Abstract
Aim:
This study aimed to evaluate the efficacy of static or step-and-shoot intensity-modulated radiotherapy (ssIMRT) and dynamic intensity-modulated radiotherapy (dIMRT) delivery techniques for various treatment sites.
Materials and methods:
The treatment planning system (TPS) was utilized to develop optimal treatment plans for twenty-seven patients selected for this comparative study, including nine with head and neck cancer, nine with prostate cancer, and nine with cervical cancer. The prescribed doses were 7000cGy/33fr, 7425cGy/33fr, and 5000cGy/25fr for the nasopharynx, prostate, and cervix cases, respectively, in both ssIMRT and dIMRT delivery techniques. Plans were generated using the Monaco treatment planning system with a 6MV photon beam and nine equidistant fields. Plan evaluation criteria included dose-volume histogram analysis, dose homogeneity index, conformity index, radiation delivery time, and monitor unit requirements.
Results:
All plans were optimized to ensure that 98% of the planning target volume (PTV) received at least 95% of the prescribed dose, while meeting the planning objectives for organs at risk. dIMRT plans exhibited superior conformity (CI = 0.85 ± 0.05) compared to ssIMRT plans (CI = 0.79 ± 0.08), with statistically significant differences (P < 0.01). Inhomogeneity within the PTV was significantly higher in ssIMRT plans (HI = 0.10 ± 0.02) compared to dIMRT plans (HI = 0.09 ± 0.01), with a significant difference (P < 0.01). Delivery time per fraction was significantly lower in dIMRT compared to ssIMRT (P < 0.01). Furthermore, dIMRT plans required a higher mean monitor unit value (1335.4 ± 172.2) compared to ssIMRT plans (974.4 ± 133.6) with a significant difference (P < 0.001).
Conclusion:
The findings of this study indicate that dIMRT provides improved target coverage, homogeneity, and conformity while reducing treatment delivery time compared to ssIMRT.
Key Words: IMRT (ssIMRT, dIMRT), Delivery Time, Monitor Unit (MU), Conformity Index (CI)
Introduction
Intensity-Modulated Radiation Therapy (IMRT) is a contemporary radiation therapy technique utilized for the treatment of both malignant and benign tumors. This advanced technology involves the manipulation of photon beams to conform to the shape of a tumor through inverse treatment planning [1]. Inverse planning in IMRT entails the utilization of multiple radiation beam directions with varying intensities to create a customized dose distribution for complex and concave shapes within a patient [2]. In this process, each radiation beam is subdivided into multiple rays or beamlets, which are the smallest units of the irradiation area. Different intensities are then assigned to these beamlets to achieve the desired dose distribution criteria [3]. These beamlets are designed to target minute sections of tissue called voxels, which are measured in cubic millimeters of space [4].
Through mathematical optimization algorithms, inverse planning enhances target dose conformality by intensifying rays within the target area while reducing intensities across critical organs, all based on a prescribed dose [5]. Dose constraint parameters are employed for targets, and dose or dose–volume constraint parameters are utilized for normal tissues to regulate dose distributions. The optimization results are significantly influenced by the parameters used to control dose distributions in inverse planning, as the shape and position of both the target and normal tissue strongly impact the optimized dose distributions [6].
IMRT is delivered using medical linear accelerators equipped with multileaf collimator systems (MLCs). These MLCs consist of numerous tungsten leaves that are computer-controlled and possess a high atomic number. They play a crucial role in generating intensity modulations and can move independently to block the radiation beam path [4].
An intensity map represents the distribution of beamlets in the irradiation field. These intensity maps are utilized in the ‘leaf sequencing’ process, where an algorithm defines the shapes of MLC leaves required to create an intensity distribution that closely matches the calculations of the optimization system. ‘Leaf sequencing’ allows for the creation of arbitrary intensity profiles by moving the MLC leaves unidirectionally in a ‘sweep’ motion from one side to the other. This manipulation of the treatment beam enables the adaptation of non-uniform radiation fluence to the complex shapes of the target volume’s dose distributions [7].
Two delivery modes for IMRT involving MLCs are step-and-shoot and dynamic modes. Both modes have their advantages and disadvantages, and there is no consensus on which delivery method is superior [8]. The step-and-shoot technique in radiation therapy involves the use of multiple fields to treat the patient, with each field divided into subfields that are delivered in discrete steps, one at a time and in a specific sequence. The radiation beam is turned off when the MLC leaves move between field segments and turned back on when they reach designated positions [9].
