Abstract
We have generated a cell line (F cells) producing a secreted form of Japanese encephalitis virus (JEV) subviral particle (extracellular particles [EPs]) that contains the JEV envelope glycoprotein (E) and a precursor (prM) of the virion membrane protein (M). The F cells were engineered to synthesize these JEV products from a cDNA encoding a mutated (furin proteinase resistant) form of prM, since stable cell lines expressing E and the authentic form of prM could not be obtained, due (in part) to the cell-fusing ability of EPs containing E and M. Our biochemical alteration of the prM protein was critical for the successful production of EP-producing cell lines. EPs produced by F cells share the biochemical properties of empty viral particles produced by JEV-infected cells, except that the F-cell EPs lack hemagglutinating activity and M. F-cell EPs were recognized by a panel of monoclonal antibodies to E, and EPs were shown to be useful as vaccine candidates in mice and as diagnostic reagents in evaluating human immune responses to JE vaccination. The amounts of E antigen released into the culture fluid of F cells were similar to those found in virion fractions of JEV-infected cell culture fluids or JEV-infected weanling mouse brains (the current source of antigen used to produce human vaccines for JE). Thus, the F-cell line would appear to be a useful source of antigen for JE vaccines and diagnostics.
Most vaccines and diagnostic reagents for viral diseases are manufactured using infectious agents, making them costly and dangerous to produce. Using recombinant DNA technology, it should be possible to overcome these problems by synthesizing viral immunogens and antigens in vitro. To be useful, these in vitro systems need to be able to produce the immunologically relevant viral components in an authentic form, which may require the in vitro systems to duplicate the posttranslational processing pathways that contribute to viral antigen formation. These posttranslational events in antigen formation may be particularly important in the synthesis of envelope glycoprotein structures, especially those that are heterodimeric. Production of recombinant DNA-derived viral surface proteins in a virus-like particulate form using eukaryotic cells has been reported for several enveloped viruses (1, 4, 5, 26, 27, 31–33). Since virion maturation may be driven by the ability of the individual envelope proteins to self-assemble, some viral proteins may self-assemble and be released from cells transfected with their genes, greatly facilitating their production and purification.
We have studied the flavivirus Japanese encephalitis virus (JEV) as a model for production of recombinant viral proteins (16–19, 21, 29). The flavivirus virion consists of a nucleocapsid structure surrounded by a lipid bilayer containing an envelope (E) glycoprotein and a nonglycosylated membrane (M) protein (6). The E protein is the major surface protein, with a role in receptor binding and membrane fusion, and it is known to contain many protective epitopes (11). The M protein is found in infected cells as a glycosylated precursor, premembrane (prM). In the process of virion maturation in vertebrate cells, provirion particles are formed when portions of endoplasmic reticulum membrane containing prM and E envelop nucleocapsids consisting of the capsid (C) protein and genomic RNA (6). These poorly infectious provirions accumulate in the lumen of the exocytic pathway, and during virion maturation, prM is cleaved to M by a cellular protease, furin, located in the trans-Golgi network (37). This maturation cleavage event is accompanied by changes in oligomerization of prM/M and E that is essential for development of the characteristics of mature virions, including high infectivity, hemagglutination (HA) activity, and fusion activity (37).
We have demonstrated that cells expressing the JEV prM and E genes are able to produce subviral extracellular particles (EPs) in a system using a vaccinia virus vector for gene delivery (18, 29). Biochemical and morphological analyses of EPs obtained from HeLa cells infected with a recombinant vaccinia virus encoding prM and E (vP829) indicated that EPs are empty viral particles composed of approximately 20-nm-diameter spherical membrane vesicles containing prM/M and E embedded in a lipid bilayer without a nucleocapsid, similar to slowly sedimenting hemagglutinin (SHA) particles found in culture fluids harvested from cells infected with JEV (19). Antigenic analyses using a panel of monoclonal antibodies indicated that E contained in EPs possesses conformational structures equivalent to those of the authentic E contained in the JEV virion (16). Mouse experiments indicated that EPs are able to induce neutralizing antibody and protective immunity (19) and, consistent with their particulate nature, virus-specific cytotoxic T lymphocytes (21). An enzyme-linked immunosorbent assay (ELISA) using EPs showed a sensitivity and specificity similar to those of the neutralization assay for testing human sera, demonstrating their usefulness as immunodiagnostic reagents (17). In spite of these promising features, however, EPs purified from culture fluid of vP829-infected cells contained small amounts of vaccinia virus antigens, including infectious virus (17, 19), that could complicate their use.
Contamination of antigens and/or infectious particles derived from the vector virus is an unavoidable problem in systems using a virus vector for gene delivery, but the problem can be solved by delivery of genes using a nonvirus vector such as a plasmid. Establishment of continuously expressing eukaryotic cell lines (14) would be an ideal way to produce viral proteins in terms of safety and yield. The ability of the cells to stably produce antigens without loss of cell viability might also provide a greater yield of viral protein products relative to transient expression systems based on virus vectors. However, a potential obstacle in the generation of stable EP-producing cell lines is that EPs could have toxic effects on cells expressing large amounts of these particles. One cause of this type of toxicity could result from the known cell-fusing activity of the flavivirus E protein. In particular, fusion from within has been reported to occur in flavivirus-infected cells (12, 39), and fusion from without has been reported for purified virions (10, 12, 38) and EPs (34). Thus, toxic effects of fusion due to EP production could prevent the establishment of a cell line stably expressing large amounts of EPs.
In this article, we report the establishment of a cell line continuously expressing JEV EPs which was developed by transfecting CHO-K1 cells with a plasmid encoding the JEV E proteins and a form of prM containing a modification of the amino acid sequence at the furin cleavage site. The mutation was designed to suppress cleavage from prM to M, eliminating the fusion activity of the EPs, allowing us to overcome the difficulty in generating cells continuously expressing EPs at a high level. Our cell line, designated the F-cell line, produced a relatively large amount of EPs without any contamination with infectious materials. EPs purified from culture fluids of F cells were immunogenic in mice and useful as an antigen for ELISA, indicating potential application of EPs to production of subunit vaccines and diagnostic tests for JE.
MATERIALS AND METHODS
Cells and conditions for cultivation.
Mammalian cell lines CHO-K1 (22) and HeLa (18) and a mosquito cell line, C6/36 (19), were grown in Eagle's minimal essential medium (MEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), nonessential amino acids, and kanamycin (60 μg/ml) at 37°C in a humidified atmosphere of 5% CO2–95% air. Another mammalian cell line, Vero (18), was grown under the same conditions without nonessential amino acids. The maintenance medium used for HeLa, C6/36, and Vero cells following infection (and for harvesting EPs from F cells) was growth medium containing 0.1% bovine serum albumin (BSA) instead of FBS. F-cell cultures were maintained by seeding the cells at a low density and then passaging them when the cells grew into colonies consisting of 16 to 32 cells. For harvesting EPs, F cells were seeded at a much higher density, allowing them to reach confluency within 2 days of cultivation.
