Abstract
Purpose
Determine whether combining magnetic resonance imaging (MRI) observations and clinical tests could substantially improve sensitivity for diagnosis of long head of the biceps tendon (LHBT) pathology.
Methods
The authors retrospectively assessed a consecutive series of 140 patients who underwent arthroscopic rotator cuff repair for isolated supraspinatus tears. The presence of LHBT pathology was assessed preoperatively on MRI using three criteria and four clinical tests specific to shoulder injuries. Binary outcomes of MRI observations and four clinical tests were combined to identify combinations resulting in the best sensitivity using intra‐operative arthroscopic findings as reference.
Results
The study cohort comprised 100 shoulders (58 men and 42 women) aged 56.6 ± 9.4 years (range, 30–76) at index surgery. A total of 29 combinations were tested to obtain the best diagnostic algorithm for LHBT pathologies. Only four combinations reached a sensitivity ≥0.75, but had a specificity <0.45. The ‘Speed or Signal’ combination achieved the highest sensitivity (Se: 0.88; 95% confidence interval [CI]: 0.73%–0.96%; Sp: 0.20; 95% CI: 0.10%–0.33%).
Conclusion
The most important findings of this study were that, for the diagnosis of LHBT pathology using clinical tests alone, the Speed test had the highest sensitivity (Se, 0.74), and using MRI observations alone, the signal intensity had the highest sensitivity (Se, 0.68). Combination of ‘Speed test or Signal intensity’ substantially improved the sensitivity (Se, 0.88) but yielded the lowest specificity (Sp, 0.20). The clinical relevance of these findings is that using the combination ‘Speed or Signal’ for preoperative diagnosis, 88% of pathologic LHBTs would be correctly diagnosed, while 80% of healthy LHBTs could be misdiagnosed as pathologic.
Level of Evidence
Diagnostic study, Level IV.
Keywords: clinical tests, long head of biceps, magnetic resonance imaging, rotator cuff, tendinopathy
Abbreviations
- CTA
computed tomography arthrography
- FN
false negative
- FP
false positive
- LHBT
long head of the biceps tendon
- MRI
magnetic resonance imaging
- NPV
negative predictive value
- PPV
positive predictive value
- RCR
rotator cuff repair
- RCT
rotator cuff tear
- Se
sensitivity
- Sp
specificity
- TN
true negative
- TP
true positive
INTRODUCTION
When repairing rotator cuff tears (RCTs), the choice of whether or not to perform tenodesis or tenotomy of the long head of the biceps tendon (LHBT) is controversial [12, 19]. While some surgeons recommend systematic tenodesis or tenotomy regardless whether the LHBT is normal or pathologic [2, 16, 29], other surgeons suggest that tenodesis or tenotomy should only be performed in shoulders with LHBT pathology [4, 13, 15, 18, 21, 28, 30].
There is no clear evidence to support systematic LHBT procedures during rotator cuff repair (RCR). Conservation of a pathologic LHBT could seriously compromise clinical and functional outcomes, while tenodesis or tenotomy of a healthy LHBT can unnecessarily extend surgery time, cause postoperative pain, or result in the undesirable Popeye sign, negatively affecting the patients' quality of life [3, 26]. Therefore, preoperative diagnosis of the biceps should maximise sensitivity (reliability at detecting/ruling‐in pathology), even if it compromises specificity (reliability at eliminating/ruling‐out pathology) [17]. Two recent systematic reviews on diagnosis of LHBT pathology revealed that magnetic resonance imaging (MRI) has low sensitivity (range, 0.52–0.56) but high specificity (range, 0.64–0.99), while clinical tests have moderate sensitivity (range, 0.17–0.71) and specificity (range, 0.38–0.92) [11, 23]. Even though studies have been published on the diagnostic accuracy of either MRI or clinical tests, to the authors' knowledge, there are no published studies that attempted to improve the sensitivity or specificity of diagnosis of LHBT pathology, using combinations of MRI observations and clinical tests.
The purpose of the present study was therefore to determine whether combining MRI observations and clinical tests could substantially improve sensitivity for diagnosis of LHBT pathology (compared to MRI alone and clinical tests alone). The hypothesis was that a combination of MRI observations and clinical tests would grant higher sensitivity compared to MRI observations alone or clinical tests alone.
METHODS
The authors retrospectively included a consecutive series of 140 patients that underwent arthroscopic RCR for isolated supraspinatus tears, by eight orthopaedic surgeons at eight centres, between November 2019 and January 2021. Patients were excluded if they did not undergo preoperative MRI assessment (n = 39), or clinical testing (n = 1) (Figure 1). This left a study cohort of 100 patients in which biceps status was confirmed intra‐operatively during arthroscopy (considered as the ‘gold‐standard’ diagnosis).
Figure 1.
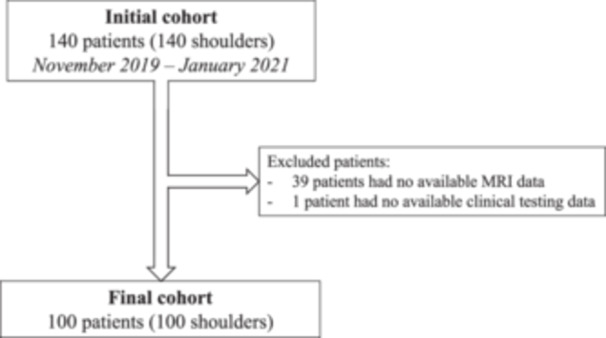
Flowchart following PRISMA guidelines.
