Abstract
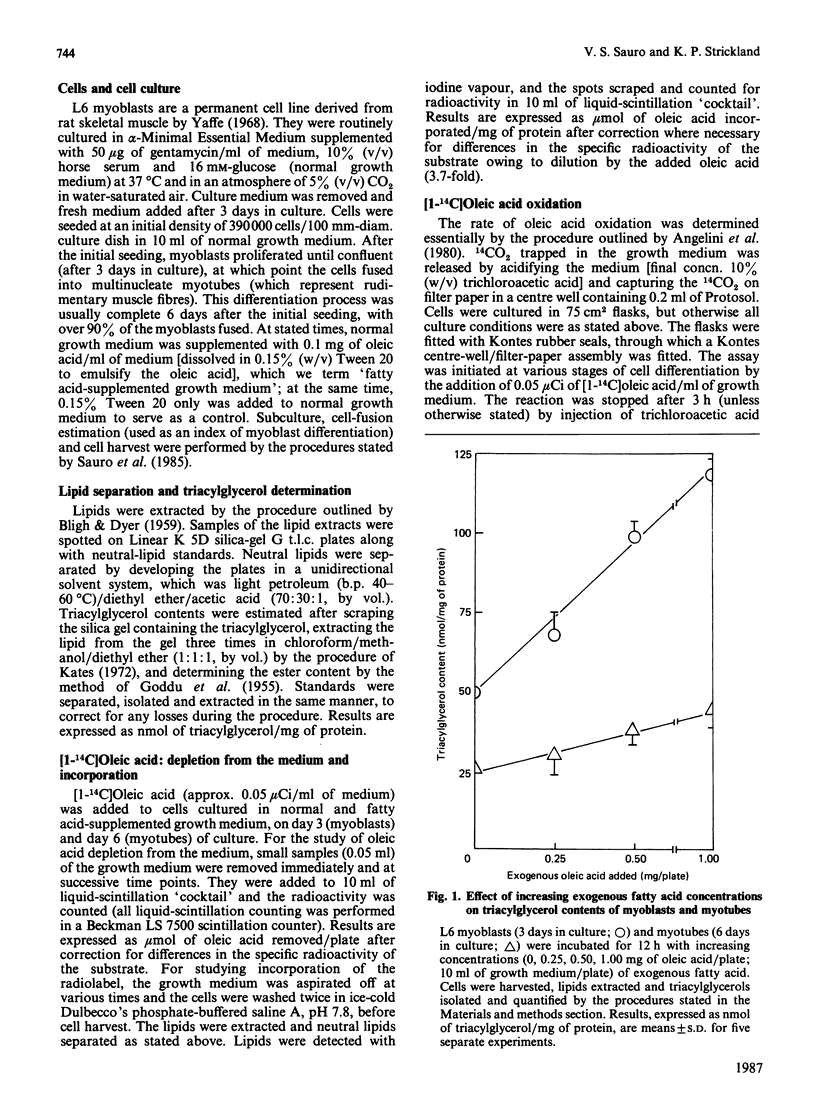
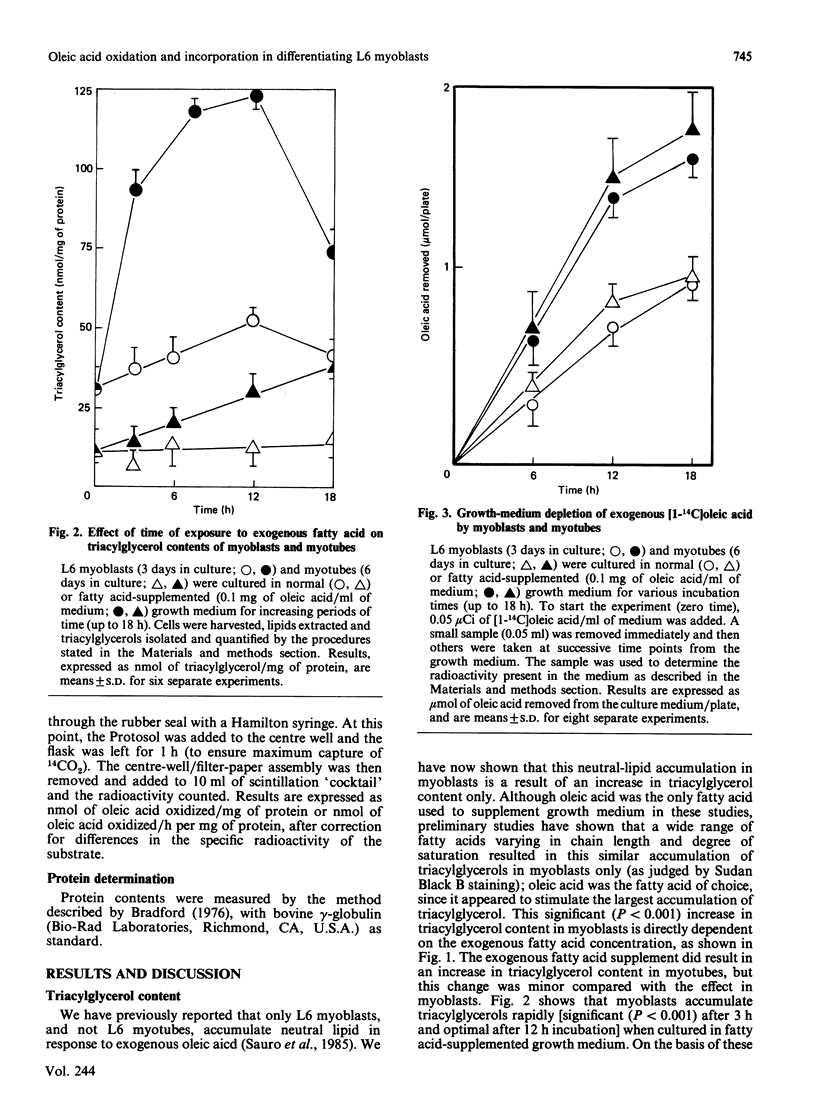
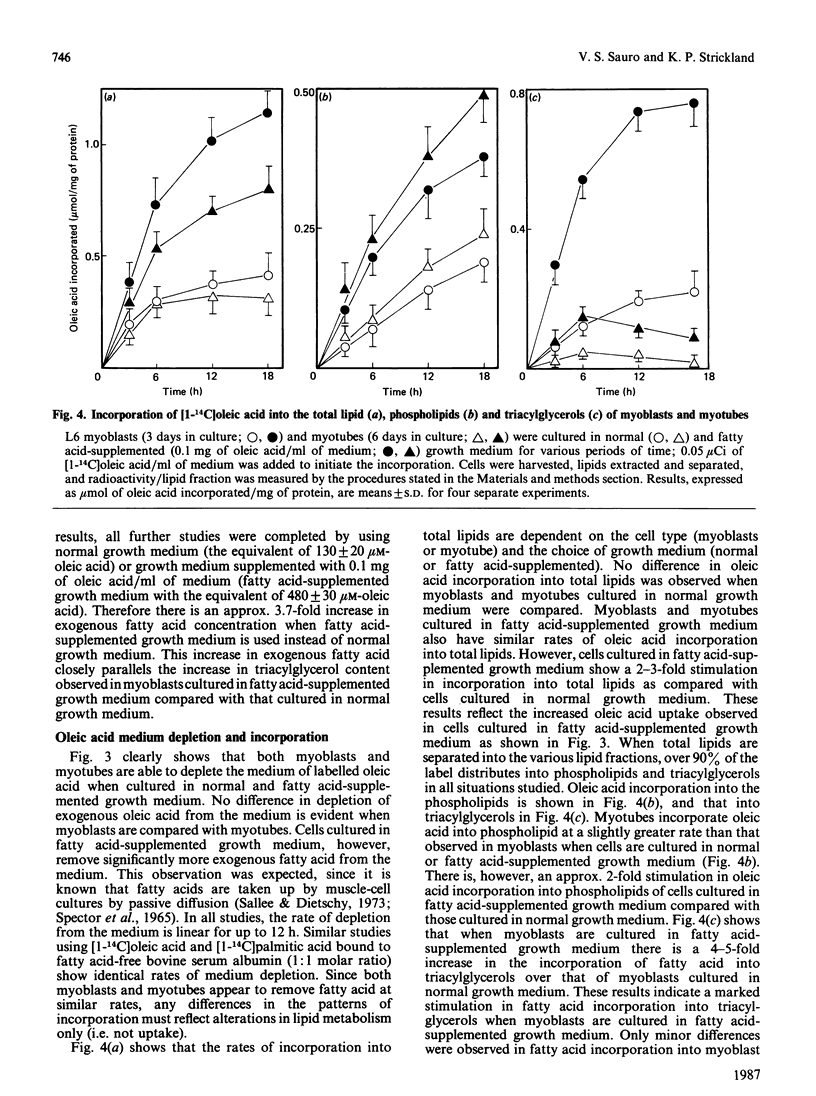
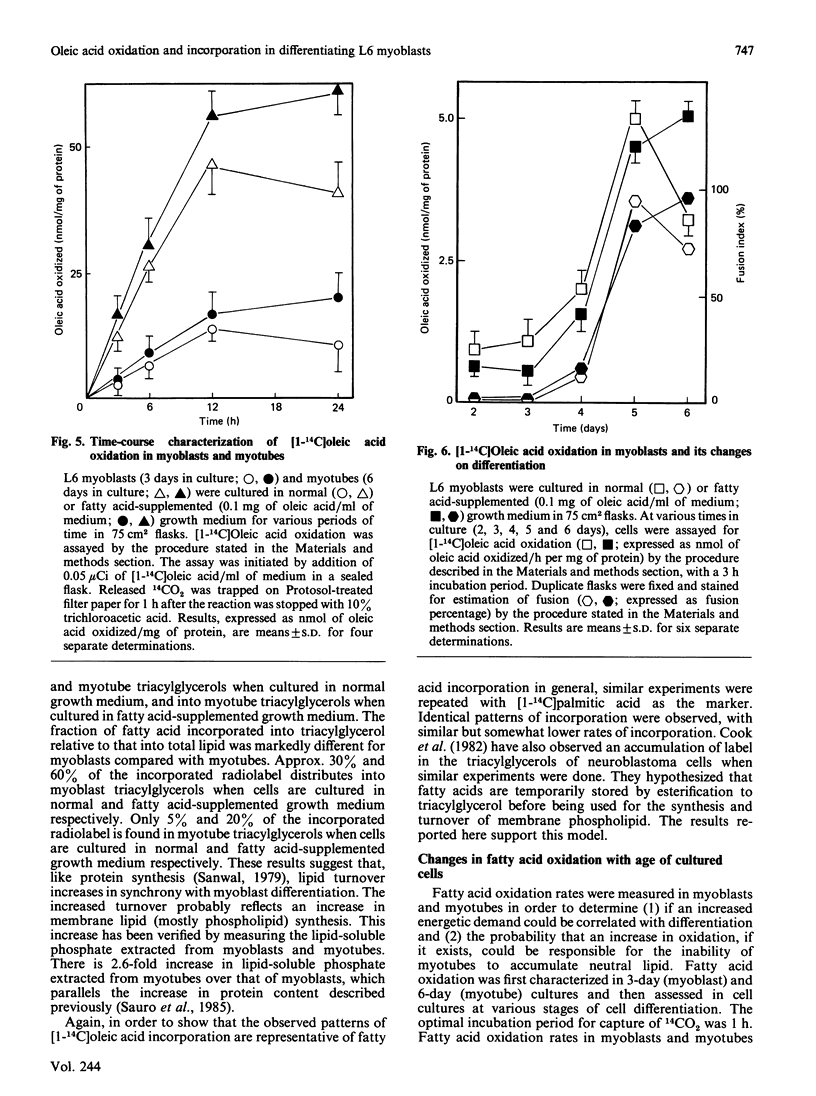
L6 myoblasts accumulate large stores of neutral lipid (predominantly triacylglycerol) when cultured in fatty acid-supplemented growth medium. No accumulation of neutral lipid was evident in myotubes (differentiated myoblasts) when treated similarly. Triacylglycerol accumulation was rapid and dependent on exogenous fatty acid concentration. Triacylglycerol content in myoblasts cultured in fatty acid-supplemented growth medium was approx. 3-fold higher than that in myotubes treated similarly and 2-3-fold higher than that in myoblasts cultured in normal growth medium. Incorporation studies using [I-14C]oleic acid showed that myoblasts and myotubes take up exogenous fatty acid at similar rates. However, cells cultured in fatty acid-supplemented growth medium remove more exogenous fatty acid than do cells cultured in normal growth medium. Over 90% of the incorporated label was found in phospholipid and triacylglycerol fractions in all situations studied. Myoblasts incorporated a more significant proportion (P less than 0.001) of label into triacylglycerol compared with that of myotubes. No differences in fatty acid oxidation rates were detected when differentiating L6 cells cultured in normal growth medium were compared with those