Viruses and conditions for infection.
The prototype Nakayama strain and the virulent Beijing P3 strain of JEV have been described (29). A recombinant vaccinia virus encoding the JEV (Nakayama strain) signal sequence of prM and the prM and E genes (18) (designated vP829) was used for providing a representative EP preparation produced by a virus vector-based transient-expression system. The parent vaccinia virus, vP410 (18), was used as a control for vP829. HeLa monolayer cells were infected with vP829 or vP410 at a multiplicity of infection of 2 PFU/cell and incubated in maintenance medium at 37°C for 24 h prior to the harvest of infected culture fluid. Vero and C6/36 monolayer cells were infected with the Nakayama strain of JEV at a multiplicity of infection of 5 PFU/cell and incubated in maintenance medium at 37°C for 48 h before the harvest of infected culture fluid. An infected mouse brain homogenate was prepared by inoculating a seed Nakayama virus intracranially into weanling (4-week-old) ICR mice, harvesting brains from moribund mice showing signs of encephalitis, and then homogenizing brains in 7.5% BSA in phosphate-buffered saline (PBS) to make a final 20% emulsion.
Plasmids.
The construction of pcJEME, which is a pcDNA3-based plasmid encoding the JEV (Nakayama strain) signal sequence of prM and the prM and E genes has been described previously (23). pcJEEP, which has a mutated pr/M cleavage site, was constructed from pcJEME by site-directed mutagenesis using the PCR method (13). Specifically, the furin cleavage site, RSRR, was changed to TSRR by performing overlap PCR mutagenesis (13) on a fragment encoding prM and a portion of E bounded by EcoRI and EcoRV restriction endonuclease sites (Fig. 1). Following substitution of the mutated fragment into pcJEME in place of the original fragment, the resulting plasmid (pcJEEP) was sequenced to ensure that only the desired mutation had been inserted during the manipulations.
FIG. 1.
Schematic diagram of the in vitro site-directed mutagenesis strategy used to create the furin cleavage site-mutated plasmid pcJEEP from pcJEME. pcJEME is a pcDNA3-based plasmid containing a strong eukaryotic promoter derived from human cytomegalovirus (CMV promoter), the JEV (Nakayama strain) signal sequence of prM, and the prM and E genes, a polyadenylation signal derived from bovine growth hormone (BGH poly A), neomycin resistance gene (NeoR), and ampicillin resistance gene (AmpR). The first round of PCR amplifications were performed with a combination of primers 1 and 3 or a combination of primers 2 and 4 using the pcJEME template with its native pr/M cleavage site (RSRR). The products of this first round of amplification were combined and reamplified with primer 1 and primer 4, yielding a fragment with the altered cleavage site (TSRR; containing an SpeI site to facilitate identification), which was then substituted for the wild-type fragment in pcJEME using the EcoRI and EcoRV restriction sites (see Materials and Methods). Nucleotide (nt) sequences from nucleotides 625 to 672 (where 1 is the first nucleotide of the C protein initiation codon) and their encoded amino acids (one-letter code) are shown for both the wild-type and mutated plasmids in the region surrounding the furin cleavage site.
Antibodies and sera.
Monoclonal antibodies to E (J3-11B9, J3-10E1, J3-11H7, and J3-12H11) and to M (J2-2F1) and polyclonal anti-JEV antibodies obtained in the form of hyperimmune mouse ascitic fluid have been described (28). Rabbit anti-JEV hyperimmune serum was provided by Yoshinobu Okuno of Osaka Prefectural Institute of Public Health, Japan. Human serum samples obtained from American vaccinees who received inactivated JE vaccine have been described elsewhere (8, 17).
Transfection and selection of cells.
CHO-K1 cells were transfected with 0.5 to 1.0 μg of pcJEME or pcJEEP by using lipopolyamine (Transfectam; Biosepra, Villeneuve-la-Garenne, France) according to the instructions supplied by the company. In some cases, cells containing the transfected plasmids were selected for their ability to express the plasmid-encoded neomycin phosphotransferase II by growth in medium supplemented with G418 (Life Technologies Inc., Gaithersburg, Md.) (3) at 400 μg/ml (a concentration that eliminated G418-sensitive CHO-K1 cells within 3 weeks of incubation). In other cases, a subcloning strategy was used to derive transfected cells displaying high-level E protein expression. For this procedure, transfected cultures were dissociated with trypsin and plated at limiting dilution (0.2 to 1 cell per well) in 96-well microplates. Following growth to 16 to 32 cells, wells containing a single colony were dissociated with trypsin and allowed to regrow in the same well to confluence. At this stage, the contents of each well were split into two similar-sized wells, and that in one well was fixed, stained, and scored for percent cells immunochemically stained (see below) with monoclonal J3-11B9 or polyclonal anti-JEV antibodies (hyperimmune mouse ascitic fluid). Based on the results of the immunochemical staining, the duplicate well containing the highest percentage of JEV antigen-expressing cells was used for subsequent limiting-dilution cloning steps as described above.
Purification of EPs, virions, and SHA particles.
F-cell cultures grown at nearly 100% confluency were rinsed three times with PBS and incubated in maintenance medium at 37°C for 22 to 28 h unless otherwise specified. Culture fluids harvested from F, vP829-infected HeLa, and JEV-infected Vero cells (see above) were clarified by centrifugation and precipitated with polyethylene glycol (PEG; molecular mass, approximately 6,000 Da) at 10% (17). The precipitate was collected by centrifugation, suspended in TN buffer (10 mM Tris-HCl [pH 7.5], 100 mM NaCl), and applied to a 10 to 40% (wt/wt) continuous sucrose gradient (prepared with TN buffer). Following centrifugation at 4°C at 35,000 rpm for 3 h in the P40ST rotor of a Himac CP70MX ultracentrifuge or at 55,000 rpm for 90 min in the S55S rotor of a Himac CS100GX microultracentrifuge (Hitachi Koki Co. Ltd., Ibaragi, Japan), fractions were collected, and the level of E antigen in each fraction was determined by ELISA (see below).
Immunochemical staining.
Cells were fixed with a mixture of cold methanol and acetone (1:1) and dried. The monolayers were rehydrated with PBS containing normal horse serum at 1% and incubated with monoclonal or polyclonal antibodies. Antigen-antibody reactions were then detected with biotinylated anti-mouse immunoglobulin G (IgG; heavy and light chain specific), the ABC (avidin-biotinylated enzyme complex) reagents, and the VIP substrate (Vector Laboratories, Inc., Burlingame, Calif.).
ELISA for quantification of E antigen.
E antigen was quantified using a sandwich ELISA (20). Briefly, 96-well microplates (Maxisorp; A/S Nunc, Roskilde, Denmark) sensitized with rabbit anti-JEV hyperimmune serum were incubated serially with test samples, monoclonal antibody J3-11B9, alkaline phosphatase-conjugated anti-mouse IgG, and p-nitrophenyl phosphate. Antigen levels were calculated from absorbance values obtained with the sample and a reference standard and were expressed as E protein amount in nanograms per milliliter, unless otherwise specified. The reference standard was prepared with a PEG concentrate of EPs obtained from culture fluids of vP829-infected HeLa cells, and the E protein amount contained in the standard EP preparation was estimated by comparison with BSA samples in Coomassie brilliant blue-stained gels.
