Abstract
Angiogenesis, the formation of new blood vessels, stimulates tumor growth and spread by delivering oxygen and nutrients, and is a key component of metastasis. This work aimed to evaluate the anti-angiogenic properties of a new synthesized compound. Rat aorta angiogenesis assay was used to evaluate the ability of the carbothioamide derivative to inhibit blood vessels sprouting. The tetrazolium (MTT) assay was used to evaluate the anti-proliferative effect of the synthetic compound on human umbilical vein endothelial cell line (HUVECs) and A549 lung cancer cells line. The (2, 2-diphenyl-1-picrylhydrazyl) DPPH was used to investigate the free radical scavenging action. The study showed that the compound has anti-angiogenic activity with IC50 56.9 µg/mL, moreover the compound managed to inhibit the proliferation of HUVECs and A549 cells (IC50 76.3 µg/mL and 45.5 µg/mL, respectively), and The IC50 concentration for free radical scavenging activity of the compound was 27.8 µg/ml. The study concluded that the compound has significant anti-angiogenic activity may be related to its significant anti-proliferative effect against HUVECs, these pharmacological effect may attributed to its potent free radical scavenging activity.
Key Words: Angiogenesis, MTT assay, Rat aorta ring, DPPH, cancer cell line
Introduction
Angiogenesis, the outgrowth and restructuring of new blood vessels from preexisting blood vasculature [1], is a fundamental physiological process but can also occur in different pathological conditions [2]. Angiogenesis process adjusts by many growth factors such as vascular endothelial growth factor (VEGF) via the receptor tyrosine kinase VEGF receptors (VEGFR-2). Moreover, VEGFA/VEGFR-2 signaling shows s very basic role in angiogenesis-related cellular responses [3]. On the other hand, the epidermal growth factor receptor (EGFR) induces cell cycle progression, proliferation, migration, and when up regulated it may leads to cancer [4]. In addition, it has been shown that the EGFR may be partially mediated by induction of angiogenesis by up regulating VEGF [5].
Over the last five decades, there has been a lot of interest in the idea that controlling angiogenesis could have therapeutic benefits. Angiogenesis stimulation had significant beneficial in treating ischemic heart disease and wound healing, while inhibiting angiogenesis can be used to treat tumors and other ailments [5].
Previous study conducted by scientists has been active in synthesizing compounds that target EGFR and VEGFR-2 [6-8]. A series of carbothioamide derivatives had been developed that have been shown to target epidermal growth factor receptor EGFR. One of these compounds is 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide [9-11]. However, the effects of these compounds on angiogenesis have not been investigated. Thus, the current study aimed to investigate the effects of this carbothioamide derivative on rat aortic angiogenesis.
Materials and Methods
Experimental animal
Male albino rats with an average weight of 250 g were used in this experiment. The animals were obtained from Al-Nahrain University / College of Pharmacy/ Animal house. The rats were kept at the Animal House Facility at 28-30 ºC and were allowed free access to food and tap water. The experiments were approved by the Animal Ethical Committee, College of Pharmacy/ Al-Nahrain University [6].
Cell culture
Human umbilical vein endothelial cells (HUVECs) and A459 lung cancer cell line were purchased from the American Type Culture (ATCC, USA). Cells were cultured in F-12K Medium and RPMI-1640 medium respectively, both mediums containing 10% FBS, 1% penicillin, 1% streptomycin, and 1% glutamine respectively. Cells were maintained at 37 °C and 5% CO2. The test started once the cells reached 70% confluence [7].
Anti-proliferation assay (MTT)
(HUVEC and A459 cells) were treated for 24 hours with serial dilution of 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide then 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide salt (MTT) was prepared as 5 mg/ml solution in Phosphate Buffer Saline (PBS). The plate was incubated for 4 hours then the absorbance was detected by ELIZA at 570 nm [8].
Angiogenesis test
The test was performed in according to Hayder and coworker 2023, [10]. The animals were sacrificed via cervical dislocation under light anesthesia with diethyl ether. Thoracic aorta was rapidly excised, rinsed with serum-free M199 medium, the blood clot had been removed from the aorta, and then aorta sectioned into rings of 1 mm in thickness. Forty eight well plates purchased to culture the aorta rings. One aortic ring was placed in each well with 500µl serum-free M199 growth medium supplemented with fibrinogen (3 mg/mL); aprotinin (5µg/mL) was added. After that, 10μl of thrombin was added to each well and the mixture was allowed to harden at 37ºC in 5% CO2 for 15min. After that, the compound had serially diluted with 300 µL of M199 medium supplemented with 20% of fetal bovine serum (FBS), 0.1% 6-aminocaproic acid, 1% L-Glutamine, 0.6% gentamicin, and the carbothioamide derivative. Then the 48 well plates incubated at 37 ºC and 5% CO2 for five days. Each concentration had been repeated six times. The blood vessels outgrowth was counted on day five by inverted microscope equipped with a camera. The extent of vessels outgrowth was determined as Hayder and colleagues did in their previous research [7] the distance of the minute vessels outgrowths from the main ex-plants had been identified. Following formula: determined the percentage of blood vessels inhibition:
% Blood vessel growth inhibition = (A) / (A0) × 100%
Where:
A: average distance of blood vessels outgrowth in the sample (µm); A0: average distance of blood vessels growth in the control.