cultured in fatty acid-supplemented growth medium. However, fatty acid oxidation rates were observed to increase 3-5-fold upon myoblast differentiation. We conclude that there is a marked change in the pattern of lipid metabolism when myoblasts (primarily triacylglycerol-synthesizing cells) differentiate into myotubes (primarily phospholipid-synthesizing cells). Understanding these changes, which coincide with normal muscle development, may be important, since a defect in this natural switch could explain the observed accumulation of lipid in muscle characteristic of some of the muscular dystrophies and other lipid-storage myopathies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelini C., Philippart M., Borrone C., Bresolin N., Cantini M., Lucke S. Multisystem triglyceride storage disorder with impaired long-chain fatty acid oxidation. Ann Neurol. 1980 Jan;7(1):5–10. doi: 10.1002/ana.410070104. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chanarin I., Patel A., Slavin G., Wills E. J., Andrews T. M., Stewart G. Neutral-lipid storage disease: a new disorder of lipid metabolism. Br Med J. 1975 Mar 8;1(5957):553–555. doi: 10.1136/bmj.1.5957.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook H. W., Clarke J. T., Spence M. W. Involvement of triacylglycerol in the metabolism of fatty acids by cultured neuroblastoma and glioma cells. J Lipid Res. 1982 Dec;23(9):1292–1300. [PubMed] [Google Scholar]