ELISA for quantification of antibody to JEV in human sera.
A conventional ELISA for quantifying IgG antibodies to JEV was performed essentially as previously described (17). Briefly, microplates (Microwell; Nunc) were sensitized with EP antigens containing 30 ng of E per well, which had been partially purified from culture fluids of F cells or vP829-infected cells by precipitation with PEG (see above) and dissolved in 0.1 M sodium carbonate containing 0.1% Triton X-100. Sensitized plates were incubated serially with test sera, alkaline phosphatase-conjugated anti-human IgG, and p-nitrophenyl phosphate.
Western blot analyses.
Sodium dodecyl sulfate (SDS)-containing polyacrylamide gel electrophoresis, Western blotting, and subsequent immunochemical staining were performed essentially as previously described (19). Briefly, samples were run on standard Laemmli gels under reducing conditions, and proteins were transferred to a polyvinylidene difluoride membrane (Millipore Corporation, Bedford, Mass.) and detected by incubation with J2-2F1 or J3-11B9, with alkaline phosphatase-conjugated anti-mouse IgG, and then with nitro blue tetrazolium–5-bromo-4-chloro-3-indolyl phosphate substrate.
Immunization and challenge of mice.
Groups of 3-week-old female ICR mice were inoculated once or twice at an interval of 2 weeks by subcutaneous (s.c.) injections with F-cell-or vP829-derived purified EP fractions containing 1 μg of E. These immunogens were emulsified with Freund's complete or, for the second immunization, incomplete adjuvant. A small amount of infectious vaccinia virus remaining in EP fractions purified from culture fluids of vP829-infected HeLa cells was inactivated with 0.06% binary ethyleneimine as previously described (19). As a control, mice were inoculated s.c. with TN buffer emulsified with adjuvant or vaccinated by intraperitoneal (i.p.) injections with a dose of 3 × 106 PFU of the Nakayama strain. Two or three weeks after immunization, retroorbital blood was collected and pooled for antibody titration. For challenge experiments, immunized mice were inoculated i.p. with 100,000 50% lethal doses (LD50) of the P3 strain of JEV and observed for 3 weeks.
HA test, plaque assay, and neutralization test.
HA tests were performed by a modification of the method described by Clarke and Casals (7). Virus titers were determined on Vero cell monolayers (18), and neutralizing antibodies were determined using plaque reduction assays (22) performed with the Nakayama strain. The neutralization titer was expressed as the maximum serum dilution yielding a 90% reduction in plaque number.
RESULTS
Attempts to establish a stable cell line expressing EPs by using pcJEME.
Our initial attempts to establish cells expressing JEV EPs used an antibiotic (G418) to select CHO-K1 cells transfected with pcJEME, based on coexpression of the plasmid-encoded neomycin phosphotransferase II. Although immunochemical staining revealed that 5 to 10% of the cells expressed E antigens 1 day after transfection, the percentage of cells expressing immunochemically reactive E protein decreased with time of cultivation, and no E-expressing cells were detectable after 4 weeks of cultivation. During these studies, all colonies selected with G418 that had cells expressing JEV antigens also contained cells with no detectable JEV protein. Similar results were obtained during multiple attempts to obtain pure populations of E-expressing cells by limiting-dilution cloning in the presence of G418. Further attempts to obtain cells stably transfected with plasmid pcJEME also failed using a different transfection reagent (Lipofectin; Life Technologies), different concentrations of G418, and different cell types (Vero and COS7) (data not shown).
When transfected cells were cloned by limiting dilution in the absence of G418, a single E-expressing clone was detected among approximately 500 clones tested. Thus, the frequency of isolating E antigen-positive clones was far lower than the percentage of expressing cells obtained 24 h after transfection (5 to 10%). While monitoring transfected cells for expression during the first 3 days following transfection, we noted that most of the E-expressing cells rounded up during this time period, suggesting that the low frequency (0.2%) of antigen-expressing cells obtained during the initial cloning step could be attributable to toxicity of the prM/E gene product. However, in contrast to the results obtained in the presence of G418 (see above), the percentage of clones containing E-expressing cells increased after the second cloning cycle performed on this positive clone. Furthermore, by the third cloning cycle, a high percentage (94%) of clones with some E-expressing cells were obtained (open circles, Fig. 2A). Although performing our repeated cloning strategy in the absence of G418 allowed the recovery of a large number of clones containing E-expressing cells, this strategy did not yield “clones” with very high percentages of cells expressing E (open circles, Fig. 2B).
FIG. 2.
Time courses of three markers used to evaluate the production of continuously expressing cell clones from cells transfected with pcJEME (open circles) or pcJEEP (solid circles) by limiting-dilution cloning cycles. Markers were percentages of clones containing E antigen-expressing cells (A), maximum percentages of expressing cells among clones (B), and percentages of polykaryoctes in total number of expressing cells (for these calculations, each expressing polykaryocyte was counted as a single cell) found in the clone that had the maximum percentage of expressing cells (C) (see Fig. 3 for representative micrographs).
While attempting to obtain clones that contained a high percentage of E-expressing cells, we observed that polykaryocytes were relatively frequent in pcJEME-transfected cell clones and that almost all of these multinuclear cells expressed JEV antigens (Fig. 3, left). During all six cloning steps that we attempted, more than 10% of the JEV antigen-positive cells were polykaryocytes (open circles, Fig. 2C), whereas polykaryocytes constituted less than 1% of antigen-negative cells. Moreover, the cell clones that contained no E-expressing cells had very few polykaryocytes (data not shown). These results suggested that a toxic effect of E antigen-induced fusion of expressing cells could have been responsible for our failure to obtain a cell clone containing a high percentage of E-expressing cells.
FIG. 3.
Micrographs of immunoperoxidase-stained cloned cell populations originated from cells transfected with pcJEME (left; after the sixth cloning cycle) or pcJEEP (right; after the fourth cloning cycle). Dark cytoplasmic staining was the result of specific immunoreactivity with an E protein-specific antibody (J3-11B9; see Materials and Methods). Note the large number of polykaryocytes present in the pcJEME-transfected cells.
Generation of E antigen-expressing cell clones by using pcJEEP.