Magnetic resonance imaging
The presence of LHBT pathology was assessed preoperatively on MRI by each surgeon using three binary observations: (i) thickening of diameter, (ii) subluxation or dislocation from the bicipital groove and (iii) presence of signal intensity (T2 hypersignal).
Clinical assessment
Patients were preoperatively assessed using four clinical tests specific to shoulder injuries: (i) Speed test [7], (ii) Yergason test [8], (iii) Kibler test [6] and (iv) bicipital groove tenderness. Each test rendered a binary outcome, positive in case of pain and therefore suspected pathologic LHBT, or negative if no pain was reported. Furthermore, the constant score and subjective shoulder value (SSV) were recorded following surgery.
Combining MRI observations with clinical tests
Considering intra‐operative arthroscopic findings as the ‘gold‐standard’, the authors tested various combinations of binary MRI observations and binary clinical test results, to identify the combination that renders the highest sensitivity. The numbers of true positives (TPs), true negatives (TNs), false positives (FPs) and false negatives (FNs) were calculated for 29 combinations of MRI observations and/or clinical tests (18 sets of two criteria, 10 sets of three criteria and 1 set of four criteria).
Ethical approval
All patients provided informed consent, and the study was approved by the local ethics committee (IRB:2018‐A01382‐53).
Statistical analysis
Descriptive statistics were used to summarise the data. The sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) were calculated for each of the 29 combinations of MRI observations and/or clinical tests. Statistical analyses were performed using R version 4.2.3 (R Foundation for Statistical Computing).
RESULTS
The study cohort comprised 100 shoulders (58 men and 42 women) aged 56.6 ± 9.4 years (range, 30–76) at index surgery, with a body mass index of 26.6 ± 4.4 (range, 18.4–42.7). Of the 100 patients, 71 were operated on their dominant shoulder (71%), and 17 were smokers (17%). Patients had a mean preoperative Constant score of 56.0 ± 12.7, and a mean preoperative SSV score of 51.7 ± 13.3 (Table 1).
Table 1.
Preoperative data.
| Final cohort (n = 100) | ||
|---|---|---|
| Mean ± SD | ||
| N (%) | Range | |
| Constant score | 56.0 ± 12.7 | 21–80 |
| SSV | 51.7 ± 13.3 | 20–80 |
| Range of motion | ||
| Passive forward elevation | 171 ± 17.0 | 90–180 |
| Passive abduction | 153 ± 24.3 | 90–180 |
| Passive external rotation 1 | 59 ± 13.7 | 30–90 |
| Passive external rotation 2 | 83 ± 11.4 | 35–90 |
| Active forward elevation | 152 ± 29.2 | 50–180 |
| Active abduction | 137 ± 31.8 | 50–180 |
| Active external rotation 1 | 50 ± 16.0 | 10–80 |
| Active external rotation 2 | 78 ± 14.7 | 30–90 |
| Active internal rotation | ||
| (0) Grand trochanter | 1 | 1% |
| (2) Buttock | 10 | 10% |
| (4) Sacrum | 5 | 5% |
| (6) L3 | 26 | 26% |
| (8) T12 | 32 | 32% |
| (10) T7 | 22 | 22% |
| C7 | 1 | 1% |
Note: External rotation 1, arm at side and elbow at 90° flexion; External rotation 2, arm at 90° abduction and elbow at 90° flexion.
Abbreviations: C7, cervical vertabra 7; L3, lumbar vertabra 3; N, cohort size; SD, standard deviation; SSV, subjective shoulder value; T7, thoracic vertabra 7; T12, thoracic vertabra 12.
Single criterion tests
Clinical testing resulted in 64 positive diagnoses for LHBT pathology using the Speed test, 28 using the Yergason test, 37 using the Kibler test and 50 using the bicipital groove tenderness test. MRI observations found 10 positive LHBT pathology diagnoses according to signal intensity, 19 with regard to the diameter and 9 with regard to position. Intra‐operative arthroscopic findings, considered as gold standard, positively identified 43 pathological LHBT (Table 2).
Table 2.
Individual diagnostic findings.
| Gold standard | Clinical tests | MRI evaluations | ||||||
|---|---|---|---|---|---|---|---|---|
| Arthroscopic findings | Speed | Yergason | Kibler | Pain on palpation | Signal anomaly | LHBT diameter | LHBT position | |
| Positive diagnosis for LHBT pathology | 43 | 64 | 28 | 37 | 50 | 10 | 19 | 9 |
| Negative diagnosis for LHBT pathology | 57 | 36 | 72 | 63 | 50 | 85 | 81 | 91 |
| True positives | 32 | 18 | 24 | 31 | 27 | 16 | 5 | |
| True negatives | 25 | 47 | 44 | 38 | 23 | 54 | 53 | |
| False positives | 32 | 10 | 13 | 19 | 32 | 3 | 4 | |
| False negatives | 11 | 25 | 19 | 12 | 13 | 27 | 38 | |
Abbreviations: LHBT, long head of the biceps tendon; MRI, magnetic resonance imaging.
The Speed's test had the highest sensitivity (0.74; 95% confidence interval [CI]: 0.59%–0.86%), followed by the bicipital groove tenderness test (0.72; 95% CI: 0.56%–0.85%) (Table 3).
Table 3.
