopic view of spleen; representative samples from each group. (D) Comparison of spleen weight. (E) Evaluation of spleen weight relative to body weight in different groups. Data are presented as the mean ± SEM. (***P < 0.001, **P < 0.01, * P < 0.05 compared to colitis group, ## P < 0.01 # P < 0.05 compared to MSZ group).
A reduction in spleen weight was observed, with lower scores noted in the treatment group. However, the inhibitory effect of Mesalazine on spleen enlargement was less pronounced than that of Dicrocoelium eggs, with no significant difference compared to the colitis group (Fig. 3C, E). These macroscopic results confirm that treatment with Dicrocoelium eggs significantly ameliorates the clinical manifestations of DSS-induced colitis.
3.2 Dicrocoelium dendriticum egg reduces inflammation in vivo
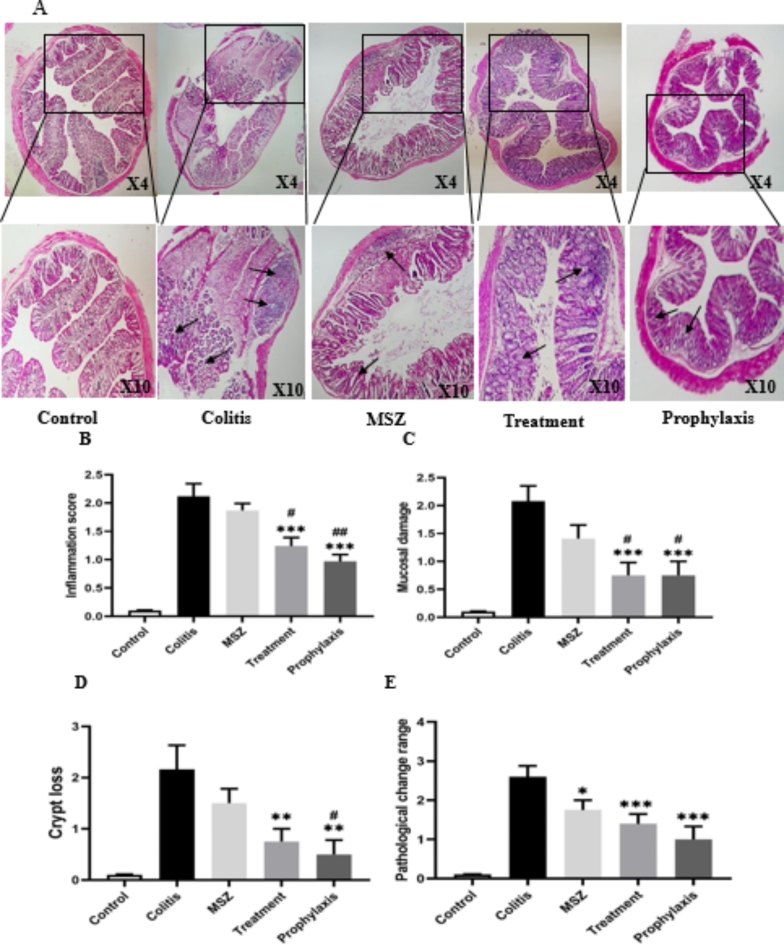
Hematoxylin-eosin staining was performed to investigate the therapeutic effect of Dicrocoelium dendriticum eggs on inflammation and tissue damage resulting from DSS-induced colitis. In the cross-sectional assessment of the colon, healthy epithelium with normal thickness was observed in the control group (Fig. 4A). In contrast, a significant increase in inflammation was observed in the colon samples of DSS-treated mice compared to the control group (Fig. 4B). Histopathological sings, such as inflammation intensity (Fig. 4B), mucosal damage (Fig. 4C), crypt loss (Fig. 4D), and pathological change (Fig. 4E), were significantly reduced in treatment and prophylaxis groups compared to the colitis and MSZ groups. Although histological changes in the large intestinal were alleviated following MSZ administration, no significant difference was observed compared to the colitis group.
Fig. 4.