To overcome the possibility that the presence of fusion-competent EPs prevented us from obtaining a stable cell line expressing EPs from pcJEME-transfected cells, we altered the biochemical properties of the plasmid-encoded prM protein to produce fusion-incompetent EPs. Specifically, we generated a plasmid, pcJEEP, with the furin cleavage site (30) mutated from RSRR to TSRR (see Materials and Methods), similar to a mutation (RKKR to TKKR) reported to alter the cleavage of influenza virus hemagglutinin by furin (40). Attempts to generate cell lines expressing EPs from cells transfected with pcJEEP also failed when transfected cells were selected using G418. As observed with cultures transfected with pcJEME (see above), the percentages of E-expressing cells observed 1 day following transfection with pcJEEP (5 to 10%) decreased with cultivation time and eventually no expressing cells were observed even when a limiting-dilution cloning protocol was used. Most of the E-expressing cells showed morphological changes, suggesting that the pcJEEP products were also toxic to transfected cells. However, when transfected cells were cloned by limiting dilution in the absence of G418, the percentage of clones containing expressing cells and the percentage of expressing cells contained in each clone increased with each cloning step (solid circles, Fig. 2A and B). After the fourth cycle of cloning, a cell line designated the F-cell line containing more than 90% E-expressing cells was obtained (solid circles, Fig. 2B). Thus, substitution of plasmid pcJEEP for pcJEME in our transfection and cloning strategy allowed us to obtain a cell line containing a high percentage of antigen-expressing cells.
In contrast to the observations obtained from the pcJEME-transfected clones, very few polykaryocytes were observed during the cloning process of pcJEEP-transfected cells (solid circles, Fig. 2C; Fig. 3, right). The difference in rate of polykaryocyte formation between pcJEEP-and pcJEME-transfected cells supports the above-mentioned hypothesis that fusion activity of E antigen produced by pcJEME contributed to our failure to generate cell clones containing high percentages of E-expressing cells.
Stability of F cells in expressing and releasing E antigens.
To evaluate how long F cells would stably release EPs, the percentage of E-expressing cells and the amount of E antigen released from cultures were monitored during several passages of three F-cell subclones. For the experiment shown in Fig. 4, passage 1 was defined as the culture grown from the 96-well plate used for the limiting-dilution cloning of the F cells. The left panel of Fig. 4 shows that over 80% of the cells present in five serial passages (note that these passages are different from the cloning cycles shown in Fig. 2) of all three subclones were positive for E antigen expression. Furthermore, the amount of E antigen released from cultures of these three subclones varied between 1.1 ± 0.2 and 0.9 ± 0.2 μg per 107 cells for the first five passages (Fig. 4, lower left panel). One of the three subclones selected for additional passages showed a drop in E-expressing cells to one-third (27%) and a reduction in E antigen release to 1/10 (100 ng) on the 30th passage. During 30 passages, these cells did not show notable variations in growth rate. These results indicate that the F-cell line subclones could maintain high levels of E antigen synthesis and release for at least five passages.
FIG. 4.
Percent E-expressing cells (A and B) and amount of E antigen (Ag) released (C and D) from three F-cell subclones (A and C) and from a single F-cell clone following recovery from cryopreservation (B and D). Data were obtained during serial passages of cells (see text for details).
Stability of EP expression by F cells was further evaluated using cryopreserved cells. Three passages after establishment of the F-cell line, aliquots of trypsinized cells were suspended in Eagle's MEM containing 50% FBS and 10% dimethyl sulfoxide and cryopreserved in liquid nitrogen for 3 months. Following thawing, cultures of these cells maintained approximately 80% expressing cells and E antigen release from 107 cells in the range from 100 to 1,000 ng during 10 passages after recovery (Fig. 4B and D). These results indicate that F cells were stable in releasing E antigens for at least 10 passages after recovery from cryopreservation and that the levels of E antigen released were not greatly affected by the cryopreservation and recovery processes.
Biochemical characterization of E antigen produced by F cells.
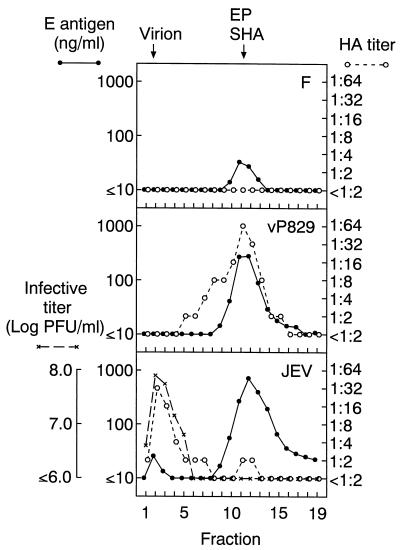
To characterize the biophysical properties of extracellular E antigens produced by F cells, the growth medium was removed from a confluent F-cell culture, and the cells were incubated in serum-free medium containing 0.1% BSA for 24 h at 37°C. This culture fluid was resolved on a 10 to 40% sucrose density gradient, and the amount of HA activity and E proteins present in each fraction were compared to those in fractions harvested from identical gradients prepared with culture fluids obtained from vP829-infected HeLa cells and JEV-infected C6/36 cells. As shown in Fig. 5, these analyses identified a peak of E antigen in the F-cell sample that comigrated with vP829-derived EPs and JEV-derived SHA particles. Although the results in Fig. 5 were obtained with a single E monoclonal antibody (J3-11B9), this density gradient peak also reacted with three other monoclonal antibodies to E (J3-10E1, J3-11H7, and J3-12H11; data not shown), indicating that E antigen molecules contained in F-cell-derived EPs had an antigenic structure similar to that of authentic JEV particles. As expected from work with a furin protease inhibitor and a furin-deficient cell line (37), the E antigen peak produced by F cells did not display any detectable levels of HA activity (Fig. 5). Furthermore, HA activity was not detected in PEG-concentrated F-cell EP samples with an E antigen level of 15 μg/ml (data not shown), indicating that the specific HA activity of EPs with a mutated pr/M cleavage site was 1,000 times lower than the HA activity in vP829-derived EPs with the natural furin cleavage site (specific activity was calculated by computing the HA activity relative to E antigen mass measured by ELISA).
FIG. 5.
Analyses of JEV activities recovered in sucrose density gradient fractions obtained from culture fluids harvested from F cells (F), vP829-infected HeLa cells (vP829), and JEV-infected C6/36 cells (JEV). Fractions were examined for E antigen amounts (solid circles), HA activity (open circles), and infectivity (multiplication signs). The arrows at the top of the figure identify peaks of virion, EP, and SHA activity; fraction 1 was harvested from the bottom of the gradient (see Materials and Methods for details).
The EP fraction purified from F-cell culture fluid was compared with purified preparations of vP829-derived EPs and JEV-derived virion and SHA particles by Western blotting. As shown in Fig. 6, EPs produced by F cells contained clearly visible amounts of prM and E, which comigrated in SDS gels with the same components found in virion, SHA, and the vP829-specified EPs. However, the M protein was present at barely detectable levels in F-cell-derived EPs, confirming that these cells produced a biochemically altered prM protein resistant to cleavage by furin.
FIG. 6.
Western blot analyses of purified fractions of fusion-incompetent EPs from F cells (F), EPs from vP829-infected HeLa cells (829), virions (V) from JEV-infected Vero cells, and SHA particles (SHA) from JEV-infected Vero cells. Samples were run on a 14% polyacrylamide gel, blotted, and stained with monoclonal antibodies (MAb) to E (J3-11B9) or M (J2-2F1).
Immunogenicity and protective efficacy of EPs produced by F cells.
