Abstract
Objectives:
Rhabdomyosarcoma (RMS) accounts for 50% of soft tissue sarcomas and 7% of pediatric malignancies. Cyclophosphamide (CPA) is the cornerstone of therapy and is a prodrug that is activated by the highly polymorphic drug-metabolizing enzyme CYP3A5. We aim to examine the possible CYP3A5 polymorphism association with CPA efficacy, survival outcomes, and toxicity in Egyptian pediatric RMS patients.
Methods:
The three non-functional SNPs, CYP3A5*3 rs776746 (C_26201809_30), CYP3A5*6 rs10264272 (C_30203950_10), and CYP3A5*7 rs41303343 (C_32287188_10) were genotyped by real-time PCR. We conducted a cohort retrospective study of 150 pediatric RMS patients treated with CPA-based first-line treatment to analyze the association between these genotypes and CPA efficacy/toxicities in RMS patients.
Key findings:
The frequency of having normal, intermediate, and poor metabolizers was 4.7%, 34%, and 61.3%, respectively. There was an association between these different phenotypes, genotypes, and CPA efficacy/toxicity. Hemorrhagic cystitis and pancytopenia were present in all patients, while nephrotoxicity incidence was 87.3%. There was a notable difference in the occurrence of hemorrhagic cystitis among CYP3A5 intermediate metabolizers *1/*3, *1/*6, and poor metabolizers *3/*3, *3/*6 with a significance level of p<0.05. Neither CYP3A5*7 polymorphism nor *6/*6 genotype was identified in our study.
Conclusion:
Our results demonstrate that CYP3A5*3 (rs776746) and CYP3A5*6 (rs10264272) have a great association with CPA efficacy and toxicity in RMS patients.
Key Words: Rhabdomyosarcoma, cyclophosphamide, CYP3A5, Pharmacogenetics, Pharmacogenomics
Introduction
Rhabdomyosarcoma (RMS) is a malignancy that primarily affects skeletal muscle tissue but can also occasionally affect organs like the bladder and uterus. RMS can affect anyone at any age, but children are more prone to developing it. It is a little spherical blue cell tumor that makes up 7% of all pediatric malignancies [1].
Rhabdomyosarcoma (RMS) cases are estimated to increase by 400–500 each year in the USA. More than half of rhabdomyosarcoma diagnoses are made in children and adolescents under the age of 10, who also account for the majority of cases. These tumors, known as embryonal rhabdomyosarcomas (ERMS), typically develop in the head and neck region, as well as the vaginal and urinary systems. All age groups are susceptible to alveolar rhabdomyosarcoma (ARMS), which is more frequently detected in the arms, legs, or trunk. The risk rating approach that guides RMS treatment takes into account both pre-treatment clinical Tumor , Node and Metastasis (TNM) staging and surgical grouping. Recovery rates increased from 25% in the early 1970s to 70% in the subsequent 40 years, thanks in part to risk stratification and multimodal care [2].
Vincristine, actinomycin, and cyclophosphamide (VAC) have been used as the standard chemotherapy regimen for treating RMS for many years, according to the Intergroup Rhabdomyosarcoma Study Group. Approximately 35% of RMS patients benefit from chemotherapy in achieving complete remission (CR) [3].
Cyclophosphamide (CPA), an oxazaphosphorine alkylating chemotherapeutic medication that is primarily used in combination with other chemotherapeutic medications to treat lymphomas, some forms of brain cancer, neuroblastoma, leukemia, and other solid tumors. [4]. It has a relatively narrow therapeutic index and a number of undesirable side effects, including cardiotoxicity, nephrotoxicity, neurotoxicity, infertility, hemorrhagic cystitis, myelosuppression/pancytopenia, and leukemogenesis [5,6]. Due to the fact that it is a prodrug, only 70–80% of the dosage is converted to its active metabolite; 4-hydroxycyclophosphamide. It is swiftly absorbed and then processed into active metabolites by the cytochrome P450 system (CYP3A5) in the liver [7]. The main active metabolite is 4-hydroxycyclophosphamide, which balances aldophosphamide that easily diffuses into cells, where it is broken down into phosphoramide mustard and acrolein [8].
Phosphoramide mustard creates DNA crosslinks between and within DNA strands causing cell death [9]. Acrolein however, can induce hemorrhagic cystitis, which is characterized by microscopic or significant hematuria and, in rare cases, dysuria [10]. Hemorrhagic cystitis can be prevented by drinking enough fluids, avoiding nighttime doses, and using MESNA (sodium 2-mercaptoethane sulfonate), a sulfhydryl donor that binds and detoxifies acrolein [11]. When cyclophosphamide is administered less frequently, the total medicine dose is decreased, and the amount of acrolein that enters the bladder is decreased [12]. Many hepatic cytochrome P450 (CYP450) enzymes, including CYP3A4/5, CYP2B6, CYP2C9, and CYP2C19, have been connected to CPA activation. According to earlier investigations, the main CYP450s involved in CPA activation include CYP3A4 and CYP3A5 [13,14]. The polymorphic enzyme CYP3A5 has a large number of recognized variations [15,16]. There are now nine recognized CYP3A5 single nucleotide polymorphisms (SNPs) in humans with various ethnic frequencies [17]. In comparison to the CYP3A5*1 wild type , polymorphic CYP3A5 variants have been shown to have less favorable metabolic features than the CYP3A5*1 [18,19].
The three allelic variations of CYP3A5 that are most prevalent in Caucasians are CYP3A5*3, CYP3A5*6, and CYP3A5*7. A single nucleotide polymorphism in intron 3 of the CYP3A5*3 causes an early termination codon, whereas studies have indicated that the allelic variants CYP3A5*6 and CYP3A5*7 produce little to no active CYP3A5 enzyme [20,21]. The remaining genotypes indicated are essentially devoid of active CYP3A5 enzyme since people without the CYP3A5*1 produce such a minimal amount of this enzyme. Cyclophosphamide-induced toxicity and efficacy were shown to be higher in non-expressers (poor metabolizers) than in expressers (normal metabolizers) [22,23]. Researchers have been examining the role of common non-functional CYP3A5 SNPs in predicting response to various treatments and assisting in the individualization of dose for each patient to improve chemotherapeutic effectiveness while reducing toxicity in response to the growing importance of personalized medicine [24].
Understanding the factors that affect how CPA is metabolized could aid in explaining why different people have varying reactions to CPA thus leading to the development of more effective CPA dosing regimens. This emphasizes the importance of dose individualization, which can enhance therapeutic efficacy and toxicity. To the best of our knowledge, there is no information regarding the impact of the CYP3A5 polymorphism on CPA efficacy and toxicity in pediatric cancer patients in Egypt. Therefore we aim to ascertain the prevalence of three CYP3A5 common non-functional SNPs in a cohort of Egyptian pediatric RMS patients CYP3A5*3 (rs776746), CYP3A5*6 (rs10264272), and CYP3A5*7 (rs41303343) and to examine the possible association of CYP3A5 polymorphism and cyclophosphamide efficacy, survival outcomes, and toxicity in Egyptian pediatric RMS cancer patients to increase treatment success with low side effects.