Individual diagnostic accuracy.
| Sensitivity | Specificity | Accuracy | PPV | NPV | Se + Sp | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | TP | FP | FN | TN | Est. | Est. | Est. | Est. | Est. | ||||||||||||||||||||||
| Clinical test | |||||||||||||||||||||||||||||||
| Speed | 100 | 32 | 32 | 11 | 25 | 0.74 | (0.59–0.86) | 0.44 | (0.31–0.58) | 0.57 | (0.47–0.67) | 0.50 | (0.37–0.63) | 0.69 | (0.52–0.84) | 1.18 | |||||||||||||||
| Yergason | 100 | 18 | 10 | 25 | 47 | 0.42 | (0.27–0.58) | 0.82 | (0.70–0.91) | 0.65 | (0.55–0.74) | 0.64 | (0.44–0.81) | 0.65 | (0.53–0.76) | 1.24 | |||||||||||||||
| Kibler | 100 | 24 | 13 | 19 | 44 | 0.56 | (0.40–0.71) | 0.77 | (0.64–0.87) | 0.68 | (0.58–0.77) | 0.65 | (0.47–0.80) | 0.70 | (0.57–0.81) | 1.33 | |||||||||||||||
| Tenderness | 100 | 31 | 19 | 12 | 38 | 0.72 | (0.56–0.85) | 0.67 | (0.53–0.79) | 0.69 | (0.59–0.78) | 0.62 | (0.47–0.75) | 0.76 | (0.62–0.87) | 1.39 | |||||||||||||||
| MRI observation | |||||||||||||||||||||||||||||||
| Signal | 95 | 27 | 32 | 13 | 23 | 0.68 | (0.51–0.81) | 0.42 | (0.29–0.56) | 0.53 | (0.42–0.63) | 0.46 | (0.33–0.59) | 0.64 | (0.46–0.79) | 1.09 | |||||||||||||||
| Diameter | 100 | 16 | 3 | 27 | 54 | 0.37 | (0.23–0.53) | 0.95 | (0.85–0.99) | 0.70 | (0.60–0.79) | 0.84 | (0.60–0.97) | 0.67 | (0.55–0.77) | 1.32 | |||||||||||||||
| Position | 100 | 5 | 4 | 38 | 53 | 0.12 | (0.04–0.25) | 0.93 | (0.83–0.98) | 0.58 | (0.68–0.68) | 0.56 | (0.21–0.86) | 0.58 | (0.47–0.68) | 1.05 | |||||||||||||||
Abbreviations: Est., estimation; FN, false negative; FP, false positive; MRI, magnetic resonance imaging; N, cohort size; NPV, negative predictive value; PPV, positive predictive value; Se, sensitivity; Sp, specificity; TN, true negative; TP, true positive.
For MRI, the signal intensity had the highest sensitivity (0.68; 95% CI: 0.51%–0.81%), while both diameter and position had low sensitivity (0.37; 95% CI: 0.23%–0.53%; and 0.12; 95% CI: 0.04%–0.25%), but high specificity (0.95; 95% CI: 0.85%–0.99%; and 0.93; 95% CI: 0.83%–0.98%) (Table 3).
Multiple criteria tests
A total of 29 combinations were tested to obtain the best diagnostic algorithm for LHBT pathologies. Only four combinations reached a sensitivity ≥0.75, but had a specificity <0.45 (Table 4). The ‘Speed or Signal’ combination achieved the highest sensitivity (Se: 0.88; 95% CI: 0.73%–0.96%; Sp: 0.20; 95% CI: 0.10%–0.33%), followed by the ‘Speed or Diameter’ combination (Se: 0.78; 95% CI: 0.63%–0.89%; Sp: 0.42; 95% CI: 0.29%–0.56%), the ‘Speed or Position or Diameter’ combination (Se: 0.77; 95% CI: 0.61%–0.88%; Sp: 0.40; 95% CI: 0.28%–0.54%), and finally the ‘Yergason or Signal or Position’ combination (Se: 0.75; 95% CI: 0.59%–0.87%; Sp: 0.33; 95% CI: 0.21%–0.47%). Of these combinations, the best balance of sensitivity and specificity was achieved for the ‘Speed or Diameter’ combination (Se + Sp, 1.20).
Table 4.
Combined diagnostic accuracy.