EPs with a mutated pr/M cleavage site were compared in immunogenicity and protective efficacy with EPs with a native cleavage site (from vP829-infected cells), which were previously demonstrated to be protective in a mouse model for JE (19). For the first experiment, groups of seven 3-week-old female ICR mice were given a single s.c. inoculation with either F-cell-or vP829-derived EP immunogens containing 1 μg of E (Table 1). These immunogens were purified EP fractions emulsified with Freund's complete adjuvant. A single immunization protocol with a dose of 1 μg of E was used, since we previously obtained partial protection under this condition using vP829-derived EPs (19) and considered that this condition would be suitable for comparison in protective efficacy between EPs with and without a mutated pr/M cleavage site. As controls, mice were inoculated s.c. with TN buffer emulsified with adjuvant or vaccinated i.p. with 3 × 106 PFU of the Nakayama strain. Fifteen mice were used for the Nakayama-inoculated group, based on our previous experience that this dose of virus corresponded to 1 LD50 in ICR mice; however, 13 of 15 mice died as a result of the Nakayama injection, indicating that this “immunizing” dose was considerably higher than 1 LD50. Three weeks after immunization, all mice were bled and challenged with the P3 strain of JEV. Although mice that received one immunization with F-cell-derived EPs did not develop detectable neutralizing antibody, these mice were partially protected at a level somewhat higher (but not significantly) than that observed in mice immunized with vP829-derived EPs (Table 1).
TABLE 1.
Immunogenicity and protective efficacy of F-cell-derived EPs in mice
| Expt no.a | Immunogenb | Dosec | No. of mice | Neutralization titerd after:
|
% Survivale (no. alive/total) | |
|---|---|---|---|---|---|---|
| 1 immunization | 2 immunizations | |||||
| 1 | F cell EPs | 1 μg of E | 7 | <1:10 | — | 50 (3/6)f |
| vP829 EPs | 1 μg of E | 7 | <1:10 | — | 29 (2/7) | |
| JEV | 3 × 106 PFU | 2g | 1:20 | — | 100 (2/2) | |
| TN buffer | 7 | <1:10 | — | 0 (0/7) | ||
| 2 | F cell EPs | 1 μg of E | 12 | <1:10 | 1:80 | — |
| vP829 EPs | 1 μg of E | 12 | 1:10 | 1:160 | — | |
| TN buffer | 12 | <1:10 | <1:10 | — | ||
Groups of 3-week-old ICR mice were immunized once (experiment 1) or twice at an interval of 2 weeks (experiment 2) with the indicated immunogens.
All immunogens except JEV were emulsified with Freund's complete adjuvant or, for the second immunization, incomplete adjuvant, and inoculated by the s.c. route. JEV was inoculated without adjuvant by the i.p. route.
The dose of EPs is represented as amount of E antigen contained in EPs.
Represented as the maximum serum dilution yielding a 90% reduction in plaque number. —, not done.
Survival 3 weeks after challenge with 100,000 LD50 of the P3 strain of JEV. —, not done.
One mouse died accidentally during administration of anesthesia.
The live Nakayama strain of JEV given at the indicated dose killed 13 of 15 mice. Therefore, serological and challenge data were derived from the remaining two mice.
For the second experiment, groups of 12 3-week-old ICR mice were immunized twice at an interval of 2 weeks with EPs containing 1 μg of E and bled 2 weeks following each immunization to evaluate EPs for their ability to induce neutralizing antibody (Table 1). Immunogens were F-cell- and vP829-derived EPs or TN buffer as a control, all of which were emulsified with Fruend's complete adjuvant for the first immunization and incomplete adjuvant for the second immunization. Both EP immunogens, which induced low or undetectable levels of neutralizing antibodies following one immunization, induced neutralizing antibodies following two immunizations. In this experiment, vP829-derived EPs and F-cell-derived EPs elicited roughly equivalent levels of neutralizing antibodies. Taken together, these results indicate that EPs with a mutated pr/M cleavage site were immunogenic and protective at levels similar to EPs with a native cleavage site.
Applicability of F-cell-derived EPs to ELISA antigen.
To evaluate EPs with a mutated pr/M cleavage site for usefulness as an antigen in immunodiagnostic tests, F-cell-derived EPs were tested in an ELISA format that we previously developed using vP829-derived EPs (17) (Fig. 7). To evaluate these two EP antigens, we used a panel of 45 human sera with known neutralization titers, which were obtained from a JE vaccine study (8, 17). The results of the ELISA performed with the F-cell antigen correlated very well with the results of the ELISA performed using vP829 antigen (correlation coefficient, 0.981; P < 0.001), indicating that EPs with a mutated pr/M cleavage site are antigenically equivalent to EPs with a native cleavage site. Furthermore, ELISA using F-cell antigen was significantly correlated with the neutralization test, with a correlation coefficient of 0.651 (P < 0.001). By setting 0.06 (mean plus 3 standard deviations of ELISA absorbance values obtained from 15 prevaccination sera) as the cut-off to differentiate positive from negative sera, all the sera positive in ELISA were positive for neutralizing antibody, and all the sera negative for ELISA were negative for neutralizing antibody. Thus, the results of our ELISA with the F-cell-derived EP antigen were in complete correlation with vaccination status established using the neutralization assay for this small group of sera, demonstrating the usefulness of the F-cell-derived EPs in immunodiagnostic tests.
FIG. 7.
Comparison of the F-cell antigen ELISA to a vP829 antigen ELISA (left panel) and to a neutralization test (right panel) using 45 human serum samples. The dashed line indicates the cut-off calculated from ELISA absorbance values obtained with 15 prevaccination samples (mean plus 3 standard deviations).
Yield of EPs from F cells in comparison with other antigen production methods.
To further assess the potential usefulness of F-cell-derived EPs as an antigen to replace inactivated vaccines or immunodiagnostic antigens, the yield of E antigen obtained from F cells was compared with the yield of virions in JEV (Nakayama)-infected C6/36 culture fluid or mouse brain homogenate. For these studies, 1 ml of F-cell culture fluid corresponds to the amount of E released from approximately 2 × 106 cells into 1 ml of BSA-free or 0.1% BSA-containing MEM during a 24-h cultivation period; 1 ml of JEV-infected C6/36 culture fluid corresponds to the amount of E antigen released by a similar number of cells into 1 ml of 0.1% BSA-containing MEM during a 48-h cultivation period, and 1 ml of 20% homogenate of JEV-infected mouse brain corresponds to the amount of E antigen present in one half of the brain of a moribund JEV-infected weanling mouse (Table 2). Comparison of these numbers shows that the amount of E harvested from the F cell (127 ng/ml) compares favorably with those from the virion fraction of cell culture (76 ng/ml) and infected mouse (120 ng/ml) sources (Table 2). The favorable comparison to the latter source is particularly important, since mice serve as the only currently approved source of commercial vaccine (inactivated virion fraction) in Japan and the United States, and each dose of the current vaccine, which contains approximately 250 to 300 ng of E antigen (determined by sandwich ELISA; results not shown), is prepared from the brains of two JEV-infected weanling mice (M. Nakayama, National Institute of Infectious Diseases, Tokyo, Japan; personal communication). Although the total amount of E harvested from JEV-infected C6/36 culture fluid within 2 days (2,182 ng/ml) is much higher than the amount harvested from F-cell cultures within 1 day (76 ng/ml), use of C6/36 cultures for producing vaccines or diagnostic reagents requires repeated procedures for infection of cells and inactivation of the harvested materials, neither of which is required for production of EPs from F cells.