Materials and Methods
Patient eligibility and treatment
This pharmacogenetic study is a cohort observational retrospective analysis on pediatric Egyptian cancer patients at Egypt’s 57357 children’s cancer hospital. A total of 150 RMS patients under the age of 18 were included in this retrospective analysis. They were recruited in January 2013 till November 2016 at the Children’s Cancer Hospital of Egypt (CCHE) and treated with a combination of vincristine, actinomycin, and cyclophosphamide (VAC) as the first-line standard clinical treatment for RMS, according to Intergroup Rhabdomyosarcoma study group (IRSG-IV) protocol. All patients took their treatment protocol and were followed-up in the past and their samples and data were collected from the hospital’s biobank and files in the hospital system. The regimen included Vincristine (1.5 mg/m2) given as an IV push (max. 2 mg), actinomycin (0.045 mg/kg) given as a 5 min intravenous infusion, and cyclophosphamide (1.2 gm/m2) infused over 30–60 min, with hydration maintained at 3000 ml/m2/day and vincristine given once a week for two weeks.
For all types of RMS, cyclophosphamide was administered every three weeks throughout the ten-month RMS protocol treatment period. According to the treatment regimen, radiotherapy or surgery was performed. Patients were followed-up on till their treatment was completed.
Clinical evaluation of cyclophosphamide-related efficacy (survival rate and response) and toxicity (grade and occurrence) were based on occurrences noted in each patient’s medical record. For the individuals in this study, one whole blood sample (5ml) had been taken from the biobank for DNA extraction and genotyping. Taqman Real-Time polymerase chain reaction assay was used to genotype the CYP3A5 (3, 6 & 7) SNPs rs776746 (C_26201809_30 CYP3A5*3), rs10264272 (C_30203950_10 CYP3A5*6), and rs41303343 (C_32287188_10 CYP3A5*7), respectively.
RMS protocol doses according to age or weight: Cyclophosphamide dose: ≥3 years were given 1200 mg/m2 IV as a 30- to 60-minute infusion with IV fluids and MESNA. Patients ≥1 year < 3 years were given 40 mg/kg/dose IV as a 30- to 60-minute infusion with IV fluids and MESNA. Patients < 1 year were given 40 mg/kg IV as a 30- to 60-minute infusion with IV fluids and MESNA, as shown in Table 1.
Table 1.
RMS Protocol Doses According to Age or Weight.
| Drug | Age | Dose | |
|---|---|---|---|
| V | Vincristine | < 1 year | 0.025 mg/kg IV x 1 |
| ≥1 year and < 3 years | 0.05 mg/kg IV x 1 (maximum dose 2mg) | ||
| ≥3 years | 1.5 mg/m2 IV x 1 (maximum dose 2mg) | ||
| A | Dactinomycin | < 1 year | 0.025 mg/kg IV x 1 |
| ≥1 year | 0.045 mg/kg (maximum dose 2.5mg) IV x 1 | ||
| C | Cyclophosphamide | < 3 years | 40 mg/kg/dose IV x 1 |
| ≥3 years | 1200 mg/m2 IV x 1 |
*Mesna and fluids will be used with cyclophosphamide.
Cyclophosphamide was combined with MESNA and fluids to avoid and indicate the incidence of hemorrhagic cystitis. The recommended total daily MESNA dose was 100% of the daily cyclophosphamide dose given at 0, 3, 6, and 9 hours after cyclophosphamide was started. Hydration with 200 mL/m2/hour fluids was done before cyclophosphamide administration and after CPA with 3L/m2 for 24 hours.
Ethics statement
This study was reviewed and approved by the Faculty of Pharmacy’s Ethical Committee (Approval number: PT 2612). The Children’s Cancer Hospital Egypt 57357 Biobank Committee has approved the sample release. Under the Biobank Research Consent, all participants’ parents or legal guardians had to sign a Biobank research consent form.
Blood sampling and DNA extraction
Participants’ peripheral blood (5 mL) was taken and collected in EDTA vacutainers. We make DNA extraction from Qiagen kits according to the manufacturing instructions. DNA was measured by the The Nanoquant TM spectrophotometer which was used to determine the purity and concentration of DNA. Until pharmacogenetic analysis, DNA was stored at –20°C.
Eligibility Criteria
Subjects between the ages of one and eighteen years who had a confirmed early diagnosis of RMS and were using cyclophosphamide as part of their treatment protocol from 2013 to 2016 were eligible for this study. Patients with a history of chronic renal disease, hepatic disease, or any other type of cancer other than RMS were excluded from the trial. Patients who were pregnant were also excluded.
CYP3A5 genotyping
Blood samples from 150 unrelated patients were used to obtain genomic DNA. For the detection of CYP polymorphisms, a real-time PCR was utilized with the TaqMan SNP Genotyping Assay (Applied Biosystems) for CYP3A5*3, CYP3A5*6, and CYP3A5*7 from thermo fisher company. All PCR amplifications were carried out according to manufacturing instructions (Table 2). The TaqMan 5’-nuclease test chemistry from Applied Biosystems makes SNP genotyping findings quick and easy. The thermal cycling conditions of both SNP and DME is presented in Table 3.
Table 2.
Primers Used for the Analysis of CYP3A5 Polymorphisms
| Allele | SNP | Polymorphism | Primers Sequence |
|---|---|---|---|
| CYP3A5*3 | Rs776746 | T/C, Transition, Substitution | ATGTGGTCCAAACAGGGAAGAGATA[T/C]TGAAAGACAAAAGAGCTCTTTAAAG |
| CYP3A5*6 | Rs10264272 | C/T, Transition, Substitution | CTAAGAAACCAAATTTTAGGAACTT[C/T]TTAGTGCTCTCCACAAAGGGGTCTT |
| CYP3A5*7 | Rs41303343 | A/-, Insertion/Deletion | CCATCTGTACCACGGCATCATAGGT[A/]AGGTGGTGCCTGGAAGGAAAGAAAC |
Table 3.
Thermal Cycling Conditions
| Steps | Predesigned SNP and Custom | DME | ||||
|---|---|---|---|---|---|---|
| Temp. | Duration | Cycles | Temp. | Duration | Cycles | |
| AmpliTaq Gold®, UP, EnzymeActivation | 95°C | 10 minutes | HOLD | 95°C | 10 minutes | HOLD |
| Denaturation | 95°C | 15 seconds | 40 | 95°C | 15 seconds | 50 |
| Annealing/Extension | 60°C | 1 minute | 60°C | 90 seconds | ||
Clinical evaluation & toxicity criteria
All patients’ tumor responses were classified as complete response (CR): Disappearance of all target lesions for a period at least one month, partial response (PR): ≥30% decrease in the sum of the longest diameters of the target lesions,taking as reference the baseline sum of the longest diameter, stable/stationary disease (StD): Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum of the longest diameter since the treatment started, or progressive disease (PD): ≥ 20% increase in the sum of the longest diameter of the target lesions, using the response evaluation criteria [25] in solid tumors (Response evaluation criteria in solid tumor-RECIST) guidelines version 1.0, and we graded each toxicity using the updated Common Terminology Criteria for Adverse Events (CTCAE) guidelines.