A leaf sequencing algorithm, referred to as the dynamic technique (dIMRT), has been developed for step-and-shoot IMRT. In this method, during treatment delivery, the beam remains on while the MLC leaves move to create the desired intensity modulation. By adjusting the MLC leaves, any field shape with varying intensity maps can be achieved, typically by keeping one leaf stationary while moving the opposite leaf towards it [9].
To assess the quality of the treatment plan, a visual dosimetric analysis was conducted by examining the spatial arrangement of the tumor, critical organs, and isodoses section by section. This analysis resulted in the creation of a three-dimensional representation that can be viewed from any angle [10]. In researching various treatment planning methods, the use of dose-volume histogram (DVH) curves is crucial. These curves evaluate treatment plans and determine the 3D distribution of doses within the treatment area, enabling the identification of the highest, lowest, and average doses delivered to each area of interest [11]. The plans were evaluated based on their adherence to dose-volume limitations, maintenance of dose homogeneity, achievement of radiation conformity, and minimization of delivery time [12].
Our institution conducted this study to evaluate and compare the effectiveness of two IMRT delivery techniques in treating three common tumor sites: head and neck, prostate, and cervical cancer. The two methods assessed were dIMRT and ssIMRT, with the goal of analyzing each technique’s performance, treatment plans, and delivery efficiency.
Materials and Methods
Patient Selection
A total of 27 patient cases were included in this study, comprising 16 males and 11 females, with 9 cases each for Nasopharynx, Prostate, and Cervix. The patients exhibited varying tumor sizes, shapes, and locations. They had undergone radiotherapy treatment at the Shefa Alorman Cancer Treatment Hospital between 2017 and 2019. The clinical prescription for each site was as follows: 7000cGy/33fr for Nasopharynx, 7425cGy/33fr for Prostate, and 5000cGy/25fr for Cervix, for both the step-and-shoot IMRT (ssIMRT) and dynamic IMRT (dIMRT) delivery techniques. Each patient underwent a computed tomography (CT) simulation in the supine position, with a 2-mm slice thickness. To ensure accuracy and precision in IMRT, the patients were immobilized in the treatment position on a flat tabletop, and the exact tumor position was determined. The images were then transferred to a virtual simulation workstation computer, where a physician contoured critical organs and targets on each axial cut. All treatment plans were calculated based on the same series of CT scans and structure set.
Planning Techniques and Planning Objectives
This study aimed to compare two types of IMRT techniques: fixed MLCs and dynamic MLCs. The comparison was conducted using IMRT plans generated on the Monaco planning system version 5.11.02 software provided by Elekta AB in Stockholm, Sweden. The software utilizes a Monte Carlo algorithm and is designed to calculate and design IMRT plans for the Elekta Synergy platform linear accelerator with clinically relevant planning constraints.
Both ssIMRT and dIMRT plans were created using a 6 MV photon beam and integrated with a calculation grid of 3mm. The therapeutic fields were identical for both methods, with nine equally spaced fixed gantry angles designated for each beam (180°, 220°, 260°, 300°, 340°, 20°, 60°, 100°, and 140°). The planning goal was to ensure that 98% of the planning target volume (PTV) received 95% of the prescribed dose.
To assess the target volume coverage, dosimetric criteria were applied based on guidelines, and the fractional volume of each critical organ that received a certain predefined threshold dose was obtained from dose-volume histograms of each organ in IMRT plans. For both ssIMRT and dIMRT plans, the planning parameters were consistent. Additionally, the critical organ dose was kept below the tolerance dose specified in Table 1 [13, 14] and according to ICRU83 [15].
Table 1.
Planning Objective for the Critical Structures
| Critical structure | Radiation limits | Critical structure | Radiation limits |
|---|---|---|---|
| Brainstem | Max. dose < 54Gy | Cochlea | Mean dose<45 |
| Spinal cord | Max. dose < 45Gy | Parotid glands | Mean dose < 26 & V30 < 50% (at least one side) |
| Lens | Max. dose < 5 | Larynx | Mean dose < 45 |
| Optic nerve | Max. dose < 55 | Optic chiasm | Max. dose < 54 |
| Rectum | V45 Gy < 50% | Mandible | Max. dose < 70 |
| Femoral head | V30 Gy < 15% | Bowel-bag | V45 Gy < 250cc |
| Bladder | V45 Gy < 35% |
Evaluation tools
Dose-volume Histogram
To assess treatment plans for different types of radiation therapy, the standard Dose-Volume Histogram (DVH) was utilized for quantitative evaluation. The DVH provided crucial information about the dose distribution, facilitating the comparison of treatment plans. Metrics such as PTV, D98%, and D2% (doses received by 98% and 2% of the volume) were derived from these histograms to determine minimum and maximum doses. Additionally, doses affecting organs at risk (OARs) were evaluated based on the specific treatment areas. Various indices, including conformity index (CI) and dose heterogeneity index (HI), were defined to provide a comprehensive assessment [16].