TABLE 2.
Yield of E antigen from F-cell cultures, JEV-infected cells, and JEV-infected micea
| E antigen source | E antigen amtb (ng/ml)
|
|||
|---|---|---|---|---|
| EP | Virion | SHA | Total | |
| F-cell culture fluid | 127c | 127 | ||
| JEV-infected C6/36 culture fluid | 76 | 2,106 | 2,182 | |
| 20% homogenate of JEV-infected mouse brains | 120 | 403 | 523 | |
F-cell culture fluids were harvested from confluent cultures grown in 25-cm2 flasks 24 h after replacement of the medium with BSA-free or 0.1% BSA-containing MEM. JEV-infected C6/36 culture fluids were obtained from confluent cultures maintained in 0.1% BSA-containing MEM for 48 h after infection with JEV. For both cells, approximately 107 cells were contained in confluent cell cultures in a 25-cm2 flask containing 5 ml of medium. A 20% homogenate of JEV-infected mouse brains was prepared from moribund weanling mice intracranially inoculated with JEV (see Materials and Methods for details).
Determined by the sandwich ELISA. EP, E antigen in F-cell culture fluid was determined from the amount of E detected in the EP fraction from sucrose density gradients (see Fig. 5), which was used as the sole source of E when calculating the total amount of E present in this source of antigen. Virion and SHA, E antigen in JEV-infected C6/36 culture fluid and 20% homogenate of JEV-infected mouse brains was determined from the amount of E detected in the virion and SHA fractions from sucrose density gradients (see Fig. 5), which were used as the combined sources of E when calculating the total amount of E present in each source of antigen.
Average of 21 values (ranging from 34 to 270 ng/ml) obtained during multiple passages of F cells and subclones.
Interestingly, the yields of EPs from F cells were not affected by the BSA concentration in the medium used for cell maintenance. Specifically, the amount of EPs released from F cells maintained in MEM containing 0% BSA (approximately 0.6 μg from 107 cells in 1 culture day) was similar to the EP release in MEM containing 0.1 to 1% BSA. The use of protein-free medium could contribute to the EP purification process. Furthermore, EPs accumulated in culture fluid with longer incubation periods. Five days after medium replacement, the amounts of EPs recovered from F-cell monolayers increased two- to fourfold over the amounts recovered at 24 h. The ability to readily recover EPs that accumulate in culture medium of F cells in the absence of BSA will certainly aid in their development as antigens for use in vaccines and diagnostic tests.
DISCUSSION
The life cycles of most lytic viruses, including JEV, do not require the long-term synthesis of their products in host cells. Thus, many viral products may be cytotoxic, for either fortuitous (the normal viral products are toxic in the process of performing essential replication functions) or specific (the virus needs to specifically alter host functions, resulting in cell death) reasons. Therefore, the use of recombinant DNA methods to express individual viral products in mammalian cells could prove difficult due to unexpected and unwanted cytotoxic effects of these products on the cells. Depending on the severity of these problems, viral protein synthesis could be impossible to achieve or could merely result in low yields of product. In cells transfected with DNA molecules engineered to produce JEV EPs, several processes required for particle production, including protein synthesis, targeting the proteins to subcellular organelles, and particle release could all have toxic effects on cells. However, the known ability of the EPs to fuse cellular membranes seems to be the most obvious and predictable cytopathic outcome of high-level expression of EPs in cell culture.
Consistent with the predicted fusogenic activity of EPs, we were initially unable to obtain stable cell lines expressing EPs by transfecting cells with a plasmid, pcJEME, encoding the two viral components (prM and E) needed to form JEV EPs. Furthermore, during these studies we noted that JEV antigen-expressing cells disappeared from cultures during cultivation, large numbers of antigen-positive cells were present in polykaryocytes, and many antigen-positive cells displayed a rounded morphology typical of virally induced cytopathic effect. We believed that these factors, taken together, strongly implicated the fusogenic activities of EPs as the major reason that we had failed to obtain stable cell lines to produce these particles. To overcome this problem, we decided to suppress fusion activity in the EP-expressing cells. Initially, fusion activity was suppressed by growing cells in a high-pH buffer (50 mM Tris-HCl) known to suppress cleavage of prM to M, resulting in the production of virions of very low infectivity and a high ratio of uncleaved prM to M (36). Although this method did not allow us to obtain stable cell lines expressing EPs, high-pH-adapted cells transfected with the prM/E-encoding plasmid pcJEME did contain higher percentages of E antigen-positive cells than had been obtained in wild-type CHO cells grown under normal conditions (results not shown).
Encouraged by the results of these high-pH experiments, we decided to genetically alter the prM/E cassette in pcJEME to suppress fusion. At least two strategies could be envisioned for this procedure; one is direct mutation of the putative fusion domain of the E protein, and another is the mutation of the furin cleavage site in prM to prevent processing to M (thus suppressing the production of fusion-competent M/E oligomers). We opted for the latter strategy for two reasons. First, alteration of the predicted fusion domain of E could have unwanted effects on its immunogenicity and antigenicity, and second, the furin cleavage recognition site is well characterized and the high-pH experiments suggested that this type of alteration would be successful. Using this strategy, we successfully obtained a cell line, the F cell, which could be used to produce usable levels of JEV EP antigen.
Despite our success in producing the F-cell line that expressed high levels of JEV EPs with an abrogated furin cleavage site, several lines of evidence suggest that expression of the fusion-incompetent EPs by CHO cells has deleterious effects on these cells. One line of evidence for residual toxicity of the fusion-incompetent EPs on cell lines comes from the finding that we were unable to obtain clones expressing high levels of EPs by selecting cells harboring the plasmid using an antibiotic, G418, that can kill eukaryotic cells. Interestingly, using the same cells, plasmid expression system (pcDNA3), and growth conditions, we readily obtained clones of cells expressing the JEV NS1 protein using limiting-dilution cloning in the presence of G418 (results not shown). Although the reason for our failure to obtain EP-expressing cells under these conditions is not completely clear, it seems likely that a toxic effect(s) of EP (even fusion-incompetent EP) synthesis added to the stress of G418 selection prevented the growth of EP-expressing cells or reduced the level of EP synthesis, so that the clones isolated under these conditions did not appear to express the E antigen in our assays.