Histopathology of colon tissue. (A) Representative histopathology images of colon with Hematoxylin and Eosin staining (H&E), (B) inflammation score, (C) mucosal damage, (D) crypt loss, and (E) pathological change range. Black arrows indicate crypt loss and inflammation. Data are presented as the mean ± SEM. (***P < 0.001, **P < 0.01, * P < 0.05 compared to colitis group, ## P < 0.01 # P < 0.05 compared to MSZ group).
Measurement of IL-4 and IFN-γ expression by real-time PCR technique
To investigate the effect of Dicrocoelium eggs on inflammatory and anti-inflammatory response, the expression of IFN-γ and IL-4 genes was measured using real-time quantitative PCR (RT-PCR). The result showed that the mRNA expression of IFN-γ in mice with DSS-induced colitis (colitis group) was significantly higher than in the other groups. The level of IFN-γ expression decreased in the prophylaxis and treatment groups compared to the colitis group, but, this decrease was significant only in the prophylaxis group (Fig. 5A).
Fig. 5.

Effect of Dicrocoelium dendriticum eggs colonization on IFN-γ (A) and IL-4 (B) level in tissue colon between different studied groups (**P < 0.01, *P < 0.05 compared to Colitis group, # P < 0.05 compared to MSZ group, + P < 0.05 compared to Prophylaxis group).
Compared to the colitis and MSZ groups, the administration of Dicrocoelium eggs in both the prophylaxis and treatment groups resulted in an increase in IL-4 expression. However, the treatment group showed a significantly higher elevation compared to the prophylaxis group (Fig. 5B). Gene expression levels were measured relative to the housekeeping gene GAPDH.
Compared to the colitis and MSZ groups, the administration of Dicrocoelium eggs in both the prophylaxis and treatment groups resulted in an increase in the IL-4. Expression. However, the treatment group showed a significantly higher elevation compared to the prophylaxis group (Fig. 5B). Gene expression levels were measured relative to the housekeeping gene, GAPDH.
Measurement of IL-4 and IFN-γ expression by the ELISA technique
Cytokine production in the cell supernatants of colon tissue was quantified using the ELISA technique. Lower levels of IFN-γ were observed in the cell supernatants of mice treated with Dicrocoelium eggs (prophylaxis and treatment groups) compared to the colitis group. Cells from the prophylaxis group exhibited lower IFN-γ secretion compared to the treatment group (Fig. 6A). Additionally, the expression of IL-4 was increased in egg-treated mice compared to the colitis group. Although the expression level of this anti-inflammatory marker was higher in the treatment group, the difference was not statistically significance (Fig. 6B).
Fig. 6.
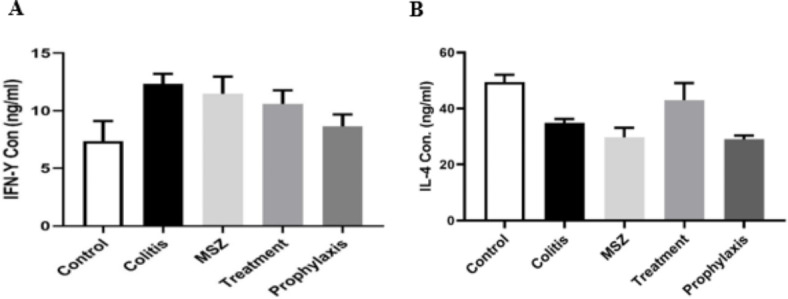
Cytokine detection. IFN-γ (A) and IL-4 (B) concentration in colon tissue homogenate supernatant were quantified using an ELISA assay. Data is represented as the mean ± SEM; not significantly different between the groups.
Discussion
The incidence of inflammatory bowel diseases (IBDs) has dramatically increased in the last two decades worldwide. Currently, the medical management of IBDs and other inflammatory diseases heavily relies on steroids and broad-spectrum immunosuppressive drugs, which, over the long term, cause severe side effects and impose substantial financial burdens25.