Conformity Indexes
The objective of IMRT is to deliver radiation more precisely, conforming to the shape of the target and minimizing damage to surrounding healthy tissues. Accurately matching the shape of the target with a radiation map can be challenging. Therefore, a conformity index is employed to measure how well the distribution of radiation conforms to the target shape. This index is crucial in selecting between two potential treatment plans for the same patient. Various conformity indices are available to describe the conformity of the prescription isodoses to the target volume.
An alternative definition of conformity index, CI98%, is the ratio of the volume of the patient that receives at least 95% of the prescribed dose to the volume of the PTV. The conformity index (CI) considers the position of the prescription isodoses volume (PIV) concerning the target volume (TV) and healthy tissues and was used to assess the conformity of the PTV dose [17].
Defined as:
Conformity Index = TV2PIV/(TV*PIV) Equation 1
Where: PI = prescription isodoses; PIV = PI volume; TV = target volume; TVPIV = TV covered by PIV.
For an accurate measure of conformity, any ratio must consider the volume of the target covered by the PI (TVPIV). If the plan is perfect, the conformity index would equal one, and the isodoses would be sculpted around the target volume.
Homogeneity index (HI)
The HI is defined as the ratio of the differences between the maximal and minimal dose to the mean dose of the TV. A HI value of zero indicates a homogeneous dose distribution to the PTV, while an increasing HI signifies greater dose heterogeneity.
In accordance with the guidelines outlined by the International Commission on Radiation Units and Measurements 83 (ICRU83) [15], the homogeneity index for the treatment plans was expressed as:
HI= (D2% − D98%)/D50% Equation 3
Where D98% represents the dose to 98% of the volume as depicted on the cumulative DVH. This indicates that 98% of the target volume receives this dose or higher, and is considered the “minimum dose.” D2% represents the dose to 2% of the target volume, as displayed on the DVH, indicating that only 2% of the target volume receives this dose or higher, and is considered the “maximum dose.”
Equation 3 demonstrates that lower HI values indicate a more homogeneous target dose [18].
Statistical analysis
The data was entered into a computer and analyzed using IBM SPSS software package version 20.0 (Armonk, NY: IBM Corp). The normality of distribution was verified using the Shapiro-Wilk test. Quantitative data were described using range (minimum and maximum), mean, and standard deviation. The significance of the obtained results was assessed at the 5% level. Paired t-tests were used for normally distributed quantitative variables to compare between two periods and examine statistical significance.
Results
All plans met the planning objectives and were deemed clinically acceptable. Table 2 presents the numerical data obtained from the PTV analysis, using an average DVH, reported as mean values ± standard deviation (SD) to assess inter-patient variability. The results indicate minimal variation between the different approaches, with small SDs. From a clinical perspective, both delivery techniques appear to be equally effective.
Table 2.
Dosimetric Results for the PTV
| Parameter | DMLC | S&S | P |
|---|---|---|---|
| Mean ± SD. | Mean ± SD. | ||
| V95% | 99.53 ± 0.47 | 99.07 ± 0.87 | <0.001 |
| MUs | 1335.4 ± 172.2 | 974.4 ± 133.6 | <0.001 |
| Delivery time | 452.8 ± 66.78 | 615.9 ± 68.27 | <0.001 |
| Segments | 259.0 ± 8.77 | 139.7 ± 19.04 | <0.001 |
| CI | 0.85 ± 0.05 | 0.79 ± 0.08 | <0.001 |
| HI98 | 0.09 ± 0.01 | 0.10 ± 0.02 | <0.001 |
PTV Coverage and Healthy tissue sparing
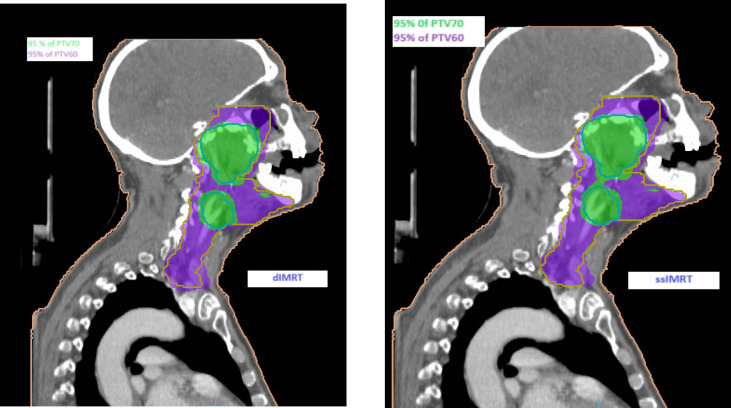
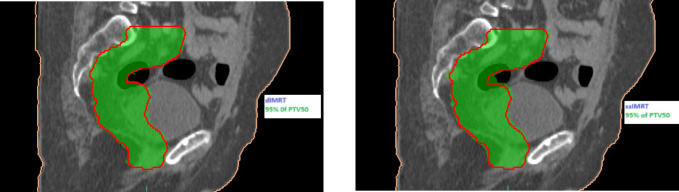
Upon comparing the final results of the two IMRT techniques across all plans for the three cases, it was observed that DIMRT achieved a PTV coverage of 99.53% ± 0.47, while step and shoot IMRT had a coverage of 99.07% ± 0.87. However, the planning objectives for healthy tissue were similar for all techniques. Figure 1 illustrates the dose distribution in coronal sections, displaying the concave PTV coverage in both methods.
