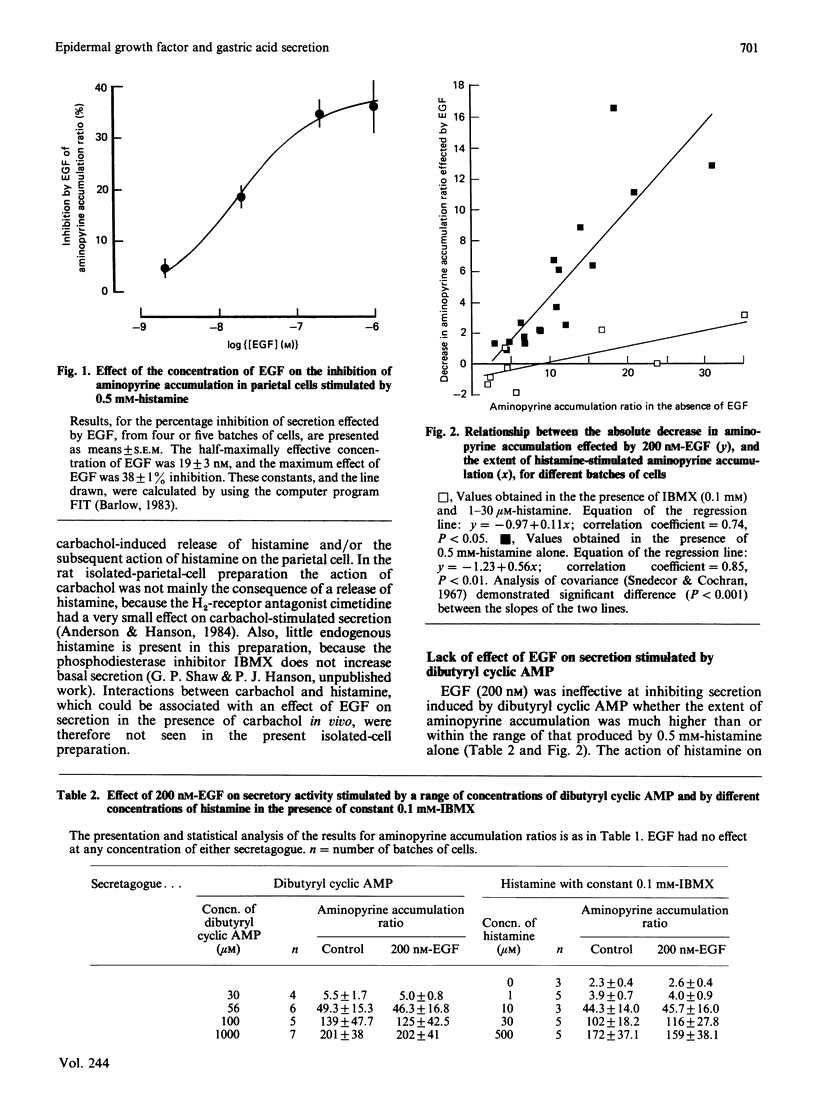
Abstract
The site and mechanism of action of epidermal growth factor (EGF) on acid secretion by rat isolated parietal cells were investigated by using the intracellular accumulation of the weak base aminopyrine as an index of secretory activity. When parietal cells were stimulated with histamine (0.5 mM), the concentration of EGF required for half-maximal inhibition of acid secretion was 19 nM, with a maximally effective concentration of EGF producing 38% inhibition of secretory activity. EGF did not inhibit secretion stimulated by 0.1 mM-carbachol, or by 30 microM-, 56 microM-, 100 microM- or 1000 microM-dibutyryl cyclic AMP, low concentrations of which produced a secretory response comparable with that obtained with 0.5 mM-histamine. Addition of 0.1 mM-3-isobutyl-1-methylxanthine (IBMX) substantially increased aminopyrine accumulation in the presence of 0.5 mM-histamine. The inhibitory action of EGF on histamine-stimulated secretion was blocked by 0.1 mM-IBMX, even if low concentrations of histamine were used to generate aminopyrine accumulation ratios similar to those obtained with 0.5 mM-histamine alone. The cyclo-oxygenase inhibitor flurbiprofen (1-100 microM) and the cyclo-oxygenase and lipoxygenase inhibitor nordihydroguaiaretic acid (10-100 microM) did not affect the inhibitory action of EGF. The pattern of inhibition of secretion produced by the activator of Ca2+-sensitive phospholipid-dependent protein kinase, 12-O-tetradecanoylphorbol 13-acetate, was markedly different from that produced by EGF. In conclusion, a major site of the action of EGF on acid secretion in the intact stomach is probably a decrease in the stimulatory effect of histamine by a mechanism which does not involve Ca2+-sensitive phospholipid-dependent protein kinase or the production of prostaglandins, but which might involve enhancement of cyclic AMP phosphodiesterase activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Hanson P. J. Inhibition of gastric acid secretion by a phorbol ester: effect of 12-O-tetradecanoylphorbol-13-acetate on aminopyrine accumulation by rat parietal cells. Biochem Biophys Res Commun. 1984 Jun 15;121(2):566–572. doi: 10.1016/0006-291x(84)90219-5. [DOI] [PubMed] [Google Scholar]
- Anderson N. G., Hanson P. J. Involvement of calcium-sensitive phospholipid-dependent protein kinase in control of acid secretion by isolated rat parietal cells. Biochem J. 1985 Dec 1;232(2):609–611. doi: 10.1042/bj2320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglindh T., Helander H. F., Obrink K. J. Effects of secretagogues on oxygen consumption, aminopyrine accumulation and morphology in isolated gastric glands. Acta Physiol Scand. 1976 Aug;97(4):401–414. doi: 10.1111/j.1748-1716.1976.tb10281.x. [DOI] [PubMed] [Google Scholar]
- Bower J. M., Camble R., Gregory H., Gerring E. L., Willshire I. R. The inhibition of gastric acid secretion by epidermal growth factor. Experientia. 1975 Jul 15;31(7):825–826. doi: 10.1007/BF01938488. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Chew C. S., Hersey S. J., Sachs G., Berglindh T. Histamine responsiveness of isolated gastric glands. Am J Physiol. 1980 Apr;238(4):G312–G320. doi: 10.1152/ajpgi.1980.238.4.G312. [DOI] [PubMed] [Google Scholar]