The idea of using helminths as a therapeutic strategy for IBD originated from epidemiological studies supporting the “hygiene hypothesis”. These studies revealed an inverse correlation between helminth infections and autoimmune disorders26. This inverse relationship may be significant because helminths induced a robust regulatory system via a Th2 response or a more generalized immune regulation or suppression25. Elliott et al. were pioneers in proposing that parasitic worms could alleviate colitis in mouse model27. Subsequently, research has focused on exploring the preventive and therapeutic effects of various helminth species on experimental colitis28,29. Substantial evidence shows that immunotherapy involving nematodes, trematodes, or cestodes can prevent the onset of IBD and reverse established disease in animal models.
In this study, we provide the first evidence indicating that Dicrocoelium dendriticum eggs have a beneficial effect on experimental colitis. The results demonstrate a significant decrease in the severity of clinical symptoms, disease activity index (DAI), and histological scores in the groups treated with Dicrocoelium dendriticum eggs. These protective effects can be attributed to the reduction of histological changes and the adjustment of the Th1/Th2 balance in the colon tissue.
Many factors influenced our choice of helminth. First, dicrocoelium dendriticum has a high prevalence among Iranian livestock and is easy to collect22. Second, due to its unique life cycle, this helminth is safe for humans, as it cannot establish infection through its eggs30. Third, given the ethical limitations and risks of helminthic infections in clinical trials31, Dicrocoelium dendriticum is to be considered a novel anti-colitic therapy, it can also be readily eradicated by administration of praziquantel at a patient’s request.
Here, we present data from a DSS model as proof-of-concept evidence supporting the anti-colitic effects of Dicrocoelium dendriticum eggs. DSS-induced colitis is a well-established model that mimics various symptoms of human ulcerative colitis14. In our experiments, DSS caused epithelial damage, diarrhea with rectal bleeding, and body weight loss in murine models32. Rectal bleeding and body weight loss were associated with spleen enlargement and colon shortening in the DSS-induced colitis group. The data show that body weight loss, spleen enlargment, and colon shortening due to DSS-induced colitis were improved by immunization with Dicrocoelium dendriticum eggs. Additionally, the DAI, macroscopic score, and colon pathology in the two therapeutic groups treated with worm eggs were significantly lower than in the colitis group. Histopathological analysis using H&E staining revealed mucosal damage, crypt loss, and inflammatory cell infiltration in the colitis group. In contrast, infection with Dicrocoelium eggs significantly improved histological scores in both the prophylaxis and treatment groups (Figs. 2 and 3). Similar results were observed in studies by Cancado et al.33, Yang et al.34, Khatri et al.35, and Cronado et al.36 which demonstrated that treatment with excreted/secreted (ES) antigens from Ancylostoma ceylanicum, Trichinella spiralis, or recombinant cystatin protein from Brugia malayi and Ascaris lumbricoides ameliorated DSS-induced colitis in mice.
Another goal of this study was to assess immunological changes in colonic tissue, focusing on CD4+ T cells. Th0 CD4+ T cells can differentiate into Th1 and Th2 cells37. CD4+ T cells, in the presence of IFN-γ and IL-2, differentiate into Th1 cells, which mediate cell immunity38. Conversely, in the presence of IL-4, CD4+ T cells differentiate into Th2 cells, which secrete IL-4, IL-5, IL-13, and IL-10 to activate B cells and mediate humoral immunity39. Recent studies suggest that colitis inflammation may result from an imbalance between Th1 and Th2 cytokines40. The treatment of ulcerative colitis with worm infections aims to balance Th1 and Th2 immune responses. Thus, IFN-γ and IL-4 were chosen as representatives of Th1 and Th2 cytokines, respectively.
IFN-γ is an inflammatory mediator that play a key role in the development of IBD41. Numreous studies have confirmed its role in causing intestinal inflammation in both human IBD42, and DSS-induced colitis in murine models25,43. In contrast, IL-4 has immunoregulatory effects, inhibiting proinflammatory mediatores such as IFN-γ and TNF-α, and limiting disease progression44. For instance, Gautam et al.45 showed that treatment of mice with monoclonal anti-IL-4 antibody nullified the protective effect of egg exposure in a TNBS challenge. Similarly, studies by studies by Takenaka et al. and Gautam et al. demonstrate that IL-4 inhinbits TNF-α, IP-10, and IL-12 production by macrophage45,46. Brunet et al.47 also showed that IL-4 knockout (KO) mice could not survive Schistosoma infection due to overproduction of inflammatory mediators like TNF-α and nitric oxide (NO).