| Sensitivity | Specificity | Accuracy | PPV | NPV | Se + Sp | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | TP | FP | FN | TN | Est. | Est. | Est. | Est. | Est. | ||||||||||||||||||||||
| Combination of two criteria | |||||||||||||||||||||||||||||||
| Speed AND Diameter | 100 | 15 | 2 | 28 | 55 | 0.35 | (0.21–0.51) | 0.96 | (0.88–1.00) | 0.70 | (0.60–0.79) | 0.88 | (0.64–0.99) | 0.66 | (0.55–0.76) | 1.31 | |||||||||||||||
| Speed OR Diameter | 100 | 33 | 33 | 10 | 24 | 0.78 | (0.63–0.89) | 0.42 | (0.29–0.56) | 0.58 | (0.48–0.68) | 0.51 | (0.39–0.64) | 0.71 | (0.53–0.85) | 1.20 | |||||||||||||||
| Speed AND Position | 100 | 5 | 3 | 38 | 54 | 0.12 | (0.04–0.25) | 0.95 | (0.85–0.99) | 0.59 | (0.49–0.69) | 0.63 | (0.24–0.91) | 0.59 | (0.48–0.69) | 1.06 | |||||||||||||||
| Speed OR Position | 100 | 32 | 33 | 11 | 24 | 0.74 | (0.59–0.86) | 0.42 | (0.29–0.56) | 0.56 | (0.46–0.66) | 0.49 | (0.37–0.62) | 0.69 | (0.51–0.83) | 1.17 | |||||||||||||||
| Speed AND Signal | 95 | 21 | 18 | 19 | 37 | 0.53 | (0.36–0.68) | 0.67 | (0.53–0.79) | 0.61 | (0.51–0.71) | 0.54 | (0.37–0.70) | 0.66 | (0.52–0.78) | 1.20 | |||||||||||||||
| Speed OR Signal | 95 | 35 | 44 | 5 | 11 | 0.88 | (0.73–0.96) | 0.20 | (0.10–0.33) | 0.48 | (0.38–0.59) | 0.44 | (0.33–0.56) | 0.69 | (0.41–0.89) | 1.08 | |||||||||||||||
| Yergason AND Diameter | 100 | 11 | 2 | 32 | 55 | 0.26 | (0.14–0.41) | 0.96 | (0.88–1.00) | 0.66 | (0.56–0.75) | 0.85 | (0.55–0.98) | 0.63 | (0.52–0.73) | 1.22 | |||||||||||||||
| Yergason OR Diameter | 100 | 23 | 11 | 20 | 46 | 0.53 | (0.38–0.69) | 0.81 | (0.68–0.90) | 0.69 | (0.59–0.78) | 0.68 | (0.49–0.83) | 0.70 | (0.57–0.80) | 1.34 | |||||||||||||||
| Yergason AND Position | 100 | 3 | 1 | 40 | 56 | 0.07 | (0.01–0.19) | 0.98 | (0.91–0.01) | 0.59 | (0.49–0.69) | 0.75 | (0.19–0.99) | 0.58 | (0.48–0.68) | 1.05 | |||||||||||||||
| Yergason OR Position | 100 | 20 | 13 | 23 | 44 | 0.47 | (0.31–0.62) | 0.77 | (0.64–0.87) | 0.64 | (0.54–0.73) | 0.61 | (0.42–0.77) | 0.66 | (0.53–0.77) | 1.24 | |||||||||||||||
| Yergason AND Signal | 95 | 14 | 4 | 26 | 51 | 0.35 | (0.21–0.52) | 0.93 | (0.82–0.98) | 0.68 | (0.58–0.78) | 0.78 | (0.52–0.94) | 0.66 | (0.55–0.77) | 1.28 | |||||||||||||||
| Yergason OR Signal | 95 | 29 | 36 | 11 | 19 | 0.73 | (0.56–0.85) | 0.35 | (0.22–0.49) | 0.51 | (0.40–0.61) | 0.45 | (0.32–0.57) | 0.63 | (0.44–0.80) | 1.07 | |||||||||||||||
| Speed AND Yergason | 100 | 17 | 9 | 26 | 48 | 0.40 | (0.25–0.56) | 0.84 | (0.72–0.93) | 0.65 | (0.55–0.74) | 0.65 | (0.44–0.83) | 0.65 | (0.53–0.76) | 1.24 | |||||||||||||||
| Speed AND Kibler | 100 | 24 | 12 | 19 | 45 | 0.56 | (0.40–0.71) | 0.79 | (0.66–0.89) | 0.69 | (0.59–0.78) | 0.67 | (0.49–0.81) | 0.70 | (0.58–0.81) | 1.35 | |||||||||||||||
| Speed AND Tenderness | 100 | 28 | 17 | 15 | 40 | 0.65 | (0.49–0.79) | 0.70 | (0.57–0.82) | 0.68 | (0.58–0.77) | 0.62 | (0.47–0.76) | 0.73 | (0.59–0.84) | 1.35 | |||||||||||||||
| Yergason AND Kibler | 100 | 13 | 8 | 30 | 49 | 0.30 | (0.17–0.46) | 0.86 | (0.74–0.94) | 0.62 | (0.52–0.72) | 0.62 | (0.38–0.82) | 0.62 | (0.50–0.73) | 1.16 | |||||||||||||||
| Yergason AND Tenderness | 100 | 17 | 7 | 26 | 50 | 0.40 | (0.25–0.56) | 0.88 | (0.76–0.95) | 0.67 | (0.57–0.76) | 0.71 | (0.49–0.87) | 0.66 | (0.54–0.76) | 1.27 | |||||||||||||||
| Kibler AND Tenderness | 100 | 21 | 7 | 22 | 50 | 0.49 | (0.33–0.65) | 0.88 | (0.76–0.95) | 0.71 | (0.61–0.80) | 0.75 | (0.55–0.89) | 0.70 | (0.57–0.80) | 1.37 | |||||||||||||||
| Combination of three criteria | |||||||||||||||||||||||||||||||
| Speed AND Position AND Diameter | 100 | 4 | 0 | 39 | 57 | 0.09 | (0.03–0.22) | 1.00 | (0.94–1.00) | 0.61 | (0.51–0.71) | 1.00 | (0.40–1.00) | 0.59 | (0.49–0.69) | 1.09 | |||||||||||||||
| Speed AND Position OR Diameter | 100 | 17 | 6 | 26 | 51 | 0.40 | (0.25–0.56) | 0.89 | (0.78–0.96) | 0.68 | (0.58–0.77) | 0.74 | (0.52–0.90) | 0.66 | (0.55–0.77) | 1.29 | |||||||||||||||
| Speed OR Position AND Diameter | 100 | 15 | 2 | 28 | 55 | 0.35 | (0.21–0.51) | 0.96 | (0.88–1.00) | 0.70 | (0.60–0.79) | 0.88 | (0.64–0.99) | 0.66 | (0.55–0.76) | 1.31 | |||||||||||||||
| Speed OR Position OR Diameter | 100 | 33 | 34 | 10 | 23 | 0.77 | (0.61–0.88) | 0.40 | (0.28–0.54) | 0.56 | (0.46–0.66) | 0.49 | (0.37–0.62) | 0.70 | (0.51–0.84) | 1.17 | |||||||||||||||
| Yergason OR Signal AND Position | 95 | 4 | 3 | 36 | 52 | 0.10 | (0.03–0.24) | 0.95 | (0.85–0.99) | 0.59 | (0.48–0.69) | 0.57 | (0.18–0.90) | 0.59 | (0.48–0.69) | 1.05 | |||||||||||||||
| Yergason OR Signal OR Position | 95 | 30 | 37 | 10 | 18 | 0.75 | (0.59–0.87) | 0.33 | (0.21–0.47) | 0.51 | (0.40–0.61) | 0.45 | (0.33–0.57) | 0.64 | (0.44–0.81) | 1.08 | |||||||||||||||