Measurements & Parameters
Radiological assessment by MRI and CT scan was done to mark the end of treatment, and was obtained from the patient’s records. Cumulative incidence of toxic mortality “overall survival” and event free survival “relapse-free survival” were recorded. Hemorrhagic cystitis was documented upon occurrence of and hematuria by urine analysis. When RBC above 50 /HPF and in case of double the standard dose of MESNA was used. Complete blood count for measuring pancytopenia/neutropenia after CPA dose administration was performed every 3 weeks along with liver function tests (ALT, AST and total bilirubin) and kidney function tests (serum creatinine and BUN). Toxicities grading were according to CTCAE guidelines. Modifications in CPA dosage was done if the patient had substantial hematuria (>50 RBCs/HPF). Cyclophosphamide was stopped and restarted when hematuria has cleared for at least 1 week at a 50% dose and escalated to 100% if tolerated. MESNA was given as a continuous infusion with its dose equivalent to double the daily CPA dose. The MESNA continuous infusion was begun at the same time as the cyclophosphamide infusion and ceased no sooner than 8 hours after the CPA infusion has finished.
Statistical Analysis
Power analysis has been conducted prior to the study, revealing that with a sample size of 150 patients and alpha targeted as 0.05, we achieve a 97% power to detect odds ratio of 3.75 [26,27]. Descriptive statistics for categorical variables included frequencies and percentages, while continuous variables were summarized using means with standard deviations or medians with interquartile ranges based on data distribution. For univariate analysis, continuous outcomes were compared using Student’s t-test / Wilcoxon test (according to distribution), while categorical outcomes were analyzed using Chi-square or Fisher’s exact test. Endpoints for overall survival (OS) are measured from the date of diagnosis to the date of death or last contact, and event-free survival (EFS) is measured from the date of diagnosis to the date of failure or last contact for failure-free patients. Multivariate logistic regression and Cox Proportional Hazard Models were used to adjust for covariates, including patient demographics, clinical characteristics, CYP3A5 mutation, and toxicities. Model selection employed backward elimination and was evaluated using the Akaike Information Criterion (AIC). A two-sided probability of p<0.05 is considered statistically significant. The analyses are conducted using SPSS version 20 and R v4.2.1.
Results
Patient demographics and characteristics
Table 4 illustrates patients’ characteristics. A total of 150 pediatric RMS patients were enrolled in receiving CPA as part of their clinical treatment protocol for RMS. The study population had a mean age of 5.72 ± 4.04 years and included 87 male and 63 female patients. The mean body weight, height, and surface area were 21.5 ± 15.6 kg, 109.7 ± 25.7 cm, and 0.96 ± 0.44 m2, respectively. The gender distribution in that cohort study was 58% male and 42% female. Frequencies of pancytopenia and hemorrhagic cystitis were 100 % while the incidence of nephrotoxicity was 87.3 %. The incidence of RMS primary sites at the abdominal wall, biliary tract, liver, chest wall, extremities, genitourinary (non-bladder or non-prostate), head &neck, pelvic, perineal region, retroperitoneal pelvic, and urinary bladder was 0.7%, 2%, 2%, 10.7%, 5.3%, 46.7%, 16.7%, 0.7%, 0.7%, and 14%, respectively. RMS stage frequencies from stages I, II, III, and IV were 15.3%, 7.3%, 53.3%, and 23.3%, respectively. The types of RMS distribution in the study were 12% alveolar and 88% embryonal, while tumor site distribution was 12% unfavorable and 88% favorable as well. RMS risk incidence was 23.3% high risk, 70.7% intermediate risk, and 5.3% low risk, respectively. There’s a 23.3% metastasis in that cohort study. RMS treatment protocol responses show 44.7% complete remission (CR), 13.3% partial remission (PR), 34% progressive disease (PD), and 4% stationary disease (StD).
Table 4.
Patient Demographics and Characteristics
| Variables | Number of patients | Percentage | |
|---|---|---|---|
| CYP3A5 Interpretation |
CYP3A5 1*1 | 7 | 4.70% |
| CYP3A5 1*3 | 33 | 22.00% | |
| CYP3A5 1*6 | 2 | 1.30% | |
| CYP3A5 3*3 | 92 | 61.30% | |
| CYP3A5 3*6 | 16 | 10.70% | |
| Metabolizing Status | Intermediate Metabolizer | 51 | 34.00% |
| Normal Metabolizer | 7 | 4.70% | |
| Poor Metabolizer | 92 | 61.30% |
Genotyping and allele frequency
The distribution of the CYP3A5 genotypes are shown in Table 5. In our cohort, 4.7% CYP3A5 *1/*1 (homozygous/normal metabolizer), 22% CYP3A5 *1/*3 (heterozygous/intermediate metabolizer), 1.3% CYP3A5 *1/*6 (heterozygous/intermediate metabolizer), 61.3% CYP3A5 *3/*3 (homozygous/poor metabolizer), and 10.7% CYP3A5 *3/*6 (heterozygous/ poor metabolizer). The frequency of having normal, intermediate, and poor metabolizers was 4.7%, 34%, and 61.3%, respectively.
Table 5.