In this study, we measured IFN-γ and IL-4 mRNA expression in colonic tissue. IFN-γ levels were significantly higher in the colitis group, while exposure to Dicrocoelium eggs significantly reduced IFN-γ expression and increased IL-4 production in both prophylaxis and treatment groups (Fig. 4). We also observed reduced IFN-γ and increased IL-4 levels in colon tissue from Dicrocoelium- treated mice (prophylaxis and treatment groups), though the difference did not reach statistical significance compared to the colitis group (Fig. 5). This data suggests that DSS-induced colitis leads to Th1-driven inflammation, with increased IFN-γ expression. Our result also highlight the important role of Th2 cytokine IL-4 in helminth protection against DSS-induced colitis, demonstrating the negative feedback of IL-4 on IFN-γ.
Studies suggest that the role of T-helper cells during acute and chronic DSS-induced colitis may differ. While acute DSS colitis is Th1-dependent48, chronic DSS-induced colitis appears to involve a Th2 response, which exacerbate disease. Our results, however, indicate that Th2 cells have anti-inflammatory properties that suppress Th1 cytokine production. This is consistent with studies showing that during chronic inflammation, the immune system initiates an anti-inflammatory state for self-healing. Hasby et al.25 reported that in chronic colitis, increased Th2 cytokines IL-4 expression correlated with DSS-induced colitis severity. Similarly, Rodrigues et al.49 reported a mixed Th1/Th2 immune response in DSS-treated mice. Our study yielded different results because Dicrocoelium eggs reduce DSS-induced acute colitis by inducing a Th2 profile. Although the Th1/Th2 balance has been proposed to explain how helminth therapy prevents experimental colitis, recent studies indicate that the Th1/Th17 balance may play a more significant role in autoimmune disease regulation, particularly in IBD. Briefly, cross-immunomodulation of Th1/Th2 in helminthic therapy remains controversial50.
Smith et al.51 and Bodammer et al.52 reported the Th2 response induced by Schistosoma mansoni eggs did not reduce DSS colitis severity. Yang et al.34 showed that Trichinella spiralis ameliorated DSS-induced colitis by increasing IL-10 mRNA expression in T cells isolated from colonic tissues. Ruyssers et al.53, Hasby et al.25, and Watanabe et al.54, also reported the immunomodulatory effect of IL-10 in suppressing Th1/Th17 responses in Schistosoma mansoni-infected mice. However, studies by Wolff et al.55 and Moreno et al.56 demonstrated that type-2 immune response alter the mucosal microbiota and play a crucial role in improving IBD condition. Other studies, showed that helminth infections reduced inflammation in DSS-treated mice without involving IL-1033,57. Smith et al.51 concluded that protection from DSS-induced colitis in Schistosoma-infected mice was not dependent on a regulatory type-2 response but rather on macrophages. Several studies have reported that helminths induce M2-type macrophage polarization, promoting anti-inflammatory modulators such as IL-10 and accelerating healing58. NF-κB is closely associated with IBD and is persistently activated in macrophages and other inflammatory cells in colitis patients59. Oh et al.60 demonstrated that NF-κB is crucial for T-helper cell differentiation and the production of inflammatory cytokines like IL-2. Our data suggest that helminth eggs or extracts may exert anti-inflammatory effects through above signaling pathway. For instance, Jinngyun et al.61 reported that the production of IL-4 in mice treated with Trichinella spiralis can reduce the expression of NF-κB, and the secretion of IFN-γ, to stabilize intestinal homeostasis.