Figure 1.
(a). Dose Distribution of the Concave PTV Coverage in Two Methods for Nasopharynx.
Case 1: Nasopharynx cancer
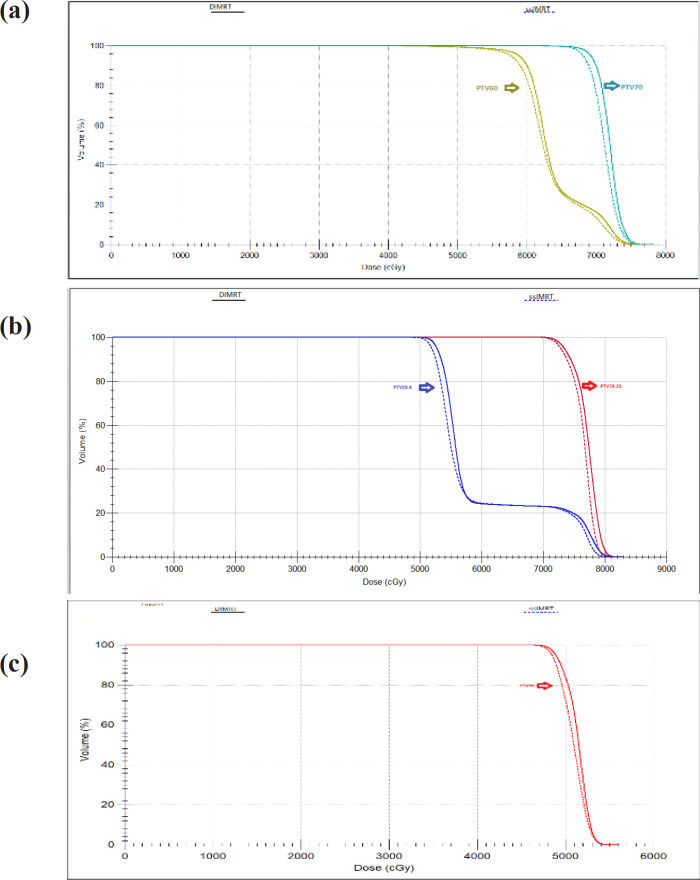
Our center treated patients with primary head and neck cancers, prescribing a dose of 70 Gy in 33 fractions for the primary tumor volume and bilateral neck nodal volume. Dose distributions are depicted in Figure 1(a) for coronal views, providing visualization of the key characteristics of dIMRT compared to ssIMRT treatment plans for a nasopharynx. The PTV coverage in dIMRT was 99.52% ± 0.42, and in ssIMRT it was 98.91% ± 1.03. Figure 2(a) presents the DVH of the PTV comparing dIMRT (solid line) to ssIMRT (dashed line). Additionally, Table 1 outlines the tolerance doses for OARs.
Figure 2.