The importance of EP toxicity in development and use of the F-cell lines was further emphasized by several additional lines of evidence collected during attempts to adapt the F-cell line to growth under different conditions. First of all, we noted that, when replacing FBS-containing growth medium with medium containing low concentrations of BSA (0.1%), the yields of EPs dropped from approximately 3 μg/ml to less than 1 μg/ml (results not shown). Second, while attempting to add additional antibiotics to the growth medium for the cells (addition of penicillin and streptomycin to kanamycin-containing medium), we noted that the percentage of cells expressing E antigen dropped from nearly 100% to less than 10% (results not shown). Finally, when we attempted to grow the F cells in suspension cultures utilizing a commercial serum-free medium (CD-CHO medium; Life Technologies), the F cells were unable to replicate at all (results not shown).
Taken together, these results suggest that the selection of cells with high-level expression of EPs must be balanced with the toxicity that the synthesis and release of these products may have on the transfected cells. Thus, the F cells may maintain a delicate balance between producing large amounts of EPs and killing themselves with the toxic effects of EP synthesis. Moreover, it is likely that the difficulty of isolating the F-cell line relates to the selection of specific cells from the transfected CHO-K1 population that were relatively resistant to the toxic effects of EP synthesis. A similar type of selection as well as balance of level of synthesis of viral products is also apparent in establishing cell lines persistently infected with JEV (9, 24, 35). In the case of isolation of persistently infected cells, multiple viral products and processes could adversely affect cell viability. However, many of the same selection criteria could determine if cells are able to survive and persistently produce viral antigens, or if they eliminate (or suppress) expression of foreign genes. From this standpoint, it is interesting that the expression of the antiapoptotic gene bcl-2 can suppress JEV-induced apoptosis, increasing the frequency of isolation of persistently infected cells from JEV-infected cultures (24, 25).
The ability to produce subviral particles of hepatitis B virus in eukaryotic cells provided a breakthrough in the development of vaccines against hepatitis B (2). In the case of hepatitis B, there was no culture system to produce vaccine antigen, so the only alternative to the recombinant DNA-derived source of antigen was blood from chronic carriers of the disease. In the case of JE, an effective vaccine can be produced from animal-derived antigen, but the production system is dangerous, and this vaccine may contain adventitious antigens that can cause unwanted reactions in vaccinees (15). Here we have described an alternative method for the production of particulate JEV antigens that could be used in the preparation of vaccines. These antigens could be produced without the safety concerns of mouse-generated antigens and could also provide advantages in the area of cost and freedom from unwanted murine antigens. Our system is likely to have applicability beyond JE. The close similarity of many other flaviviruses to JEV, particularly those in the JEV/West Nile virus subgroup, make it likely that our strategy for making flavivirus EPs in cell culture could be used to make vaccines and diagnostic reagents for several important diseases.
ACKNOWLEDGMENTS
We thank Yoshinobu Okuno for providing rabbit anti-JEV hyperimmune serum.
This investigation received financial support from the Vaccine Research and Development Unit of the WHO Global Programme for Vaccines and Immunization.
REFERENCES
- 1.Allison S L, Stadler K, Mandl C W, Kunz C, Heinz F X. Synthesis and secretion of recombinant tick-borne encephalitis virus protein E in soluble and particulate form. J Virol. 1995;69:5816–5820. doi: 10.1128/jvi.69.9.5816-5820.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assad S, Francis A. Over a decade of experience with a yeast recombinant hepatitis B vaccine. Vaccine. 1999;18:57–67. doi: 10.1016/s0264-410x(99)00179-6. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1994. [Google Scholar]
- 4.Baumert T F, Vergalla J, Satoi J, Thomson M, Lechmann M, Herion D, Greenberg H B, Ito S, Liang T J. Hepatitis C virus-like particles synthesized in insect cells as a potential vaccine candidate. Gastroenterology. 1999;117:1397–1407. doi: 10.1016/s0016-5085(99)70290-8. [DOI] [PubMed] [Google Scholar]
- 5.Betenbaugh M, Yu M, Kuehl K, White J, Pennock D, Spik K, Schmaljohn C. Nucleocapsid- and virus-like particles assemble in cells infected with recombinant baculoviruses or vaccinia viruses expressing the M and the S segments of Hantaan virus. Virus Res. 1995;38:111–124. doi: 10.1016/0168-1702(95)00053-s. [DOI] [PubMed] [Google Scholar]
- 6.Chambers T J, Hahn C S, Galler R, Rice C M. Flavivirus genome organization, expression, and replication. Annu Rev Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 7.Clarke D H, Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 8.Defraites R F, Gambel J M, Hoke C H, Jr, Sanchez J L, Withers B G, Karabatsos N, Shope R E, Tirrell S, Yoshida I, Takagi M, Meschievitz C K, Tsai T F. Japanese encephalitis vaccine (inactivated, BIKEN) in U.S. soldiers: immunogenicity and safety of vaccine administered in two dosing regimens. Am J Trop Med Hyg. 1999;61:288–293. doi: 10.4269/ajtmh.1999.61.288. [DOI] [PubMed] [Google Scholar]
- 9.Fu D W, Zhang P F. Establishment and characterization of Japanese B encephalitis virus persistent infection in the Sf9 insect cell line. Biologicals. 1996;24:225–233. doi: 10.1006/biol.1996.0031. [DOI] [PubMed] [Google Scholar]
- 10.Guirakhoo F, Heinz F X, Mandl C W, Holzmann H, Kunz C. Fusion activity of flaviviruses: comparison of mature and immature (prM-containing) tick-borne encephalitis virions. J Gen Virol. 1991;72:1323–1329. doi: 10.1099/0022-1317-72-6-1323. [DOI] [PubMed] [Google Scholar]
- 11.Heinz F X. Epitope mapping of flavivirus glycoproteins. Adv Virus Res. 1986;31:103–168. doi: 10.1016/s0065-3527(08)60263-8. [DOI] [PubMed] [Google Scholar]
- 12.Higgs S, Gould E A. Differences in fusogenicity and mouse neurovirulence of Japanese encephalitis viruses. Arch Virol. 1991;119:119–133. doi: 10.1007/BF01314328. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi R, Krummel B, Saiki R K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaufman R J. Strategies for obtaining high level expression in mammalian cells. J Methods Cell Mol Biol. 1990;2:221–236. [Google Scholar]
- 15.Konishi E, Kurane I, Mason P W. Immune response to traditional and genetically engineered Japanese encephalitis vaccines. Recent Res Dev Virol. 2000;2:1–21. [Google Scholar]