Furthermore, it has been reported that the activation of NF-κB leads to inflammation and apoptosis of colonic epithelial cells62. In line with this, Liu et al.63 tested the expression of TUNEL, C-C3, BAX, and Bcl-2 to assess apoptosis in the colonic tissues of treated mice. Their results showed the highest expression level of TUNEL, C-C3, and BAX and lowest Bcl-2 levels in DSS groups. In contrast, the lowest BAX levels and the highest Bcl-2 levels were observed in mice infected with Schistosoma japonicum. Thus, the decreased apoptosis in colonic tissues may be related to diminished NF-κB activation. Additionally, ulcerative colitis is associated with the TLR4 /MYD88 signaling pathway64. In this regard, the findings by Zhao et al.65 showed that the type-2 immune response induced by Schistosoma japonicum eggs could alleviate TNBS-induced colitis in mice by regulating TLR4 expression and reducing IFN-γ and TNF-α levels. Moreover, several immune pathways have demonstrated that helminths and proteins released by helminths stimulate the expression of TLR2 in mice, and the ligands recognized by TLR2 activate Th2 or Treg responses66,67.
On the other hand, several studies have demonstrated a positive correlation between the severity colitis in human IBD or in DSS-induced colitis and the infiltration of neutrophils and eosinophils68. Additionally, the result of the study by Rodrigues et al. could explain the modulation of inflammatory cytokines in mice infected with Strongyloides venezuelensis during DSS-induced colitis, highlighting the reduced granulocyte infiltration, decreased levels of CCL11—a chemokine involved in eosinophil engagement—as well as lower levels of EPO and MPO49. The eggs of Dicrocoelium may play a role in reducing the production of IFN-γ and inducing the expression of IL-4 by regulating or stimulating M2 macrophages and signaling pathways, as well as decreasing inflammatory markers. However, our present data are not sufficient to confirm the role of Dicrocoelium eggs in these cases. Therefore, further analysis will be required.
In conclusion, due to the differences in cytokines functions, it is difficult to obtain a definite conclusion and interpretation of the condition but, our findings suggest that DSS can disrupt the balance of Th1/Th2 expression in the colon. Dicrocoelium eggs can ameliorate DSS-induced colitis in mice by down-regulate IFN-γ and up-regulate IL-4 levels in the colonic tissue to modulate the Th1/Th2 balance and enhance immunity. The ability of Dicrocoelium eggs to inhibit inflammation suggests their therapeutic potential in reducing the risk of developing colitis. Therefore, it could be considered as a novel biological strategy for ameliorating inflammation in autoimmune disease.
Acknowledgements
This work was part of Leila Mighani’s master’s thesis and was financially supported by Mashhad University of Medical Sciences. We extend our sincere gratitude to the Research Office of the Faculty of Medicine for providing the project grant (4001761).
Author contributions
L.M.: Investigation, writing original draft, writingMaliheh Eilakinezhad: Investigation. S.-A.E.: Advisor, Formal analysis, Conceptualization. M.K.: Advisor, methodology. M.E.: Methodology, collecting data, Software. S.E.N.: Methodology, collecting data, software. M.M.B.: Data Analysis. K.K.: collecting data. E.M.: Conceptualization, Methodology, Resources, Supervision, Funding acquisition, Reviewing and Editing. M.Z.: Conceptualization, Methodology, Resources, Supervision, Funding acquisition, Reviewing and Editing.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Leila Mighani and Malihe Eilakinezhad.
Contributor Information
Elham Moghaddas, Email: MoghaddasE@mums.ac.ir.
Mehdi Zarean, Email: ZareanM@mums.ac.ir.