(a), DVH of PTV for Nasopharynx; (b), DVH of PTV for Prostate; (c), DVH of PTV Cervix cancer
Analysis revealed that the Dmax to the brain stem was 51.11 Gy ± 4.67 in dIMRT and 52.54 Gy ± 2.91 in ssIMRT, and the Dmax to the chiasm was 47.67 Gy ± 5.19 in dIMRT and 46.94 Gy ± 5.67 in ssIMRT. For the spinal cord, the Dmax was 37.75 Gy ± 2.85 in dIMRT and 37.47 Gy ± 2.27 in ssIMRT. The maximum doses to the left lens were 5.58 Gy ± 2.02 in dIMRT and 5.62 Gy ± 1.56 in ssIMRT, and to the right lens were 5.38 Gy ± 1.58 in dIMRT and 5.62 Gy ± 1.85 in ssIMRT. The mean doses to the left cochlea were 44.46 Gy ± 2.90 in dIMRT and 44.30 Gy ± 3.96 in ssIMRT, and to the right cochlea were 43.93 Gy ± 2.92 in dIMRT and 43.39 Gy ± 2.56 in ssIMRT. Similarly, the mean doses to the left and right parotid were 26.45 Gy ± 1.14 and 25.96 Gy ± 1.40 in dIMRT, and 25.40 Gy ± 2.14 and 25.28 Gy ± 1.99 in ssIMRT.
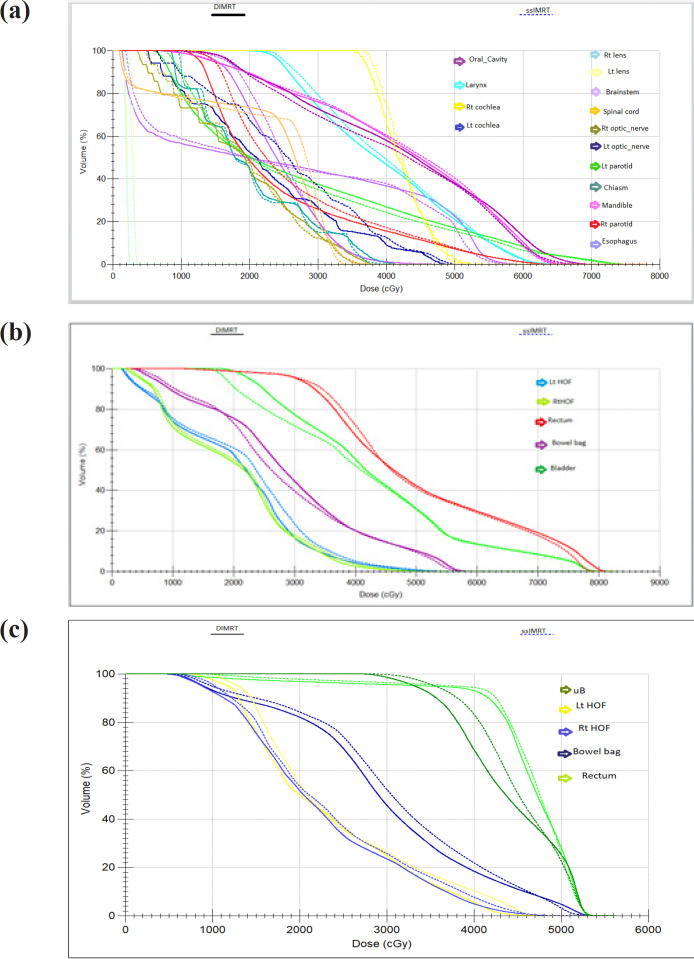
Figure 3(a) presents the average DVH computed for various OARs and healthy tissue, comparing dIMRT (solid lines) to ssIMRT (dashed lines).
Figure 3.
(a), DVH of OAR for Nasopharynx; (b), DVH of OAR for Prostate; (c), DVH of OAR for Cervix cancer
Case 2: Prostate cancer
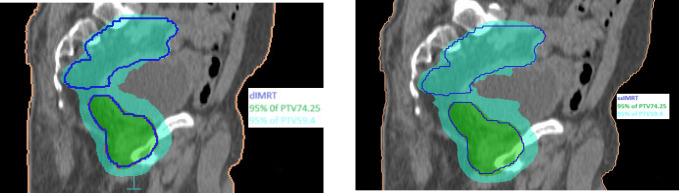
The dose distribution for a single patient is depicted in Figure 1(b) for coronal views, providing a visual comparison of the key characteristics of dIMRT and ssIMRT plans for a patient with prostate cancer. The total prescribed dose for this case was 74.25 Gy in 33 fractions. The PTV coverage achieved in dIMRT was 99.32% ± 0.61, while ssIMRT achieved a coverage of 98.97% ± 1.05. Tolerance doses for organs at risk (OARs) are detailed in Table 1. The mean doses to the left and right femoral heads were 2.05 Gy ± 1.32 and 1.40 Gy ± 1.0 with dIMRT, and 1.83 Gy ± 1.00 and 1.76 Gy ± 1.43 with ssIMRT. Furthermore, the mean bladder dose was 33.07 Gy ± 5.40 with dIMRT, and 32.50 Gy ± 6.01 with ssIMRT, the rectum mean dose was 40.90 Gy ± 6.28 with dIMRT, and 42.39 Gy ± 4.64 with ssIMRT, and the penile bulb mean dose was 32.11 Gy ± 7.56 with dIMRT, and 34.78 Gy ± 9.42 with ssIMRT. The average DVH for the PTV comparing dIMRT (solid lines) to ssIMRT (dashed lines) is presented in Figure 2(b), while Figure 3(b) illustrates the average DVH computed for various OARs and healthy tissue, comparing dIMRT (solid lines) to ssIMRT (dashed lines).