- Chiba T., Hirata Y., Taminato T., Kadowaki S., Matsukura S., Fujita T. Epidermal growth factor stimulates prostaglandin E release from isolated perfused rat stomach. Biochem Biophys Res Commun. 1982 Mar 15;105(1):370–374. doi: 10.1016/s0006-291x(82)80054-5. [DOI] [PubMed] [Google Scholar]
- Dembiński A., Gregory H., Konturek S. J., Polański M. Trophic action of epidermal growth factor on the pancreas and gastroduodenal mucosa in rats. J Physiol. 1982 Apr;325:35–42. doi: 10.1113/jphysiol.1982.sp014133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. B., Williams G., Lacey E., Gregory H. Cellular localisation of human urogastrone/epidermal growth factor. Nature. 1978 Feb 2;271(5644):466–467. doi: 10.1038/271466a0. [DOI] [PubMed] [Google Scholar]
- Finke U., Rutten M., Murphy R. A., Silen W. Effects of epidermal growth factor on acid secretion from guinea pig gastric mucosa: in vitro analysis. Gastroenterology. 1985 May;88(5 Pt 1):1175–1182. doi: 10.1016/s0016-5085(85)80077-9. [DOI] [PubMed] [Google Scholar]
- Gerber J. G., Fadul S., Payne N. A., Nies A. S. Adenosine: a modulator of gastric acid secretion in vivo. J Pharmacol Exp Ther. 1984 Oct;231(1):109–113. [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Ito S., Lacy E. R. Morphology of rat gastric mucosal damage, defense, and restitution in the presence of luminal ethanol. Gastroenterology. 1985 Jan;88(1 Pt 2):250–260. doi: 10.1016/s0016-5085(85)80178-5. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Brzozowski T., Piastucki I., Dembinski A., Radecki T., Dembinska-Kiec A., Zmuda A., Gregory H. Role of mucosal prostaglandins and DNA synthesis in gastric cytoprotection by luminal epidermal growth factor. Gut. 1981 Nov;22(11):927–932. doi: 10.1136/gut.22.11.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek S. J., Cieszkowski M., Jaworek J., Konturek J., Brzozowski T., Gregory H. Effects of epidermal growth factor on gastrointestinal secretions. Am J Physiol. 1984 May;246(5 Pt 1):G580–G586. doi: 10.1152/ajpgi.1984.246.5.G580. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Radecki T., Brzozowski T., Piastucki I., Dembiński A., Dembińska-Kieć A., Zmuda A., Gryglewski R., Gregory H. Gastric cytoprotection by epidermal growth factor. Role of endogenous prostaglandins and DNA synthesis. Gastroenterology. 1981 Sep;81(3):438–443. [PubMed] [Google Scholar]
- MacAdams M. R., Pek S. B., Lands W. E. The effect of flurbiprofen, a potent inhibitor of prostaglandin synthesis, on insulin and glucagon release from isolated rat pancreas. Endocrinology. 1984 Apr;114(4):1364–1370. doi: 10.1210/endo-114-4-1364. [DOI] [PubMed] [Google Scholar]
- Major J. S., Scholes P. The localization of a histamine H2-receptor adenylate cyclase system in canine parietal cells and its inhibition by prostaglandins. Agents Actions. 1978 Jun;8(4):324–331. doi: 10.1007/BF01968611. [DOI] [PubMed] [Google Scholar]