References
- 1.Aldars-García, L., Marin, A. C., Chaparro, M. & Gisbert, J. P. The interplay between immune system and microbiota in inflammatory bowel disease: A narrative review. Int. J. Mol. Sci.22(6) (2021). [DOI] [PMC free article] [PubMed]
- 2.Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet390(10114), 2769–2778 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Sýkora, J. et al. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J. Gastroenterol.24(25), 2741–2763 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long, S. R., Liu, R. D., Kumar, D. V., Wang, Z. Q. & Su, C. W. Immune protection of a helminth protein in the DSS-induced colitis model in mice. Front. Immunol.12, 664998 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taghipour, N. et al. Immunomodulatory effect of Syphacia obvelata in treatment of experimental DSS-induced colitis in mouse model. Sci. Rep.9(1), 19127 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Y. F. et al. Inonotus obliquus polysaccharide ameliorates dextran sulphate sodium induced colitis involving modulation of Th1/Th2 and Th17/Treg balance. Artif. Cells Nanomed. Biotechnol.47(1), 757–766 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Hirahara, K. & Nakayama, T. CD4+ T-cell subsets in inflammatory diseases: Beyond the Th1/Th2 paradigm. Int. Immunol.28(4), 163–171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi, W. et al. Helminth therapy for immune-mediated inflammatory diseases: Current and future perspectives. J. Inflamm. Res.15, 475–491 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai, T. & Lopes, F. Potential of human helminth therapy for resolution of inflammatory bowel disease: The future ahead. Exp. Parasitol.232, 108189 (2022). [DOI] [PubMed] [Google Scholar]
- 10.Lee, H. S., Park, S. K. & Park, D. I. Novel treatments for inflammatory bowel disease. Korean J. Intern. Med.33(1), 20–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matisz, C. E. et al. Suppression of colitis by adoptive transfer of helminth antigen-treated dendritic cells requires interleukin-4 receptor-α signaling. Sci. Rep.7, 40631 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heylen, M. et al. Treatment with egg antigens of Schistosoma mansoni ameliorates experimental colitis in mice through a colonic T-cell-dependent mechanism. Inflamm. Bowel Dis.21(1), 48–59 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Navi, Z. et al. Dicrocoelium ova can block the induction phase of experimental autoimmune encephalomyelitis. Parasite Immunol.42(12), e12792 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Chassaing, B., Aitken, J. D., Malleshappa, M. & Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. ;104:15.25.1-15.25.14. (2014). [DOI] [PMC free article] [PubMed]
- 15.Broadhurst, M. J. et al. IL-22 + CD4+ T cells are associated with therapeutic trichuris trichiura infection in an ulcerative colitis patient. Sci. Transl. Med.2(60), 60ra88 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Hou, X. et al. Protective effect of Schistosoma japonicum eggs on TNBS-induced colitis is associated with regulating Treg/Th17 balance and reprogramming glycolipid metabolism in mice. Front. Cell. Infect. Microbiol.12, 1028899 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croese, J. et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut55(1), 136–137 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reardon, C., Sanchez, A., Hogaboam, C. M. & McKay, D. M. Tapeworm infection reduces epithelial ion transport abnormalities in murine dextran sulfate sodium-induced colitis. Infect. Immun.69(7), 4417–4423 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum, A. M. et al. Heligmosomoides polygyrus bakeri induces tolerogenic dendritic cells that block colitis and prevent antigen-specific gut T cell responses. J. Immunol.189(5), 2512–2520 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taghipour, N. et al. Syphacia obvelata: A new Hope to induction of intestinal immunological tolerance in C57BL/6 mice. Korean J. Parasitol.55(4), 439–444 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malekzadeh, M. M. et al. Iranian Registry of Crohn’s and colitis: Study profile of first nation-wide inflammatory bowel disease registry in Middle East. Intest. Res.17(3), 330–339 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbabi, M., Dalimi, A., Ghafarifar, F. & Moghadam, M. F. Prevalence and intensity of Dicrocoelium dendriticum in sheep and goats of Iran. Res. J. Parasitol.6(5), 160–167 (2011). [Google Scholar]
- 23.Altun, S. K., Barlik, F., Aydemir, M. E. & Alkan, S. A bibliometric analysis on Dicrocoelium dendriticum. Iran. J. Parasitol.18(2), 193–201 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eskandari, M. et al. Mebendazole, an anti-helminth drug, suppresses inflammation, oxidative stress and injury in a mouse model of ulcerative colitis. Sci. Rep.12(1), 10249 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasby, E. A., Hasby Saad, M. A., Shohieb, Z. & El Noby, K. FoxP3+ T regulatory cells and immunomodulation after Schistosoma mansoni egg antigen immunization in experimental model of inflammatory bowel disease. Cell. Immunol.295(1), 67–76 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Mules, T. C. et al. Controlled hookworm infection for medication-free maintenance in patients with ulcerative colitis: A pilot, double-blind, randomized control trial. Inflamm. Bowel Dis. (2023). [DOI] [PMC free article] [PubMed]
- 27.Elliott, D. E., Urban, J. J., Argo, C. K. & Weinstock, J. V. Does the failure to acquire helminthic parasites predispose to Crohn’s disease? Faseb j.14(12), 1848–1855 (2000). [DOI] [PubMed] [Google Scholar]
- 28.Khan, W. I. et al. Intestinal nematode infection ameliorates experimental colitis in mice. Infect. Immun.70(11), 5931–5937 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elliott, D. E. et al. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol.284(3), G385–G391 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Olsen, O. W. Animal Parasites: Their life Cycles and Ecology (Courier Corporation, 1986).