- Engel A. G., Angelini C. Carnitine deficiency of human skeletal muscle with associated lipid storage myopathy: a new syndrome. Science. 1973 Mar 2;179(4076):899–902. doi: 10.1126/science.179.4076.899. [DOI] [PubMed] [Google Scholar]
- Geyer R. P. Uptake and retention of fatty acids by tissue culture cells. Wistar Inst Symp Monogr. 1967;6:33–47. [PubMed] [Google Scholar]
- Ionasescu V., Monaco L., Sandra A., Ionasescu R., Burmeister L., Deprosse C., Stern L. Z. Alterations in lipid incorporation in Duchenne muscular dystrophy. Studies of fresh and cultured muscle. J Neurol Sci. 1981 May;50(2):249–251. doi: 10.1016/0022-510x(81)90171-4. [DOI] [PubMed] [Google Scholar]
- Lin C. H., Hudson A. J., Strickland K. P. Fatty acid metabolism in dystrophic muscle in vitro. Life Sci. 1969 Jan 15;8(2):21–26. doi: 10.1016/0024-3205(69)90112-x. [DOI] [PubMed] [Google Scholar]
- Mackenzie C. G., Mackenzie J. B., Reiss O. K. Regulation of cell lipid metabolism and accumulation. V. Quantitative and structural aspects of triglyceride accumulation caused by lipogenic substances. Wistar Inst Symp Monogr. 1967;6:63–83. [PubMed] [Google Scholar]
- Miranda A., DiMauro S., Eastwood A., Hays A., Johnson W. G., Olarte M., Whitlock R., Mayeux R., Rowland L. P. Lipid storage myopathy, ichthyosis, and steatorrhea. Muscle Nerve. 1979 Jan-Feb;2(1):1–13. doi: 10.1002/mus.880020102. [DOI] [PubMed] [Google Scholar]