Genotyping and Allele Frequency
| Variables | Total number of patients = 150 |
||
|---|---|---|---|
| Age (years) | Mean ± SD | 5.72 ± 4.04 | |
| Weight (Kg) | Mean ± SD | 21.5 ± 15.6 | |
| Height (cm) | Mean ± SD | 109.7 ± 25.7 | |
| BSA(m2) | Mean ± SD | 0.96 ± 0.44 | |
| Number of patients | Percentage | ||
| Gender | Male | 87 | 58% |
| Female | 63 | 42% | |
| Primary site | Abdominal Wall | 1 | 0.70% |
| Biliary Tract Liver | 3 | 2.00% | |
| Chest Wall | 3 | 2.00% | |
| Extremities | 16 | 10.70% | |
| Genitourinary (Non-Bladder/Non-Prostate) | 8 | 5.30% | |
| Head & Neck | 70 | 46.70% | |
| Pelvic | 25 | 16.70% | |
| Perineal Region | 1 | 0.70% | |
| Retroperitoneal Pelvic | 1 | 0.70% | |
| Urinary bladder | 21 | 14.00% | |
| Missing data | 1 | 0.70% | |
| Stage | Stage I | 23 | 15.30% |
| Stage II | 11 | 7.30% | |
| Stage III | 80 | 53.30% | |
| Stage IV | 35 | 23.30% | |
| Missing data | 1 | 0.70% | |
| Type of RMS | Alveolar | 18 | 12.00% |
| (Histopathology) | Embryonal | 132 | 88.00% |
| Tumor Site | Favorable | 132 | 88.00% |
| Unfavorable | 18 | 12.00% | |
| Initial risk | High Risk | 35 | 23.30% |
| Intermediate Risk | 106 | 70.70% | |
| Low Risk | 8 | 5.30% | |
| Missing data | 1 | 0.70% | |
| Metastasis | No | 114 | 76.00% |
| Yes | 35 | 23.30% | |
| Missing data | 1 | 0.70% | |
| Response | Complete Remission | 67 | 44.70% |
| Partial Remission | 20 | 13.30% | |
| Progressive Disease | 51 | 34.00% | |
| Stationary Disease | 6 | 4.00% | |
| Missing data | 6 | 4.00% | |
| Nephrotoxicity | No | 19 | 12.70% |
| Yes | 131 | 87.30% | |
| Hemorrhagic cystitis | Yes | 150 | 100% |
| Pancytopenia | Yes | 150 | 100% |
Factors influencing the 5 years Overall Survival (OS)
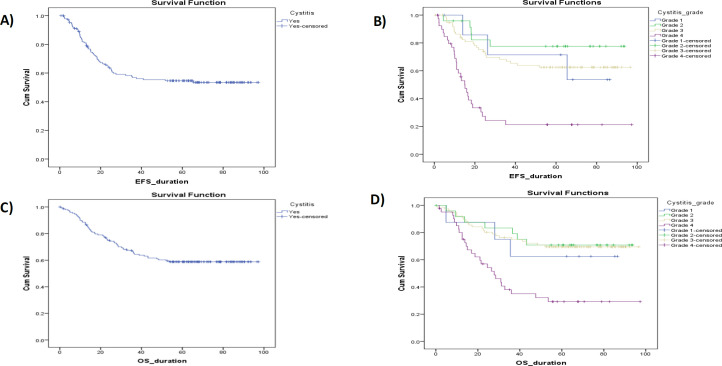
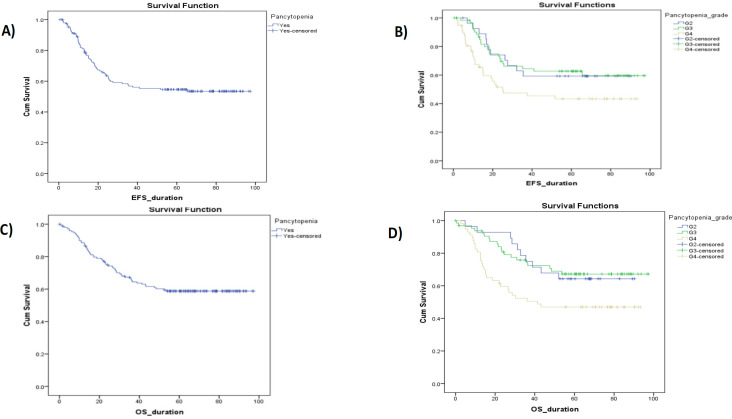
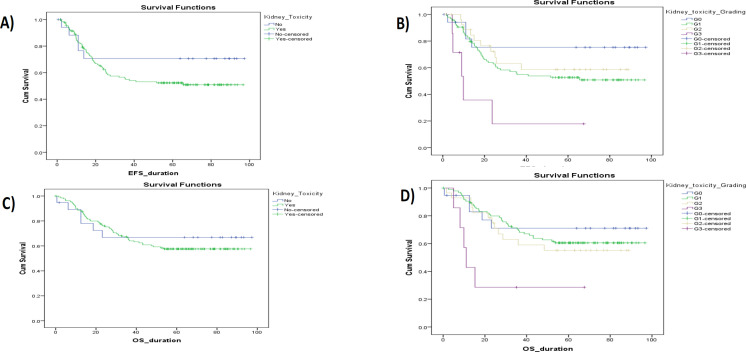
Figures 1, 2, and 3 show Kaplan-Meier illustrating OS with the hemorrhagic cystitis and its toxicity grading, the pancytopenia and its toxicity, and the kidney toxicity and its toxicity grading of the study cohort, respectively. There’s a significant difference between OS and gender, tumor stage, initial risk, metastasis, response, hemorrhagic cystitis grade, pancytopenia grade, and nephrotoxicity grade, with a p<0.05. No significant difference was observed with types of RMS, tumor site, CYP3A5 interpretations, or metabolizing status, with p values of 0.06, 0.06, 0.28, and 0.15, respectively (Table 6).
Figure 1.
Kaplan-Meier Illustrating OS and EFS with the Hemorrhagic Cystitis & Its Grading Toxicity of the Study Cohort
Figure 2.
Kaplan-Meier Illustrating OS and EFS with the Pancytopenia & Its Grading Toxicity of the Study Cohort.
Figure 3.
Kaplan-Meier Illustrating OS and EFS with the Kidney Toxicity & Its Grading Toxicity of the Study Cohort.
Table 6.
Factors Influencing the 5 Years Overall Survival
| Factors influencing the OS | Case Processing Summary | OS (5 years) | 95% CI Mean survival duration | P value | |||
|---|---|---|---|---|---|---|---|
| Event number | Censored Number | Probability % | Lower | Upper | |||
| Gender | Female | 32 | 31 | 49.3 | 48.078 | 67.384 | < 0.05* |
| Male | 30 | 57 | 65.7 | 62.669 | 78.301 | ||
| Primary site | Abdominal Wall | 0 | 1 | 100 | - | - | 0.079 |
| Biliary Tract Liver | 2 | 1 | 33.3 | - | - | ||
| Chest Wall | 1 | 2 | 66.7 | - | - | ||
| Extremities | 8 | 8 | 50 | - | - | ||
| Genitourinary | 1 | 7 | 87.5 | - | - | ||
| Head & Neck | 27 | 43 | 62.9 | - | - | ||
| Pelvic | 16 | 9 | 35.2 | - | - | ||
| Perineal Region | 1 | 0 | 100 | - | - | ||
| Retroperitoneal | 1 | 0 | 100 | - | - | ||
| Urinary bladder | 5 | 16 | 57.6 | - | - | ||
| Stage | Stage I | 7 | 16 | 73.2 | 62.176 | 85.644 | < 0.05* |
| Stage II | 3 | 8 | 70.7 | 61.622 | 97.396 | ||
| Stage III | 26 | 54 | 68.4 | 64.587 | 80.205 | ||
| Stage IV | 26 | 9 | 21.6 | 22.5 | 42.007 | ||
| Type of RMS | Alveolar | 8 | 10 | 55.6 | 38.315 | 73.251 | 0.06 |
| (Histopathology) | Embryonal | 54 | 78 | 59.1 | 59.468 | 72.48 | |
| Tumor site | Favorable | 54 | 78 | 59.1 | 59.468 | 72.48 | 0.06 |
| Unfavorable | 8 | 10 | 55.6 | 38.315 | 73.251 | ||
| Initial risk | High Risk | 26 | 9 | 21.6 | 22.5 | 42.007 | < 0.05* |
| Intermediate Risk | 35 | 71 | 68.3 | 66.429 | 79.814 | ||
| Low Risk | 1 | 7 | 87.5 | 68.156 | 99.098 | ||
| CYP3A5 Interpretation | CYP3A5 1*1 | 5 | 2 | 42.9 | - | - | 0.28 |
| CYP3A5 1*3 | 14 | 19 | 57.6 | - | - | ||
| CYP3A5 1*6 | 0 | 2 | 100 | - | - | ||
| CYP3A5 3*3 | 39 | 53 | 57.6 | - | - | ||
| CYP3A5 3*6 | 4 | 12 | 71.8 | - | - | ||
| Intermediate Metabolizer | 18 | 33 | 63.3 | 61.47 | 80.689 | 0.15 | |
| Metabolizing Status | Normal Metabolizer | 5 | 2 | 0 | 18.279 | 75.744 | |
| Poor Metabolizer | 39 | 53 | 57.6 | 55.5 | 71.762 | ||
| Metastasis | No | 36 | 78 | 67.9 | 67.839 | 80.578 | < 0.05* |
| Yes | 26 | 9 | 21.6 | 22.5 | 42.007 | ||
| Response | CR | 7 | 60 | 92.4 | 85.902 | 95.355 | < 0.05* |
| Partial Remission | 7 | 13 | 65 | 44.533 | 65.598 | ||
| Progressive Disease | 42 | 9 | 13.8 | 24.705 | 39.66 | ||
| Stationary Disease | 2 | 4 | 66.7 | 33.605 | 85.521 | ||
| Yes | 5 | 6 | 54.5 | 35.844 | 75.697 | ||
| Hemorrhagic cystitis | Yes | 60 | 90 | 58.8 | 59.895 | 72.304 | - |
| Grade | Grade 1 | 3 | 5 | 62.5 | 40.514 | 84.65 | < 0.05* |