| Speed AND Yergason AND Kibler | 100 | 13 | 8 | 30 | 49 | 0.30 | (0.17–0.46) | 0.86 | (0.74–0.94) | 0.62 | (0.52–0.72) | 0.62 | (0.38–0.82) | 0.62 | (0.50–0.73) | 1.16 | |||||||||||||||
| Speed AND Yergason AND Tenderness | 100 | 17 | 6 | 26 | 51 | 0.40 | (0.25–0.56) | 0.90 | (0.78–0.96) | 0.68 | (0.58–0.77) | 0.74 | (0.52–0.90) | 0.66 | (0.55–0.77) | 1.29 | |||||||||||||||
| Speed AND Kibler AND Tenderness | 100 | 21 | 7 | 22 | 50 | 0.49 | (0.33–0.65) | 0.88 | (0.76–0.95) | 0.71 | (0.61–0.80) | 0.75 | (0.55–0.89) | 0.70 | (0.57–0.80) | 1.37 | |||||||||||||||
| Yergason AND Kibler AND Tenderness | 100 | 13 | 5 | 30 | 52 | 0.30 | (0.17–0.46) | 0.91 | (0.81–0.97) | 0.65 | (0.55–0.74) | 0.72 | (0.47–0.90) | 0.63 | (0.52–0.74) | 1.22 | |||||||||||||||
| Combination of four criteria | |||||||||||||||||||||||||||||||
| Speed AND Yergason AND Kibler AND Tenderness | 100 | 13 | 5 | 30 | 52 | 0.30 | (0.17–0.46) | 0.91 | (0.81–0.97) | 0.65 | (0.55–0.74) | 0.72 | (0.47–0.90) | 0.63 | (0.52–0.74) | 1.22 | |||||||||||||||
Abbreviations: Est., estimation; FN, false negative; FP, false positive; MRI, magnetic resonance imaging; N, cohort size; NPV, negative predictive value; PPV, positive predictive value; Se, sensitivity; Sp, specificity; TN, true negative; TP, true positive.
DISCUSSION
The most important findings of this study were that, for the diagnosis of LHBT pathology the combination of ‘Speed test or Signal intensity’ substantially improved the sensitivity (Se, 0.88) but yielded the lowest specificity (Sp, 0.20). These findings confirm the hypothesis that a combination of MRI observations and clinical tests grants higher sensitivity compared to MRI observations alone or clinical tests alone. The clinical relevance of these findings is that using the combination ‘Speed or Signal’ for preoperative diagnosis, 88% of pathologic LHBTs would be correctly diagnosed, while 80% of healthy LHBTs could be misdiagnosed as pathologic.
The best clinical test for preoperative assessment of the state of the LHBT has been previously investigated, and a number of tests have been created for the shoulder or the biceps. Holtby et al. [20] reported diagnostic values for the Speed and Yergason tests, and found a sensitivity of 0.32 and 0.43, respectively. In 2007, Gill et al. [15] investigated the diagnostic values of 10 clinical tests for LHBT tear, and found sensitivity values ranging from 0.17 to 0.68, the highest being for the active compression palm down test. The diagnostic values reported in the studies are insufficient for reliable preoperative diagnosis of LHBT pathology. In a systematic review by Rosas et al. [25], the authors developed a practical, evidence‐based clinical examination algorithm to accurately diagnose patients with LHBT pathology. Rosas et al. found the highest sensitivity (Se, 0.88) by combining bicipital groove tenderness (Se, 0.57) with the uppercut test (Se, 0.73). In contrast, the present study found sensitivities ranging from 0.42 to 0.74 for individual clinical tests, while the combination that provided the highest sensitivity of 0.65 was obtained when using ‘Speed and Tenderness’. The present study indicates that combining clinical tests only does not provide adequate sensitivity for the assessment of LHBT pathology.
As for clinical tests, the sensitivity of MRI findings has been investigated in the literature. In 2019, Kim et al. [22] assessed the diagnostic value for MRI findings of abnormal signs (diameter, contour irregularity, and signal alteration), which resulted in a sensitivity of 0.52–0.67 in the parasagittal view, and 0.58–0.67 in the axial view. Shibayama et al. [27] investigated LHBT diameter and change in signal intensity as diagnostic criteria using MRI and found a higher sensitivity using diameter (Se, 0.84) compared to change in signal intensity (Se, 0.52). Finally, in a meta‐analysis by Lalevée et al. [23], the sensitivity of MRI for diagnosis of LHBT pathologies was investigated by type of pathology. Lalevée et al. [23] reported a pooled sensitivity of 0.56 for full‐thickness LHBT tears, 0.52 for partial‐thickness LHBT tears and 0.58 for any LHBT tear. In the present study, the sensitivity of individual MRI observations ranged from 0.12 to 0.68. Individual MRI findings seemingly have low to moderate sensitivity in diagnosing any type of LHBT lesion, while the combination of MRI findings and clinical tests substantially improved the sensitivity (Se, 0.88).