- 31.Edwards, L. J. & Constantinescu, C. S. Parasite immunomodulation in autoimmune disease: Focus on multiple sclerosis. Expert Rev. Clin. Immunol.5(5), 487–489 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Okayasu, I. et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology98(3), 694–702 (1990). [DOI] [PubMed] [Google Scholar]
- 33.Cançado, G. G. et al. Hookworm products ameliorate dextran sodium sulfate-induced colitis in BALB/c mice. Inflamm. Bowel Dis.17(11), 2275–2286 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Yang, X. et al. Excretory/secretory products from Trichinella Spiralis adult worms ameliorate DSS-induced colitis in mice. PLoS One9(5), e96454 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khatri, V., Amdare, N., Tarnekar, A., Goswami, K. & Reddy, M. V. Brugia malayi cystatin therapeutically ameliorates dextran sulfate sodium-induced colitis in mice. J. Dig. Dis.16(10), 585–594 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Coronado, S. et al. A recombinant cystatin from Ascaris lumbricoides attenuates inflammation of DSS-induced colitis. Parasite Immunol.39(4). (2017). [DOI] [PubMed]
- 37.Nemoto, Y. & Watanabe, M. The Th1, Th2, and Th17 paradigm in inflammatory bowel disease. Crohn’s Disease and ulcerative colitis: From epidemiology and immunobiology to a rational diagnostic and therapeutic approach. 183–94 (2012).
- 38.Workman, A. M., Jacobs, A. K., Vogel, A. J., Condon, S. & Brown, D. M. Inflammation enhances IL-2 driven differentiation of cytolytic CD4 T cells. PLoS One9(2), e89010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, Y., Zhang, Y., Gu, W., He, L. & Sun, B. Th1/Th2 cell’s function in immune system. Adv. Exp. Med. Biol.841, 45–65 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Tang, F., Wang, F., An, L. & Wang, X. Upregulation of Tim-3 on CD4(+) T cells is associated with Th1/Th2 imbalance in patients with allergic asthma. Int. J. Clin. Exp. Med.8(3), 3809–3816 (2015). [PMC free article] [PubMed] [Google Scholar]
- 41.Garside, P. Cytokines in experimental colitis. Clin. Exp. Immunol.118(3), 337–339 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh, U. P. et al. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine77, 44–49 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MacDonald, T. T. Effector and regulatory lymphoid cells and cytokines in mucosal sites. Curr. Top. Microbiol. Immunol.236, 113–135 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Műzes, G., Molnár, B., Tulassay, Z. & Sipos, F. Changes of the cytokine profile in inflammatory bowel diseases. World J. Gastroenterol.18(41), 5848–5861 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gautam, S., Tebo, J. M. & Hamilton, T. A. IL-4 suppresses cytokine gene expression induced by IFN-gamma and/or IL-2 in murine peritoneal macrophages. J. Immunol.148(6), 1725–1730 (1992). [PubMed] [Google Scholar]
- 46.Takenaka, H. et al. Regulation of T cell-dependent and -independent IL-12 production by the three Th2-type cytokines IL-10, IL-6, and IL-4. J. Leukoc. Biol.61(1), 80–87 (1997). [DOI] [PubMed] [Google Scholar]
- 47.Brunet, L. R., Finkelman, F. D., Cheever, A. W., Kopf, M. A. & Pearce, E. J. IL-4 protects against TNF-alpha-mediated cachexia and death during acute schistosomiasis. J. Immunol.159(2), 777–85 (1950). [PubMed]
- 48.Smallwood, T. B. et al. Helminth immunomodulation in autoimmune disease. Front. Immunol.8, 453 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodrigues, V. F. et al. Acute infection with Strongyloides venezuelensis increases intestine production IL-10, reduces Th1/Th2/Th17 induction in colon and attenuates Dextran Sulfate Sodium-induced colitis in BALB/c mice. Cytokine111, 72–83 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Britton, G. J. et al. Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt(+) regulatory T cells and exacerbate colitis in mice. Immunity50(1), 212–24e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith, P. et al. Infection with a helminth parasite prevents experimental colitis via a macrophage-mediated mechanism. J. Immunol.178(7), 4557–4566 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Bodammer, P. et al. Schistosoma mansoni infection but not egg antigen promotes recovery from colitis in outbred NMRI mice. Dig. Dis. Sci.56(1), 70–78 (2011). [DOI] [PubMed] [Google Scholar]