| Grade 2 | 7 | 18 | 70.8 | 60.434 | 86.421 | ||
| Grade 3 | 23 | 53 | 69.4 | 65.847 | 81.768 | ||
| Grade 4 | 27 | 14 | 29.2 | 31.602 | 54.782 | ||
| Pancytopenia | Yes | 60 | 90 | 58.8 | 59.895 | 72.304 | - |
| Grade | G2 | 10 | 18 | 64.3 | 57.94 | 79.941 | < 0.05* |
| G3 | 20 | 45 | 67.2 | 64.531 | 82.172 | ||
| G4 | 30 | 27 | 47 | 42.628 | 62.907 | ||
| Nephrotoxicity | No | 19 | 6 | 66.9 | 50.643 | 87.663 | 0.63 |
| Yes | 131 | 54 | 57.6 | 58.749 | 71.795 | ||
| Grade | G1 | 96 | 37 | 60.5 | 60.886 | 75.547 | < 0.05* |
| G2 | 28 | 12 | 55.1 | 45.724 | 71.749 | ||
| G3 | 7 | 5 | 28.6 | 6.914 | 45.78 | ||
Factors influencing the 5 years Event Free Survival (EFS)
Figures 1, 2, and 3 show Kaplan-Meier illustrating OS with the hemorrhagic cystitis and its toxicity grading, the pancytopenia and its toxicity, and the kidney toxicity and its toxicity grading of the study cohort, respectively. There’s a significant difference between EFS and primary site, tumor stage, initial risk, metastasis, response, hemorrhagic cystitis grade, pancytopenia grade, and nephrotoxicity grade, with p<0.05. There was no significant difference with gender, types of RMS, tumor site, CYP3A5 interpretations, or metabolizing status, with p values of 0.38, 0.38, 0.41, and 0.28, respectively (Table 7).
Table 7.
Factors influencing the 5 years Event Free Survival
| Factors influencing the EFS | Case Processing Summary | EFS (5 years) | 95% CI Mean survival duration |
P value | |||
|---|---|---|---|---|---|---|---|
| Event number | Censored Number | Probability % | Lower | Upper | |||
| Gender | Female | 27 | 36 | 50 | 46.06 | 67.617 | 0.51 |
| Male | 36 | 50 | 58.1 | 53.848 | 71.27 | ||
| Primary site | Abdominal Wall | 0 | 1 | 100 | - | - | < 0.05* |
| Biliary Tract Liver | 1 | 2 | 50 | - | - | ||
| Chest Wall | 1 | 2 | 66.7 | - | - | ||
| Extremities | 9 | 7 | 34.9 | - | - | ||
| Genitourinary | 1 | 7 | 87.5 | - | - | ||
| Head & Neck | 25 | 45 | 59.9 | - | - | ||
| Pelvic | 17 | 8 | 29.3 | - | - | ||
| Perineal Region | 1 | 0 | 0 | - | - | ||
| Retroperitoneal | 1 | 0 | 0 | - | - | ||
| Urinary bladder | 7 | 14 | 66.3 | - | - | ||
| Stage | Stage I | 6 | 17 | 72.7 | 57.962 | 85.716 | < 0.05* |
| Stage II | 3 | 8 | 68.6 | 50.099 | 95.97 | ||
| Stage III | 27 | 53 | 64.6 | 60.172 | 77.266 | ||
| Stage IV | 27 | 8 | 14.7 | 13.935 | 32.381 | ||
| Type of RMS | Alveolar | 9 | 9 | 50.7 | 32.628 | 68.682 | 0.38 |
| (Histopathology) | Embryonal | 54 | 77 | 55.6 | 54.28 | 68.724 | |
| Tumor site | Favorable | 54 | 77 | 55.6 | 54.28 | 68.724 | 0.38 |
| Unfavorable | 9 | 9 | 50.7 | 32.628 | 68.682 | ||
| Initial risk | High Risk | 27 | 8 | 14.7 | 13.935 | 32.381 | < 0.05* |
| Intermediate Risk | 35 | 71 | 65 | 62.153 | 77.068 | ||
| Low Risk | 1 | 7 | 87.5 | 63.035 | 100.605 | ||
| CYP3A5 Interpretation |
CYP3A5 1*1 | 4 | 3 | 51.4 | - | - | 0.41 |
| CYP3A5 1*3 | 14 | 19 | 55.1 | - | - | ||
| CYP3A5 1*6 | 0 | 2 | 100 | - | - | ||
| CYP3A5 3*3 | 41 | 50 | 51.2 | - | - | ||
| CYP3A5 3*6 | 4 | 12 | 72.7 | - | - | ||
| Intermediate Metabolizer | 18 | 33 | 62.2 | 55.785 | 77.772 | 0.28 | |
| Metabolizing Status | Normal Metabolizer | 4 | 3 | 51.4 | 21.171 | 71.436 | |
| Poor Metabolizer | 41 | 50 | 51.2 | 48.743 | 66.442 | ||
| Metastasis | No | 36 | 78 | 66.8 | 63.785 | 77.996 | < 0.05* |
| Yes | 27 | 8 | 14.7 | 13.935 | 32.381 | ||
| Response | CR | 7 | 60 | 90.8 | 84.855 | 95.268 | < 0.05* |
| Partial Remission | 6 | 14 | 64.7 | 43.848 | 66 | ||
| Progressive Disease | 49 | 2 | 0 | 11.718 | 16.49 | ||
| Stationary Disease | 1 | 5 | 80 | 47.174 | 91.317 | ||
| Hemorrhagic cystitis | Yes | 64 | 85 | 54.6 | 53.338 | 66.932 | - |
| Grade | Grade 1 | 3 | 5 | 53.6 | 42.211 | 85.314 | < 0.05* |
| Grade 2 | 5 | 19 | 77.6 | 63.318 | 89.839 | ||
| Grade 3 | 27 | 49 | 62.5 | 58.831 | 76.439 | ||
| Grade 4 | 29 | 12 | 21.2 | 19.38 | 42.443 | ||
| Pancytopenia | Yes | 64 | 85 | 54.6 | 53.338 | 66.932 | - |
| Grade | G2 | 11 | 17 | 59.3 | 48.52 | 74.854 | < 0.05* |
| G3 | 23 | 41 | 62.7 | 56.717 | 76.525 | ||
| G4 | 30 | 27 | 43.4 | 37.433 | 59.201 | ||
| Nephrotoxicity | No | 19 | 5 | 70.6 | 52.095 | 90.454 | 0.23 |
| Yes | 130 | 59 | 52.3 | 50.965 | 65.319 | ||
| Grade | G1 | 95 | 44 | 52.7 | 50.119 | 66.668 | < 0.05* |
| G2 | 28 | 10 | 58.6 | 46.029 | 73.843 | ||
| G3 | 7 | 5 | 17.9 | 2.609 | 39.338 | ||
CYP3A5 Interpretation
As shown in Supplementary Table 8, there’s a significant difference between hemorrhagic cystitis grade and CYP3A5 polymorphism with a p value <0.05, and the metabolizing status had a significant difference with the CYP3A5 polymorphism with a p value <0.05. There is no significant difference between nephrotoxicity and pancytopenia with a p value >0.05. Also, there’s no significant difference between gender, RMS tumor primary site, tumor stage (from stage I to IV), type of RMS (alveolar or embryonal), tumor site (favorable or unfavorable), initial tumor risk (high, intermediate, or low risk), cancer metastasis, clinical treatment response, and the CYP3A5 interpretations of the following CYP3A5 *1/*1, *1/*3, *1/*6, *3/*3, and *3/*6, respectively, with a p value >0.05.
Discussion