Figure 1.
(b). Dose Distribution of the Concave PTV Coverage in Two Methods for Prostate
Case 3: Cervical cancer
For patients with cervical cancer, dose distributions for one example are displayed in Figure 1(c) for coronal views, offering a visual comparison of the main characteristics of dIMRT and ssIMRT. The prescribed dose for this case was 50 Gy in 25 fractions, and the PTV coverage achieved in dIMRT was 99.75% ± 0.23, while ssIMRT achieved a coverage of 99.34% ± 0.42. Tolerance limits for OARs are provided in Table 1, with the mean doses to the left and right femoral heads being 45.52 Gy ± 2.58 and 47.29 Gy ± 3.03 with dIMRT, and 45.80 Gy ± 1.64 and 47.11 Gy ± 2.28 with ssIMRT, the mean bladder dose being 53.17 Gy ± 0.38 with dIMRT, and 53.07 Gy ± 0.56 with ssIMRT, the rectum mean dose being 53.13 ± 0.26 with dIMRT, and 51.58 ± 3.40 with ssIMRT, and the bowel bag doses being 53.31 Gy ± 1.23 with dIMRT, and 52.43 Gy ± 1.73 with ssIMRT. The average DVH for the PTV comparing dIMRT (solid lines) to ssIMRT (dashed lines) is presented in Figure 2(c), while Figure 3(c) reports the average DVH computed for various OARs and healthy tissue, comparing dIMRT (solid lines) to ssIMRT (dashed lines).
Figure 1.
(c). Dose Distribution of the Concave PTV Coverage in Two Methods for Cervix.
Monitor units, segments and delivery time
For the delivery of a 2 Gy dose per fraction, the average monitor units (MUs) required were 974.4 ± 133.6 for ssIMRT and 1335.4 ± 172.2 for dIMRT. Notably, dIMRT plans exhibited a significantly lower average beam-on time (452.8 ± 66.78) compared to ssIMRT (615.9 ± 68.27). The number of segments in dIMRT plans averaged 259.0 ± 8.77, while ssIMLC had an average of 139.7 ± 19.04.
Conformity index and homogeneity index
The results from dIMRT plans demonstrated superior conformity (CI98% = 0.85 ± 0.05) compared to that achieved in ssIMRT plans (CI98% = 0.79 ± 0.08). However, the dose homogeneity within the PTVs was higher for ssIMRT plans, with an HI of 0.10 ± 0.02 compared to dIMRT’s HI of 0.09 ± 0.01.
Discussion
This study compared dIMRT with ssIMRT for 27 patients at different sites. In all treatment plans, nine fields were applied with equidistant gantry angles using a 6MV energy. Radiation oncologists established DVCs in IMRT methods based on acceptable radiation doses for PTV and OARs. Target coverage in dIMRT and ssIMRT plans was extracted from DVH curves, and subsequently, HI and CI were calculated for both methods.
Our results indicate that both IMRT methods can effectively achieve the primary study goals with reduced damage to OARs. However, dIMRT significantly outperformed ssIMRT in achieving 95% of the PTV receiving 98% of the prescribed dose (p value<0.001).
A study by [19] and [20] compared dynamic and static modes, revealing that the dynamic mode provided better target coverage, while the static mode offered slightly better protection for critical organs. The number of monitor units delivered for each plan was lower in the static mode compared to the dynamic mode. However, the actual delivery time in minutes was longer in the static mode than in the dynamic mode.
When comparing dIMRT and ssIMRT, we observed a significant reduction in treatment times with dIMRT (p value<0.001). This can be attributed to the dynamic delivery mode, where radiation is active during the movement of the MLC to create a new segment, resulting in decreased beam-on time. This reduction has a substantial impact on clinical throughput, potentially leading to an increased number of patients treated per day and a decrease in the waitlist. Additionally, shorter time on the treatment couch reduces the risk of geometric miss due to intra-fractional movement. The saved time can be utilized to implement more online imaging technologies without increasing the total time spent in the treatment room.