Lesions of the LHBT are a common cause of pain and dysfunction in shoulders with RCTs, and early diagnosis and treatment can prevent further degeneration of the LHBT [6, 9, 10, 23, 24]. The most common treatment options are tenotomy or tenodesis [5, 18]. In 2005, Walch et al. [29] reported that spontaneous rupture of the LHBT during the evolution of RCTs resulted in pain relief, and henceforth popularised systematic tenodesis or tenotomy of the LHBT during RCR [18], even when the LHBT showed no macroscopic signs of pathology. While satisfactory outcomes have been reported for both tenodesis and tenotomy, there is yet no consensus regarding the best treatment, as both can lead to complications [1, 28]. Tenotomy is often associated with the occurrence of a Popeye sign and a decrease in strength, while tenodesis requires longer recovery time, and can result in cramps [5, 14, 18]. There is a general consensus, however, that tenotomy or tenodesis is necessary if the LHBT is pathologic during RCR, to avoid residual bicipital pain and the need for reoperation, which is why diagnosis requires high sensitivity [15, 20, 22, 23, 25, 27].
The present study revealed that a combination of clinical tests and MRI findings (Se, 0.88) resulted in a higher sensitivity than clinical tests (Se, 0.74) or MRI findings alone (Se, 0.68). Correct preoperative assessment is important, as it allows to manage patients' expectations and intra‐operative time. Nonetheless, intra‐operative arthroscopic findings could serve as the final assessment of the intra‐articular portion of LHBT, during which a pathologic LHBT that was misdiagnosed as healthy, would still be correctly treated. To the author's knowledge, no other study has investigated the combination of clinical tests and MRI to detect LHBT pathologies. The present study, however, found a sensitivity of 0.88 by using the ‘Speed or Signal’. In a meta‐analysis by Courage et al. [11], comparing diagnostic values of clinical tests versus ultrasound findings, the pooled sensitivity of ultrasound assessment of LHBT was 0.70, which is more than what was found in the literature, as well as in the present study for MRI assessment. Even though the present study found a high sensitivity, it is possible that future studies using other imaging modalities or combinations thereof, find even higher sensitivities. Future studies should evaluate the reliability of ultrasound, as an alternative to MRI, to maximise sensitivity of diagnosis of LHBT pathologies, as ultrasound is considerably faster and cheaper to perform, and therefore could facilitate decision‐making during initial patient assessment.
The findings of the present study should be interpreted with the following limitations in mind. First, the number of patients excluded for missing MRI assessment was approximately 40%, as some patients had computed tomography arthrography (CTA) instead of MRI. Second, for five patients, signal intensity was not measured on MRI, which could leave doubts on the reliability of this single criterion, and its use as diagnostic criteria. Third, as some MRI criteria can be subjective to interpret, it is possible that MRI readings might have differed between the eight surgeons since interobserver repeatability was not assessed, and the ultimate diagnostic repeatability of the combinations used cannot be ascertained, although the authors had sufficient confidence in the repeatability of MRI as reported in numerous previous studies [22, 27]. Finally, the present study is based on MRI assessments by surgeons, which could differ from assessments by radiologists. It is worth noting, however, that the eight surgeons had agreed on common assessment criteria, while the undetermined number of radiologists could have increased heterogeneity. Nonetheless, the strength of the present study is that it is the first to investigate the combination of clinical tests and MRI observations for the diagnosis of LHBT pathology, which could provide greater diagnostic value for clinicians.
CONCLUSION
For the diagnosis of LHBT pathologies, the combination ‘Speed or Signal’ could diagnose 88% of pathologic LHBTs correctly, while 80% of healthy LHBTs could be misdiagnosed as pathologic.
AUTHOR CONTRIBUTIONS
David Gallinet and Jacques Guery: Funding acquisition; data collection; methodology; supervision; validation. Maxime Antoni, Julien Berhouet and Christophe Charousset: Data collection; methodology; validation. Chinyelum Agu, Floris van Rooij and Mo Saffarini: Methodology; manuscript writing; formal analysis; validation.
CONFLICT OF INTEREST STATEMENT
David Gallinet reports consulting and royalties from moveUP outside the submitted work. Maxime Antoni reports fees from ConMed and fees and royalties FX Shoulder Solutions outside the submitted work. Julien Berhouet reports consulting for Wright Medical outside the submitted work. Jacques Guery reports fees from moveUP outside of the submitted work. The remaining authors declare no conflict of interest.
ETHICS STATEMENT
All patients provided informed consent and the study was approved by the local ethics committee (IRB: 2018‐A01382‐53).
ACKNOWLEDGMENTS
The authors are grateful to ‘ELSAN’ for funding statistical analyses and manuscript writing.
Gallinet, D. , Antoni, M. , ReSurg, Berhouet, J. , Charousset, C. & Guery, J. (2024) MRI findings and clinical testing for preoperative diagnosis of long head of the biceps pathology. Journal of Experimental Orthopaedics, 11, e70050. 10.1002/jeo2.70050
Contributor Information
ReSurg:
DATA AVAILABILITY STATEMENT
All data are available upon reasonable request.