Cyclophosphamide (CPA) is an anticancer prodrug that must be bioactivated to become the active alkylating metabolite, 4-OH-CPA, in order to exercise its antitumor activity [28]. The response to CPA treatment varies significantly. There is a significant heterogeneity in CPA treatment responses. CYP3A5 was identified as the major CPA 4-hydroxylase catalyzing the metabolism of CPA [13,29]. Decisions on treatment doses, whether to strengthen or decrease chemotherapy dosages, or the length of treatment may benefit from strategies to identify individuals at risk of treatment failure, high toxicity incidence, and those who will need further dose adjustment. Several studies looked into how CYP3A5 affected various medicines worked clinically [30].
Our study strategy was focused on specific SNPs in the CPA activation pathway that are expected to affect response and toxicity to CPA treatment. The three non-functional SNPs identified in CYP3A5 exons 3, 6, and 7 are rs776746 (C_26201809_30/CYP3A5*3), rs10264272 (C_30203950_10/CYP3A5*6), and rs41303343 (C_32287188_10/CYP3A5*7). They were carefully chosen based on evidence that they are widespread in Caucasians, they alter CYP3A5 coding, which could modify the expression or activity of the CYP3A5 enzyme, and they are linked to a change in CPA activation and metabolism. Our study states that CYP3A5 functioning may affect the pharmacological activity and toxicity of CPA, which may have an impact on how well a patient responds to treatment. Hence, the CYP3A5 polymorphic variations may change CPA metabolism, which could account for some of the interpatient heterogeneity in CPA responsiveness. In light of these considerations, the aim of this study was to study the effect of genetic polymorphisms in CYP3A5 on the efficacy and toxicity of cyclophosphamide in Egyptian cancer patients. As a predictive biomarker for response and toxicity, toxicity grades can also be used to assess if they affect treatment efficacy and survival outcomes. A total of 150 RMS-affected kids were enlisted, and genotyping results for the chosen SNPs together with all of the clinical information and results were examined.
Although CYP3A5 is known to exhibit inter-ethnic variation, there is a significant level of genetic diversity among various groups. In our cohort, the percentage of patients carrying at least one copy of CYP3A5*3 (rs776746) and CYP3A5*6 (rs10264272) was 94% and 12%, respectively, as shown in Table 4. The main finding in our study was that in RMS patients treated with VAC as the first line of treatment, there was an association between rs776746 and rs10264272 and response to CPA-based treatment. We found that patients who received cyclophosphamide-based adjuvant chemotherapy for RMS cancer and had the CYP3A5 *1/*3, *1/*6, *3/*3, *3/*6 had significantly greater toxicity and efficacy than those who were CYP3A5 *1/*1 wild-type. These findings support our hypothesis that reduced phase-I enzyme activity (via the CYP3A5 polymorphism) leads to huge toxicity with lower efficacy after cyclophosphamide-based treatment chemotherapy, presumably as a result of slower activation of cyclophosphamide to the active form 4-hydroxycyclophosphamide.
CYP3A5 polymorphism affects the survival rates as there’s a significant difference between OS and gender, tumor stage, initial risk, metastasis, response, hemorrhagic cystitis grade, pancytopenia grade, and nephrotoxicity grade and a significant difference between EFS and primary site, tumor stage, initial risk, metastasis, response, hemorrhagic cystitis grade, pancytopenia grade, and nephrotoxicity grade. A previous study by Labib et al., [26] examined the association of CYP2B6 single nucleotide polymorphisms (SNPs) with the survival of RMS in a cohort of 73 pediatric RMS patients treated with CPA-based first-line treatment. They analyzed the association between those genotypes and the survival outcome of RMS. They demonstrated that CYP2B6 rs2279343 may predict EFS in RMS patients and warrant future studies to clarify the pharmacogenetics of CPA in pediatrics which enforced our results.
Gor et al., [31] evaluated the cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer. They performed a retrospective cohort study of 350 women enrolled in a multicenter, randomized adjuvant breast cancer chemotherapy trial. Cox regression models were computed to determine associations between genotypes (individually or in combination) and disease-free survival (DFS) or overall survival (OS), adjusting for confounding clinical variables. Pinto et al., [6] analyzed the associations between event-free survival and 394 single-nucleotide polymorphisms (SNP) in 14 drug metabolizing enzymes or transporters involved in CPA pharmacokinetics which suggested that a pharmacogenomic approach to therapy personalization of cyclophosphamide in intermediate-risk rhabdomyosarcoma.
Another study suggests that genetic polymorphisms in enzymes involved in the activation of cyclophosphamide, such as CYP3A4, and CYP3A5, can lead to decreased enzyme activity and an increased risk of developing graft-versus-host disease (GVHD). Understanding these genetic factors may assist in improving the clinical management of transplant recipients by better predicting and managing the risks associated with cyclophosphamide therapy [12].