An IMRT field and a given Isodose consist of multiple segments that are delivered based on the required intensity MUs. Each segment is composed of small beamlets defined by the MLC. Therefore, with each beam angle, the MLC can change the segment’s shape with a given MUs to provide a suitable isodose. Table 2 shows that ssIMRT has a lower number of segments compared to dIMRT, resulting in a decrease in the monitor unit for ssIMRT and an increase for dIMRT. The p-value is 0.001, which supports the use of ssIMRT over dIMRT. Alaei et al. [21] also reported that the total number of MUs delivered for an ssIMRT treatment was fewer, but ssIMRT treatments span an average of 15% longer time to deliver than the dIMRT mode. The issue of MUs is just one of the many concurrent aspects that need to be considered when comparing dIMRT and ssIMRT. However, it’s not accurate to consider the issue of the higher MUs associated with dIMRT as a decisive factor leading us to conclude that dIMRT is worse than ssIMRT.
Additionally, there are differences in the plans produced using these IMRT techniques seen in indicators such as CI and HI. In this study, calculated HI and CI by DVH curves were assessed in dIMRT and compared with the ssIMRT method for prostate, cervix, and head and neck cancer. It is typically reported that each of the target volume conformity and homogeneity are closely related to the complexity of target volume geometry in relation with adjacent OARs, the delivery equipment, and technique.
In our study, The HI and CI were significantly improved in dIMRT (p value<0.001), attributed to the ability of dIMRT to create multiple segments with different shapes and conform the prescribed dose to the target volume and spare the OARs. Also, the arrangement of beams used in IMRT improves homogeneity and conformity while reducing the volume of OARs. In a study conducted by [22], the performance of three radiotherapy techniques, namely volumetric arc therapy (VMAT), dynamic intensity-modulated radiation therapy (dIMRT), and step-and-shoot IMRT (ssIMRT), was evaluated in nasopharyngeal cancer patients. The results indicated that the conformity indices of VMAT and dIMRT plans were superior to those of ssIMRT. Furthermore, the monitor units (MUs) for dIMRT were notably higher compared to VMAT and ssIMRT. A more recent study by Raina et al. (2020) demonstrated that both dIMRT and VMAT techniques offer improved sparing of normal tissue, as well as enhanced homogeneity and conformity compared to ssIMRT, accompanied by reduced treatment delivery time.
In conclusion, the findings suggest that dIMRT outperforms ssIMRT, providing superior target coverage, dose homogeneity, and dose conformity, while also reducing treatment delivery time. This leads to enhanced treatment efficiency and minimization of patient discomfort.
Acknowledgements
I extend my sincere gratitude to Dr. Hany Ammar, my paper supervisor, for his invaluable mentorship and guidance throughout my research. I also wish to thank Dr. Mohamed Mahmoud for his clinical insights and feedback as a member of my committee. Additionally, I acknowledge Dr. Gammal Yahia, the third reader, for their review and critique of the paper. My colleagues have been an invaluable resource on the technical aspects of radiation treatment, and I appreciate their genuine interest in my paper and professional development.
Author Contribution Statement
All authors contributed equally in this study.