REFERENCES
- 1. Abraham, V.T. , Tan, B.H.M. & Kumar, V.P. (2016) Systematic review of biceps tenodesis: arthroscopic versus open. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 32(2), 365–371. Available from: 10.1016/j.arthro.2015.07.028 [DOI] [PubMed] [Google Scholar]
- 2. Ahrens, P.M. & Boileau, P. (2007) The long head of biceps and associated tendinopathy. The Journal of Bone and Joint Surgery. British Volume, 89(8), 1001–1009. Available from: 10.1302/0301-620X.89B8.19278 [DOI] [PubMed] [Google Scholar]
- 3. Aldon‐Villegas, R. , Perez‐Cabezas, V. & Chamorro‐Moriana, G. (2021) Efficacy of management of associated dysfunctions on rotator cuff and long head of the biceps: systematic review. Journal of Orthopaedic Surgery and Research, 16(1), 501. Available from: 10.1186/s13018-021-02621-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baumgarten, K.M. , Chang, P.S. & Foley, E.K. (2019) Patient‐determined outcomes after arthroscopic rotator cuff repair with and without biceps tenodesis utilizing the PITT technique. Journal of Shoulder and Elbow Surgery, 28(6), 1049–1055. Available from: 10.1016/j.jse.2019.01.024 [DOI] [PubMed] [Google Scholar]
- 5. Belk, J.W. , Kraeutler, M.J. , Houck, D.A. , Chrisman, A.N. , Scillia, A.J. & McCarty, E.C. (2021) Biceps tenodesis versus tenotomy: a systematic review and meta‐analysis of level I randomized controlled trials. Journal of Shoulder and Elbow Surgery, 30(5), 951–960. Available from: 10.1016/j.jse.2020.11.012 [DOI] [PubMed] [Google Scholar]
- 6. Ben Kibler, W. , Sciascia, A.D. , Hester, P. , Dome, D. & Jacobs, C. (2009) Clinical utility of traditional and new tests in the diagnosis of biceps tendon injuries and superior labrum anterior and posterior lesions in the shoulder. The American Journal of Sports Medicine, 37(9), 1840–1847. Available from: 10.1177/0363546509332505 [DOI] [PubMed] [Google Scholar]
- 7. Bennett, W. (1998) Specificity of the Speed's test: arthroscopic technique for evaluating the biceps tendon at the level of the bicipital groove. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 14(8), 789–796. Available from: 10.1016/S0749-8063(98)70012-X [DOI] [PubMed] [Google Scholar]
- 8. Calis, M. (2000) Diagnostic values of clinical diagnostic tests in subacromial impingement syndrome. Annals of the Rheumatic Diseases, 59(1), 44–47. Available from: 10.1136/ard.59.1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen, H.S. , Lin, S.H. , Hsu, Y.H. , Chen, S.C. & Kang, J.H. (2011) A comparison of physical examinations with musculoskeletal ultrasound in the diagnosis of biceps long head tendinitis. Ultrasound in Medicine & Biology, 37(9), 1392–1398. Available from: 10.1016/j.ultrasmedbio.2011.05.842 [DOI] [PubMed] [Google Scholar]
- 10. Churgay, C.A. (2009) Diagnosis and treatment of biceps tendinitis and tendinosis. American Family Physician, 80(5), 470–476. [PubMed] [Google Scholar]
- 11. Courage, O. , van Rooij, F. & Saffarini, M. (2023) Ultrasound is more reliable than clinical tests to both confirm and rule out pathologies of the long head of the biceps: a systematic review and meta‐analysis. Knee Surgery, Sports Traumatology, Arthroscopy, 31(2), 662–671. Available from: 10.1007/s00167-022-07154-5 [DOI] [PubMed] [Google Scholar]
- 12. Descamps, J. , Kierszbaum, E. , Protais, M. , Marion, B. , Bouché, P.A. & Aïm, F. (2023) Outcomes of isolated biceps tenodesis/tenotomy or partial rotator cuff repair associated with biceps tenodesis/tenotomy for massive irreparable tears: a systematic review. Journal of Clinical Medicine, 12(7), 2565. Available from: 10.3390/jcm12072565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erickson, B.J. , Basques, B.A. , Griffin, J.W. , Taylor, S.A. , O'Brien, S.J. , Verma, N.N. et al. (2017) The effect of concomitant biceps tenodesis on reoperation rates after rotator cuff repair: a review of a large private‐payer database from 2007 to 2014. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 33(7), 1301–1307.e1. Available from: 10.1016/j.arthro.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 14. Forsythe, B. , Patel, H.H. , Berlinberg, E.J. , Forlenza, E.M. , Okoroha, K.R. , Williams, B.T. et al. (2023) A radiostereometric analysis of tendon migration after arthroscopic and mini‐open biceps tenodesis: interference screw versus single suture anchor fixation. The American Journal of Sports Medicine, 51(11), 2869–2880. Available from: 10.1177/03635465231187030 [DOI] [PubMed] [Google Scholar]
- 15. Gill, H.S. , El Rassi, G. , Bahk, M.S. , Castillo, R.C. & McFarland, E.G. (2007) Physical examination for partial tears of the biceps tendon. The American Journal of Sports Medicine, 35(8), 1334–1340. Available from: 10.1177/0363546507300058 [DOI] [PubMed] [Google Scholar]