Abuelsoud & El Khateeb, 2023 investigated the association between the CYP2B6 c.516G>T polymorphism (rs 3745274) and various parameters related to the efficacy and tolerability of cyclophosphamide (CPA) in Egyptian patients with lupus nephritis (LN). The study findings suggest that pre-treatment evaluation of CYP2B6 rs 3745274 may help account for individual differences in treatment response for LN patients receiving cyclophosphamide therapy. This highlights the potential importance of genetic factors in predicting treatment outcomes and guiding personalized treatment strategies.
Also, CYP3A5 polymorphism showed an effect on the hemorrhagic cystitis grade and CYP3A5 metabolizing status. Other researchers examined cyclophosphamide-induced hemorrhagic cystitis. A series of 100 patients with hemorrhagic cystitis induced by cyclophosphamide was studied. [33]. While several medications may induce CYP activity, potentially modifying the expected effect of genotype, one would anticipate that such effects would be relevant only if a large proportion of the study subjects were taking such medications during chemotherapy. CYP3A5 polymorphism poor and intermediate metabolizers of CYP3A5*3 and CYP3A5*6 showed higher toxicity with lower efficacy than the normal metabolizers. CYP3A5*7 wasn’t present in the study participants; CYP3A5*3/rs776746 (homozygous and heterozygous) showed more association with cyclophosphamide efficacy and toxicity than CYP3A5*6/rs10264272 (homozygous and heterozygous).
In Conclusion, in summary, our findings paint a picture of the role of CYP3A5 polymorphisms in RMS. This study shows that among patients receiving cyclophosphamide as a component of chemotherapy used to treat RMS, a polymorphism in the cyclophosphamide-metabolizing enzyme CYP3A5 independently influences the results from cyclophosphamide-based RMS chemotherapy. When considered in conjunction with the results of earlier studies, these findings support retrospective research to further examine the connection between genetic variation, metabolite levels, and outcome to see if pharmacogenetic dosing regimens can enhance the efficacy and toxicity of this therapy. Considering CYP3A5 rs776746 and rs10264272 prior to therapy may help to explain some inter-individual variations in treatment response, according to our data. The integration of genetic factors with clinical and molecular characteristics could be applied. Additionally, this might simplify clinical judgment, enhance CPA therapy, and hence enhance clinical success. The pharmacokinetic modeling of CPA activation will help stratify RMS patients according to their genetic composition and provide a clearer understanding of the inter-individual diversity in medication response. It is estimated that the non-functional CYP3A5 *1/*3, *1/*6, *3/*3, and *3/*6 were shown to have higher cyclophosphamide efficacy and toxicity than the functional CYP3A5*1/*1.
Limitation
Due to low participants of the normal CYP3A5 *1/*1 wild, we need more normal samples to make more accurate correlations. We also need to perform additional research on all the medications used in RMS protocols to obtain more accurate clinical investigations and interpretations of the RMS patients.
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors
Data availability
The data underlying this article are available in the article
Abbreviations
CCHE: Children’s Cancer Hospital of Egypt; RMS: rhabdomyosarcoma; VAC: vincristine, actinomycin, cyclophosphamide; MESNA: sodium 2-mercaptoethane sulfonate; CYP: cytochrome P450; CPA: cyclophosphamide; DME: drug metabolizing enzymes; SNPs: Single nucleotide polymorphisms; rs: reference SNP cluster ID for every allele; ES: event-free survival; OS: overall survival; CR: complete remission; PR: partial remission; PD: progressive disease; StD: stationary disease; ERMS: embryonal rhabdomyosarcomas; ARMS: alveolar rhabdomyosarcomas; TNM staging: Tumor, Node and Metastasis staging; EDTA: Ethylenediaminetetraacetic acid; CTCAE: Common Terminology Criteria for Adverse Events; RECIST: Response evaluation criteria in solid tumors; DFS: disease-free survival; AIC: Akaike Information Criterion; IRSG-IV protocol: Intergroup Rhabdomyosarcoma Study Group Protocol; PCR: polymerase chain reaction ; ALT: alanine transaminase; AST: aspartate aminotransferase; GVHD: graft-versus-host disease; LN: lupus nephritis.
Conflict of interest
None.
Author Contribution Statement
Cherine E. ElShereef; Writing-Original draft preparation, Investigation. Hala F. Zaki; Validation, Writing-Review and Editing. Osama A. Badary; Conceptulization, Methodology. Sherif kamal; Data Curation. Mohamed Nagy; Formal analysis. Dalia Makhlouf; Investigation. Mohamed Kamal; Formal analysis. Inas elnady; Resources. Sameh A.Abdelshafi; Project administration. Sherif Abou El Naga; Supervision. Mona M. Saber; Writing-Review and Editing, Formal analysis.
Supplementary materials
References
- 1.Radzikowska J, Kukwa W, Kukwa A, Czarnecka A, Krzeski A. Rhabdomyosarcoma of the head and neck in children. Contemp Oncol (Pozn) 2015;19(2):98–107. doi: 10.5114/wo.2015.49158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okcu f. Rhabdomyosarcoma in childhood and adolescence: Epidemiology, pathology, and molecular pathogenesis. 2016. Up to date. Available online: http://www.Uptodate.Com/contents/rhabdomyosarcoma-in-childhood-and-adolescence-epidemiology-pathology-and-molecularpathogenesis.