- 16.Konishi E, Mason P W. Proper maturation of the Japanese encephalitis virus envelope glycoprotein requires cosynthesis with the premembrane protein. J Virol. 1993;67:1672–1675. doi: 10.1128/jvi.67.3.1672-1675.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konishi E, Mason P W, Shope R E. Enzyme-linked immunosorbent assay using recombinant antigens for serodiagnosis of Japanese encephalitis. J Med Virol. 1996;48:76–79. doi: 10.1002/(SICI)1096-9071(199601)48:1<76::AID-JMV12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Konishi E, Pincus S, Fonseca B A L, Shope R E, Paoletti E, Mason P W. Comparison of protective immunity elicited by recombinant vaccinia viruses that synthesize E or NS1 of Japanese encephalitis virus. Virology. 1991;185:401–410. doi: 10.1016/0042-6822(91)90788-d. [DOI] [PubMed] [Google Scholar]
- 19.Konishi E, Pincus S, Paoletti E, Shope R E, Burrage T, Mason P W. Mice immunized with a subviral particle containing the Japanese encephalitis virus prM/M and E proteins are protected from lethal JEV infection. Virology. 1992;188:714–720. doi: 10.1016/0042-6822(92)90526-u. [DOI] [PubMed] [Google Scholar]
- 20.Konishi E, Takahashi J. Detection of chikungunya virus antigen in Aedes albopictus mosquitoes by enzyme-linked immunosorbent assay. J Virol Methods. 1985;12:279–285. doi: 10.1016/0166-0934(85)90139-9. [DOI] [PubMed] [Google Scholar]
- 21.Konishi E, Win K S, Kurane I, Mason P W, Shope R E, Ennis F A. Particulate vaccine candidate for Japanese encephalitis induces long-lasting virus-specific memory T lymphocytes in mice. Vaccine. 1997;15:281–286. doi: 10.1016/s0264-410x(96)00180-6. [DOI] [PubMed] [Google Scholar]
- 22.Konishi E, Yamaoka M, Kurane I, Mason P W. A DNA vaccine expressing dengue type 2 virus premembrane and envelope genes induces neutralizing antibody and memory B cells in mice. Vaccine. 2000;18:1133–1139. doi: 10.1016/s0264-410x(99)00376-x. [DOI] [PubMed] [Google Scholar]
- 23.Konishi E, Yamaoka M, Win K S, Kurane I, Mason P W. Induction of protective immunity against Japanese encephalitis in mice by immunization with a plasmid encoding Japanese encephalitis virus premembrane and envelope genes. J Virol. 1998;72:4925–4930. doi: 10.1128/jvi.72.6.4925-4930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao C L, Lin Y L, Shen S C, Shen J Y, Su H L, Huang Y L, Ma S H, Sun Y C, Chen K P, Chen L K. Antiapoptotic but not antiviral function of human bcl-2 assists establishment of Japanese encephalitis virus persistence in cultured cells. J Virol. 1998;72:9844–9854. doi: 10.1128/jvi.72.12.9844-9854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao C L, Lin Y L, Wang J J, Huang Y L, Yeh C T, Ma S H, Chen L K. Effect of enforced expression of human bcl-2 on Japanese encephalitis virus-induced apoptosis in cultured cells. J Virol. 1997;71:5963–5971. doi: 10.1128/jvi.71.8.5963-5971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo L, Li Y, Kang C Y. Expression of gag precursor protein and secretion of virus-like gag particles of HIV-2 from recombinant baculovirus-infected insect cells. Virology. 1990;179:874–880. doi: 10.1016/0042-6822(90)90159-o. [DOI] [PubMed] [Google Scholar]
- 27.Maeda J, Maeda A, Makino S. Release of coronavirus E protein in membrane vesicles from virus-infected cells and E protein-expressing cells. Virology. 1999;263:265–272. doi: 10.1006/viro.1999.9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason P W, Dalrymple J M, Gentry M K, McCown J M, Hoke C H, Jr, Burke D S, Fournier M J, Mason T L. Molecular characterization of a neutralizing domain of the Japanese encephalitis virus structural glycoprotein. J Gen Virol. 1989;70:2037–2049. doi: 10.1099/0022-1317-70-8-2037. [DOI] [PubMed] [Google Scholar]
- 29.Mason P W, Pincus S, Fournier M J, Mason T L, Shope R E, Paoletti E. Japanese encephalitis virus-vaccinia recombinants produce particulate forms of the structural membrane proteins and induce high levels of protection against lethal JEV infection. Virology. 1991;180:294–305. doi: 10.1016/0042-6822(91)90034-9. [DOI] [PubMed] [Google Scholar]
- 30.Matthews D J, Goodman L J, Gorman C M, Wells J A. A survey of furin substrate specificity using substrate phage display. Protein Sci. 1994;3:1197–1205. doi: 10.1002/pro.5560030805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuigan L C, Stallard V, Roos J M, Payne L G. Recombinant-expressed virus-like particle pseudotypes as an approach to vaccine development. Vaccine. 1993;11:675–678. doi: 10.1016/0264-410x(93)90316-p. [DOI] [PubMed] [Google Scholar]
- 32.Pincus S, Mason P W, Konishi E, Fonseca B A L, Shope R E, Rice C M, Paoletti E. Recombinant vaccinia virus producing the prM and E proteins of yellow fever virus protects mice from lethal yellow fever encephalitis. Virology. 1992;187:290–297. doi: 10.1016/0042-6822(92)90317-i. [DOI] [PubMed] [Google Scholar]
- 33.Qiu Z, Ou D, Hobman T C, Gillam S. Expression and characterization of virus-like particles containing rubella virus structural proteins. J Virol. 1994;68:4086–4091. doi: 10.1128/jvi.68.6.4086-4091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schalich J, Allison S L, Stiasny K, Mandl C W, Kunz C, Heinz F X. Recombinant subviral particles from tick-borne encephalitis virus are fusogenic and provide a model system for studying flavivirus envelope glycoprotein functions. J Virol. 1996;70:4549–4557. doi: 10.1128/jvi.70.7.4549-4557.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah P S, Gadkari D A. Persistent infection of porcine kidney cells with Japanese encephalitis virus. Indian J Med Res. 1987;85:481–491. [PubMed] [Google Scholar]
- 36.Shapiro D, Brandt W E, Russell P K. Change involving a viral membrane glycoprotein during morphogenesis of group B arboviruses. Virology. 1972;50:906–911. doi: 10.1016/0042-6822(72)90445-x. [DOI] [PubMed] [Google Scholar]
- 37.Stadler K, Allison S L, Schalich J, Heinz F X. Proteolytic activation of tick-borne encephalitis virus by furin. J Virol. 1997;71:8475–8481. doi: 10.1128/jvi.71.11.8475-8481.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Summers P L, Cohen W H, Ruiz M M, Hase T, Eckels K H. Flaviviruses can mediate fusion from without in Aedes albopictus mosquito cell cultures. Virus Res. 1989;12:383–392. doi: 10.1016/0168-1702(89)90095-6. [DOI] [PubMed] [Google Scholar]
- 39.Ueba N, Kimura T. Polykaryocytosis induced by certain arboviruses in monolayers of BHK-21-528 cells. J Gen Virol. 1977;34:369–373. doi: 10.1099/0022-1317-34-2-369. [DOI] [PubMed] [Google Scholar]
- 40.Walker J A, Molloy S S, Thomas G, Sakaguchi T, Yoshida T, Chambers T M, Kawaoka Y. Sequence specificity of furin, a proprotein-processing endoprotease, for the hemagglutinin of a virulent avian influenza virus. J Virol. 1994;68:1213–1218. doi: 10.1128/jvi.68.2.1213-1218.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]