References
- 1.Raina P, Singh S, Tudu R, Singh DR, Kumar A. Volumetric modulated arc therapy: A dosimetric comparison with dynamic imrt and step-and-shoot imrt. Journal of Radiotherapy in Practice. 2019;19(4):393–8. [Google Scholar]
- 2.Nutting C. Intensity-modulated radiotherapy (imrt): The most important advance in radiotherapy since the linear accelerator? Br J Radiol. 2003;76(910) doi: 10.1259/bjr/14151356. [DOI] [PubMed] [Google Scholar]
- 3.Webb S. The physical basis of imrt and inverse planning. Br J Radiol. 2003;76(910):678–89. doi: 10.1259/bjr/65676879. [DOI] [PubMed] [Google Scholar]
- 4.Elith C, Dempsey SE, Findlay N, Warren-Forward HM. An introduction to the intensity-modulated radiation therapy (imrt) techniques, tomotherapy, and vmat. J Med Imaging Radiat Sci. 2011;42(1):37–43. doi: 10.1016/j.jmir.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Bortfeld T. Imrt: A review and preview. Phys Med Biol. 2006;51(13):R363 . doi: 10.1088/0031-9155/51/13/R21. [DOI] [PubMed] [Google Scholar]
- 6.Hunt MA, Hsiung CY, Spirou SV, Chui CS, Amols HI, Ling CC. Evaluation of concave dose distributions created using an inverse planning system. Int J Radiat Oncol Biol Phys. 2002;54(3):953–62. doi: 10.1016/s0360-3016(02)03004-3. [DOI] [PubMed] [Google Scholar]
- 7.Cho B. Intensity-modulated radiation therapy: A review with a physics perspective. Radiat Oncol J. 2018;36(1):1–10. doi: 10.3857/roj.2018.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martens C, De Gersem W, De Neve W, De Wagter C. Combining the advantages of step-and-shoot and dynamic delivery of intensity-modulated radiotherapy by interrupted dynamic sequences. Int J Radiat Oncol Biol Phys. 2001;50(2):541–50. doi: 10.1016/s0360-3016(01)01486-9. [DOI] [PubMed] [Google Scholar]
- 9.Rehman Ju, Ahmad N, Khalid M, Noor ul Huda Khan Asghar HM, Gilani ZA, et al. Intensity modulated radiation therapy: A review of current practice and future outlooks. J Radiat Res Appl Sci. 2018;11(4):361–7. [Google Scholar]
- 10.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: A review. Int J Radiat Oncol Biol Phys. 2006;64(2):333–42. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Salimi M, Shirani K, Nedaie H, Hassani H, Gharaati H, Samei M, et al. Assessment and comparison of homogeneity and conformity indexes in step-and-shoot and compensator-based intensity modulated radiation therapy (imrt) and three-dimensional conformal radiation therapy (3d crt) in prostate cancer. J Med Signals sens. 2017;7:102–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver M, Ansbacher W, Beckham WA. Comparing planning time, delivery time and plan quality for imrt, rapidarc and tomotherapy. J Appl Clin Med Phys. 2009;10(4):117–31. doi: 10.1120/jacmp.v10i4.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu JJ. Target volume delineation and field setup-a practical guide for conformal. Springer-verlag berlin; 2012. [Google Scholar]
- 14.Lee AW, Ng WT, Pan JJ, Chiang CL, Poh SS, Choi HC, et al. International guideline on dose prioritization and acceptance criteria in radiation therapy planning for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2019;105(3):567–80. doi: 10.1016/j.ijrobp.2019.06.2540. [DOI] [PubMed] [Google Scholar]
- 15.Das IJ, Andersen A, Chen ZJ, Dimofte A, Glatstein E, Hoisak J, et al. State of dose prescription and compliance to international standard (icru-83) in intensity modulated radiation therapy among academic institutions. Pract Radiat Oncol. 2017;7(2):e145–e55. doi: 10.1016/j.prro.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Cozzi L, Dinshaw KA, Shrivastava SK, Mahantshetty U, Engineer R, Deshpande DD, et al. A treatment planning study comparing volumetric arc modulation with rapidarc and fixed field imrt for cervix uteri radiotherapy. Radiother Oncol. 2008;89(2):180–91. doi: 10.1016/j.radonc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans Technical note. J Neurosurg. 2000;93(Suppl 3):219–22. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 18.Wu QR, Wessels BW, Einstein DB, Maciunas RJ, Kim EY, Kinsella TJ. Quality of coverage: Conformity measures for stereotactic radiosurgery. J Appl Clin Med Phys. 2003;4(4):374–81. doi: 10.1120/jacmp.v4i4.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chui CS, Chan MF, Yorke E, Spirou S, Ling CC. Delivery of intensity-modulated radiation therapy with a conventional multileaf collimator: Comparison of dynamic and segmental methods. Med Phys. 2001;28(12):2441–9. doi: 10.1118/1.1418018. [DOI] [PubMed] [Google Scholar]
- 20.Iqbal K, Isa M, Buzdar SA, Gifford KA, Afzal M. Treatment planning evaluation of sliding window and multiple static segments technique in intensity modulated radiotherapy. Rep Pract Oncol Radiother. 2012;18(2):101–6. doi: 10.1016/j.rpor.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alaei P, Higgins PD, Weaver R, Nguyen N. Comparison of dynamic and step-and-shoot intensity-modulated radiation therapy planning and delivery. Med Dosim. 2004;29(1):1–6. doi: 10.1016/j.meddos.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Ozdemir S, Coban Y, Akin M, Ambarcioglu P, Onay O, Yildirim C, et al. Dosimetric evaluation of nasopharyngeal carcinomas irradiated with different imrt techniques. J buon. 2014;19(4):953–7. [PubMed] [Google Scholar]