- Moskowitz M. S. Fatty acid-induced steatosis in monolayer cell cultures. Wistar Inst Symp Monogr. 1967;6:49–62. [PubMed] [Google Scholar]
- SPECTOR A. A., STEINBERG D., TANAKA A. UPTAKE OF FREE FATTY ACIDS BY EHRLICH ASCITES TUMOR CELLS. J Biol Chem. 1965 Mar;240:1032–1041. [PubMed] [Google Scholar]
- Sallee V. L., Dietschy J. M. Determinants of intestinal mucosal uptake of short- and medium-chain fatty acids and alcohols. J Lipid Res. 1973 Jul;14(4):475–484. [PubMed] [Google Scholar]
- Sandra A., Ionasescu V. V. Alterations in lipid turnover in developing muscle. Biochem Biophys Res Commun. 1980 Apr 14;93(3):898–905. doi: 10.1016/0006-291x(80)91160-2. [DOI] [PubMed] [Google Scholar]
- Sauro V. S., Klamut H. J., Lin C. H., Strickland K. P. Lysosomal triacylglycerol lipase activity in L6 myoblasts and its changes on differentiation. Biochem J. 1985 Apr 15;227(2):583–589. doi: 10.1042/bj2270583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin G., Wills E. J., Richmond J. E., Chanarin I., Andrews T., Stewart G. Morphological features in a neutral lipid storage disease. J Clin Pathol. 1975 Sep;28(9):701–710. doi: 10.1136/jcp.28.9.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. B., Finch R. A. Alterations in lipid metabolism of developing muscle cells in culture. Biochim Biophys Acta. 1979 Jan 29;572(1):139–145. doi: 10.1016/0005-2760(79)90208-x. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]