- 53.Ruyssers, N. E. et al. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm. Bowel Dis.15(4), 491–500 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Watanabe, K. et al. T regulatory cell levels decrease in people infected with Schistosoma mansoni on effective treatment. Am. J. Trop. Med. Hyg.77(4), 676–682 (2007). [PMC free article] [PubMed] [Google Scholar]
- 55.Wolff, M. J., Broadhurst, M. J. & Loke, P. Helminthic therapy: Improving mucosal barrier function. Trends Parasitol.28(5), 187–194 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moreno, N. F. et al. 444 Deciphering the role of IL-4 in post-colitis repair. J. Clin. Transl. Sci.8(s1), 132 (2024). [Google Scholar]
- 57.Elliott, D. E. et al. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur. J. Immunol.34(10), 2690–2698 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Borchard, J. L., Conrad, N. L., Pinto, N. B., Moura M.Q., Berne, M. E. A. & Leite, F. P. L. Acute and chronic immunomodulatory response mechanisms against Toxocara canis larvae infection in mice. Rev. Bras. Parasitol. Vet.31(4), e012522 (2022). [Google Scholar]
- 59.Wei, J. & Feng, J. Signaling pathways associated with inflammatory bowel disease. Recent Pat. Inflamm. Allergy Drug Discov.4(2), 105–117 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Oh, H. & Ghosh, S. NF-κB: Roles and regulation in different CD4(+) T-cell subsets. Immunol. Rev.252(1), 41–51 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu, J., Yu, P., Wu, L., Liu, M. & Lu, Y. Effect of Trichinella spiralis intervention on TNBS-induced experimental colitis in mice. Immunobiology224(1), 147–153 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Chen, G. et al. Maternal diabetes modulates offspring cell proliferation and apoptosis during odontogenesis via the TLR4/NF-κB signalling pathway. Cell. Prolif.50(3). (2017). [DOI] [PMC free article] [PubMed]
- 63.Liu, Y. et al. Schistosoma japonicum attenuates dextran sodium sulfate-induced colitis in mice via reduction of endoplasmic reticulum stress. World J. Gastroenterol.23(31), 5700–5712 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bing, X., Xuelei, L., Wanwei, D., Linlang, L. & Keyan, C. EGCG maintains Th1/Th2 balance and mitigates ulcerative colitis induced by dextran sulfate sodium through TLR4/MyD88/NF-κB signaling pathway in rats. Can. J. Gastroenterol. Hepatol.2017, 3057268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao, Y., Zhang, S., Jiang, L., Jiang, J. & Liu, H. Preventive effects of Schistosoma japonicum ova on trinitrobenzenesulfonic acid-induced colitis and bacterial translocation in mice. J. Gastroenterol. Hepatol.24(11), 1775–1780 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Wang, X. et al. CD4+CD25+ Treg induction by an HSP60-derived peptide SJMHE1 from Schistosoma japonicum is TLR2 dependent. Eur. J. Immunol.39(11), 3052–3065 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Dillon, S. et al. A toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J. Immunol.172(8), 4733–4743 (2004). [DOI] [PubMed] [Google Scholar]
- 68.Carlson, M., Raab, Y., Peterson, C., Hällgren, R. & Venge, P. Increased intraluminal release of eosinophil granule proteins EPO, ECP, EPX, and cytokines in ulcerative colitis and proctitis in segmental perfusion. Am. J. Gastroenterol.94(7), 1876–1883 (1999). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.