- Nylander O., Berglindh T., Obrink K. J. Prostaglandin interaction with histamine release and parietal cell activity in isolated gastric glands. Am J Physiol. 1986 May;250(5 Pt 1):G607–G616. doi: 10.1152/ajpgi.1986.250.5.G607. [DOI] [PubMed] [Google Scholar]
- Oka Y., Orth D. N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983 Jul;72(1):249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce J. B., Baird A. W., Williamson E. M., Evans F. J. Inhibition of histamine-induced acid secretion in rat isolated gastric mucosa by esters of phorbol and 12-deoxyphorbol. J Pharm Pharmacol. 1981 Nov;33(11):737–738. doi: 10.1111/j.2042-7158.1981.tb13919.x. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K. Epidermal growth factor-dependent phosphorylation of lipocortin. Nature. 1986 May 1;321(6065):81–84. doi: 10.1038/321081a0. [DOI] [PubMed] [Google Scholar]
- Sahai A., Smith K. B., Panneerselvam M., Salomon D. S. Activation of calcium and phospholipid-dependent protein kinase by epidermal growth factor (EGF) in A431 cells: attenuation by 12-0-tetradecanoylphorbol-13-acetate (TPA). Biochem Biophys Res Commun. 1982 Dec 31;109(4):1206–1214. doi: 10.1016/0006-291x(82)91905-2. [DOI] [PubMed] [Google Scholar]
- Schepp W., Ruoff H. J., Maśliński S. Aminopyrine accumulation of isolated parietal cells from the rat stomach. Effect of histamine and interaction with endogenous inhibitors. Arch Int Pharmacodyn Ther. 1983 Oct;265(2):293–308. [PubMed] [Google Scholar]
- Shaw G. P., Anderson N. G., Hanson P. J. Metabolism and gastric acid secretion. Substrate-dependency of aminopyrine accumulation in isolated rat parietal cells. Biochem J. 1985 Apr 1;227(1):223–229. doi: 10.1042/bj2270223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. P., Hanson P. J. Inhibitory effect of 12-O-tetradecanoylphorbol 13-acetate on acid secretion by rat stomach in vivo. FEBS Lett. 1986 Jun 9;201(2):225–228. doi: 10.1016/0014-5793(86)80613-5. [DOI] [PubMed] [Google Scholar]
- Soll A. H. Potentiating interactions of gastric stimulants on [14 C] aminopyrine accumulation by isolated canine parietal cells. Gastroenterology. 1982 Jul;83(1 Pt 2):216–223. [PubMed] [Google Scholar]
- Soll A. H. Secretagogue stimulation of [14C]aminopyrine accumulation by isolated canine parietal cells. Am J Physiol. 1980 Apr;238(4):G366–G375. doi: 10.1152/ajpgi.1980.238.4.G366. [DOI] [PubMed] [Google Scholar]
- Soll A. H. Specific inhibition by prostaglandins E2 and I2 of histamine-stimulated [14C]aminopyrine accumulation and cyclic adenosine monophosphate generation by isolated canine parietal cells. J Clin Invest. 1980 May;65(5):1222–1229. doi: 10.1172/JCI109777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H., Wollin A. Histamine and cyclic AMP in isolated canine parietal cells. Am J Physiol. 1979 Nov;237(5):E444–E450. doi: 10.1152/ajpendo.1979.237.5.E444. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Kramer G. L. Phosphodiesterase inhibitors as tools in cyclic nucleotide research: a precautionary comment. Mol Cell Endocrinol. 1981 Jul;23(1):1–9. doi: 10.1016/0303-7207(81)90112-x. [DOI] [PubMed] [Google Scholar]