- 3.Murshed H. Chapter 26 - radiation treatment, toxicity, and their management the unreferenced contents are primarily an organized compilation of the author’s (t.W. dziuk, m.D.) personal prescribing notes and practices; no warranty, expressed or implied, is made as to accuracy. Although every attempt has been made, both in writing and in editing, to ensure accuracy, the reader is urged to carefully review information. In: Murshed H, editor. Fundamentals of radiation oncology. third edition. Academic Press; 2019. pp. 961–74. [Google Scholar]
- 4.Soni H. Martindale: The complete drug reference brayfield alison (ed) martindale: The complete drug reference £459 4,688pp pharmaceutical press 9780857111395 0857111396 [formula: See text] Emergency nurse : the journal of the RCN Accident and Emergency Nursing Association. 2014;22:5–12. doi: 10.7748/en.22.5.12.s13. [DOI] [PubMed] [Google Scholar]
- 5.Boddy AV, Yule SM. Metabolism and pharmacokinetics of oxazaphosphorines. Clin Pharmacokinet. 2000;38(4):291–304. doi: 10.2165/00003088-200038040-00001. [DOI] [PubMed] [Google Scholar]
- 6.Pinto N, Navarro SL, Rimorin C, Wurscher M, Hawkins DS, McCune JS. Pharmacogenomic associations of cyclophosphamide pharmacokinetic candidate genes with event-free survival in intermediate-risk rhabdomyosarcoma: A report from the children’s oncology group. Pediatr Blood Cancer. 2021;68(11):e29203. doi: 10.1002/pbc.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huttunen KM, Raunio H, Rautio J. Prodrugs--from serendipity to rational design. Pharmacol Rev. 2011;63(3):750–71. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- 8.Herrmann J, Sahni G, Gallardo A, Spahillari A, Galsky M, Eschenhagen T, Schaffer W, Neilan TG, Ak G, Donisan T, Balanescu DV. Cardio-Oncology Practice Manual: A Companion to Braunwald’s Heart Disease . Elsevier; 2022. Cancer therapeutic drug guide; pp. 451–506. [Google Scholar]
- 9.Stice SA, Beedanagari SR, Vulimiri SV, Bhatia SP, Mahadevan B. Biomarkers in Toxicology . Academic Press; 2019. Genotoxicity biomarkers: Molecular basis of genetic variability and susceptibility; pp. 807–821. [Google Scholar]
- 10.Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: Golden anniversary. Nat Rev Clin Oncol. 2009;6(11):638–47. doi: 10.1038/nrclinonc.2009.146. [DOI] [PubMed] [Google Scholar]
- 11.Monach PA, Arnold LM, Merkel PA. Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases: A data-driven review. Arthritis Rheum. 2010;62(1):9–21. doi: 10.1002/art.25061. [DOI] [PubMed] [Google Scholar]
- 12.Muñiz P, Andrés-Zayas C, Carbonell D, Chicano M, Bailén R, Oarbeascoa G, et al. Association between gene polymorphisms in the cyclophosphamide metabolism pathway with complications after haploidentical hematopoietic stem cell transplantation. Front Immunol. 2022;13:1002959. doi: 10.3389/fimmu.2022.1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie HJ, Yasar U, Lundgren S, Griskevicius L, Terelius Y, Hassan M, et al. Role of polymorphic human cyp2b6 in cyclophosphamide bioactivation. Pharmacogenomics J. 2003;3(1):53–61. doi: 10.1038/sj.tpj.6500157. [DOI] [PubMed] [Google Scholar]
- 14.Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, et al. Hepatic cytochrome p450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37(10):2087–94. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang T, Klein K, Fischer J, Nüssler AK, Neuhaus P, Hofmann U, et al. Extensive genetic polymorphism in the human cyp2b6 gene with impact on expression and function in human liver. Pharmacogenetics. 2001;11(5):399–415. doi: 10.1097/00008571-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Xu C, Quinney SK, Guo Y, Hall SD, Li L, Desta Z. Cyp2b6 pharmacogenetics-based in vitro-in vivo extrapolation of efavirenz clearance by physiologically based pharmacokinetic modeling. Drug Metab Dispos. 2013;41(12):2004–11. doi: 10.1124/dmd.113.051755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zanger UM, Klein K, Saussele T, Blievernicht J, Hofmann MH, Schwab M. Polymorphic cyp2b6: Molecular mechanisms and emerging clinical significance. Pharmacogenomics. 2007;8(7):743–59. doi: 10.2217/14622416.8.7.743. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Sakuyama K, Sasaki T, Ishii Y, Ishikawa M, Hirasawa N, et al. Functional characterization of 26 cyp2b6 allelic variants (cyp2b6 2-cyp2b6 28, except cyp2b6 22) Pharmacogenet Genomics. 2010;20(7):459–62. doi: 10.1097/FPC.0b013e32833bba0e. [DOI] [PubMed] [Google Scholar]
- 19.Turpeinen M, Zanger UM. Cytochrome p450 2b6: Function, genetics, and clinical relevance. Drug Metabol Drug Interact. 2012;27(4):185–97. doi: 10.1515/dmdi-2012-0027. [DOI] [PubMed] [Google Scholar]
- 20.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in cyp3a promoters and characterization of the genetic basis of polymorphic cyp3a5 expression. Nat Genet. 2001;27(4):383–91. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 21.Xie HG, Wood AJ, Kim RB, Stein CM, Wilkinson GR. Genetic variability in cyp3a5 and its possible consequences. Pharmacogenomics. 2004;5(3):243–72. doi: 10.1517/phgs.5.3.243.29833. [DOI] [PubMed] [Google Scholar]
- 22.Langman LJ, Dasgupta A. Pharmacogenomics in clinical therapeutics. John Wiley & Sons; 2012 . [Google Scholar]
- 23.Langman L, van Gelder T, van Schaik RH. Chapter 5 - pharmacogenomics aspect of immunosuppressant therapy. In: Oellerich M, Dasgupta A, editors. Personalized immunosuppression in transplantation. San Diego: Elsevier; 2016. pp. 109–24. [Google Scholar]
- 24.Preissner SC, Hoffmann MF, Preissner R, Dunkel M, Gewiess A, Preissner S. Polymorphic cytochrome p450 enzymes (cyps) and their role in personalized therapy. PLoS One. 2013;8(12):e82562. doi: 10.1371/journal.pone.0082562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Labib RM, ME AA, Elnadi E, Hesham RM, Yassin D. Cyp2b6rs2279343 is associated with improved survival of pediatric rhabdomyosarcoma treated with cyclophosphamide. PLoS One. 2016;11(7):e0158890. doi: 10.1371/journal.pone.0158890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez EA, Kassira N, Cheung MC, Koniaris LG, Neville HL, Sola JE. Rhabdomyosarcoma in children: A seer population based study. J Surg Res. 2011;170(2):e243–51. doi: 10.1016/j.jss.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Zanger UM, Klein K. Pharmacogenetics of cytochrome p450 2b6 (cyp2b6): Advances on polymorphisms, mechanisms, and clinical relevance. Front Genet. 2013;4:24. doi: 10.3389/fgene.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy P, Yu LJ, Crespi CL, Waxman DJ. Development of a substrate-activity based approach to identify the major human liver p-450 catalysts of cyclophosphamide and ifosfamide activation based on cdna-expressed activities and liver microsomal p-450 profiles. Drug Metab Dispos. 1999;27(6):655–66. [PubMed] [Google Scholar]
- 30.Timm R, Kaiser R, Lötsch J, Heider U, Sezer O, Weisz K, et al. Association of cyclophosphamide pharmacokinetics to polymorphic cytochrome p450 2c19. Pharmacogenomics J. 2005;5(6):365–73. doi: 10.1038/sj.tpj.6500330. [DOI] [PubMed] [Google Scholar]
- 31.Gor PP, Su HI, Gray RJ, Gimotty PA, Horn M, Aplenc R, et al. Cyclophosphamide-metabolizing enzyme polymorphisms and survival outcomes after adjuvant chemotherapy for node-positive breast cancer: A retrospective cohort study. Breast Cancer Res. 2010;12(3):R26. doi: 10.1186/bcr2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abuelsoud NN, El Khateeb EM. Genetic polymorphisms effect on cyclophosphamide’s tolerability and clinical efficacy in egyptian patients with lupus nephritis. Pharmacogenet Genomics. 2023;33(8):172–80. doi: 10.1097/FPC.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 33.Sherif I. Uroprotective mechanisms of natural products against cyclophosphamide-induced urinary bladder toxicity: A comprehensive review. Acta Sci Pol Technol Aliment. 2020;19(3):333–46. doi: 10.17306/J.AFS.0832. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article