- 16. Gill, T.J. , McIrvin, E. , Mair, S.D. & Hawkins, R.J. (2001) Results of biceps tenotomy for treatment of pathology of the long head of the biceps brachii. Journal of Shoulder and Elbow Surgery, 10(3), 247–249. Available from: 10.1067/mse.2001.114259 [DOI] [PubMed] [Google Scholar]
- 17. Gilmer, B.B. , DeMers, A.M. , Guerrero, D. , Reid, J.B. , Lubowitz, J.H. & Guttmann, D. (2015) Arthroscopic versus open comparison of long head of biceps tendon visualization and pathology in patients requiring tenodesis. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 31(1), 29–34. Available from: 10.1016/j.arthro.2014.07.025 [DOI] [PubMed] [Google Scholar]
- 18. Godenèche, A. , Kempf, J.F. , Nové‐Josserand, L. , Michelet, A. , Saffarini, M. , Hannink, G. et al. (2018) Tenodesis renders better results than tenotomy in repairs of isolated supraspinatus tears with pathologic biceps. Journal of Shoulder and Elbow Surgery, 27(11), 1939–1945. Available from: 10.1016/j.jse.2018.03.030 [DOI] [PubMed] [Google Scholar]
- 19. Hartland, A.W. , Islam, R. , Teoh, K.H. & Rashid, M.S. (2022) Clinical effectiveness of tenotomy versus tenodesis for long head of biceps pathology: a systematic review and meta‐analysis. BMJ Open, 12(10), e061954. Available from: 10.1136/bmjopen-2022-061954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holtby, R. & Razmjou, H. (2004) Accuracy of the Speed's and Yergason's tests in detecting biceps pathology and SLAP lesions: comparison with arthroscopic findings. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 20(3), 231–236. Available from: 10.1016/j.arthro.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 21. Keong, M.W. & Tjoen, D.L.T. (2018) Does bicep pathology affect rotator cuff repair outcomes? Journal of Orthopaedic Surgery, 26(1), 2309499018762852. Available from: 10.1177/2309499018762852. [DOI] [PubMed] [Google Scholar]
- 22. Kim, J.Y. , Rhee, S.M. & Rhee, Y.G. (2019) Accuracy of MRI in diagnosing intra‐articular pathology of the long head of the biceps tendon: results with a large cohort of patients. BMC Musculoskeletal Disorders, 20(1), 270. Available from: 10.1186/s12891-019-2654-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lalevée, M. , van Rooij, F. , Nover, L. , Kumble, A. , Saffarini, M. & Courage, O. (2022) 3D imaging has good specificity but poor sensitivity for the diagnosis of pathologies of the long head of the biceps: a systematic review and meta‐analysis. Knee Surgery, Sports Traumatology, Arthroscopy, 30(7), 2510–2520. Available from: 10.1007/s00167-022-06873-z [DOI] [PubMed] [Google Scholar]
- 24. Nascimento, A.T. & Claudio, G.K. (2017) Avaliação da ressonância magnética sem contraste como método para diagnóstico de lesões parciais do tendão da cabeça longa do bíceps. Revista Brasileira de Ortopedia, 52(1), 40–45. Available from: 10.1016/j.rbo.2016.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rosas, S. , Krill, M.K. , Amoo‐Achampong, K. , Kwon, K. , Nwachukwu, B.U. & McCormick, F. (2017) A practical, evidence‐based, comprehensive (PEC) physical examination for diagnosing pathology of the long head of the biceps. Journal of Shoulder and Elbow Surgery, 26(8), 1484–1492. Available from: 10.1016/j.jse.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rudisill, S.S. , Best, M.J. & O'Donnell, E.A. (2021) Clinical outcomes of revision biceps tenodesis for failed long head of biceps surgery: a systematic review. Arthroscopy: The Journal of Arthroscopic & Related Surgery, 37(12), 3529–3536. Available from: 10.1016/j.arthro.2021.04.063 [DOI] [PubMed] [Google Scholar]
- 27. Shibayama, Y. , Hirose, T. , Sugi, A. , Mizushima, E. , Watanabe, Y. , Tomii, R. et al. (2022) Diagnostic accuracy of magnetic resonance imaging for partial tears of the long head of the biceps tendon in patients with rotator cuff tears. JSES International, 6(4), 638–642. Available from: 10.1016/j.jseint.2022.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vigié, R. , Bonnevialle, N. , Hao, K.A. , Berhouet, J. & Charousset, C. (2023) Tenotomy or tenodesis versus conservation of the long head of the biceps tendon in the repair of isolated supraspinatus tears: a systematic review of the literature. Orthopaedics & Traumatology: Surgery & Research, 109(8s), 103673. Available from: 10.1016/j.otsr.2023.103673. [DOI] [PubMed] [Google Scholar]
- 29. Walch, G. , Edwards, T.B. , Boulahia, A. , Nové‐Josserand, L. , Neyton, L. & Szabo, I. (2005) Arthroscopic tenotomy of the long head of the biceps in the treatment of rotator cuff tears: clinical and radiographic results of 307 cases. Journal of Shoulder and Elbow Surgery, 14(3), 238–246. Available from: 10.1016/j.jse.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 30. Watson, S.T. , Robbins, C.B. , Bedi, A. , Carpenter, J.E. , Gagnier, J.J. & Miller, B.S. (2017) Comparison of outcomes 1 year after rotator cuff repair with and without concomitant biceps surgery. Arthroscopy, 33(11), 1928–1936. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon reasonable request.