Free radical scavenging activity with 1, 1-diphenyl -2-picrylhydrazyl (DPPH)
The antioxidant activity of the test compound was evaluated by quantifying the free radical scavenging activity using the DPPH. The sample was prepared in methanol in six different concentrations (500, 250, 125, 62.5, 31.25 and 15.6µg/mL). 200µl of DPPH solution in methanol was added to 100µl of each dilution of the compound. After 30 min of incubation in the dark, absorbance was measured at 517 nm using a plate reader. The tests were performed in triplicate. The free radical scavenging activity (F) was calculated using the following formula [11]: F = [(A) / A0] × 100%
Where: A0: Absorbance of the control; A: Absorbance of the sample.
Results
Anti-proliferative activity of 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide
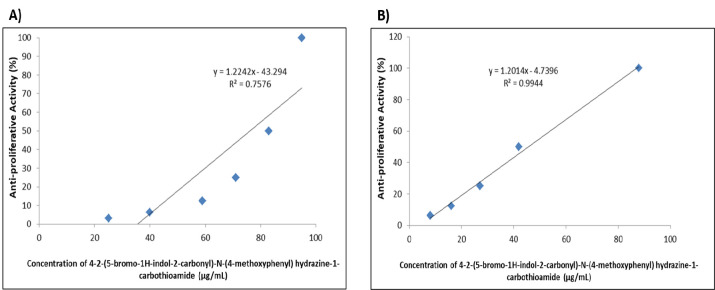
The anti-proliferative activity of the 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide was evaluated against HUVEC (Figure 1A) and A549 lung cancer cells (Figure 1B) via means of the MTT assay. The carbothioamide derivative showed a dose response anti-proliferative activity against both cell types. 100, 50, 25, 12.5, 6.25 and 3.125µg/ml inhibit the HUVEC cell line by 95, 83, 71, 59, 40 and 25% respectively The IC50 concentration against HUVECs was 76.4µg/mL, while the serial dilution concentrations showed anti-proliferation activity against A549 cell line by 88, 42, 27, 16, 8 and 2% IC50 was 45.5µg/ml.
Figure 1.
Dose Response Curve of the Anti-Proliferative Aactivity of 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide against (A) HUVEC cell line; and (B) A549 lung cancer cell line.
Rat aorta angiogenesis assay
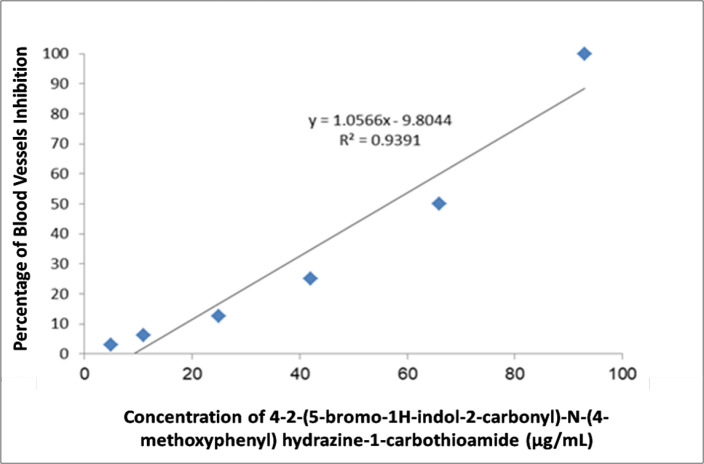
The results of the rat aorta angiogenesis assay (Figure 2) indicate that 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide showed a significant dose response inhibition of blood vessel growth, 100, 50, 25, 12.5, 6.25, 3, 12 µg/mL of the carbothioamide derivative inhibited blood vessels growth by 93%, 66%, 42%, 25%, 11%, and 5%, respectively (Figure 3). The IC50 concentration from the dose response curve of blood vessels growth inhibition is 56.9µg/ml. Suramin the positive control inhibited the blood vessels out growth by 99%, however there was no significant differences between the concentration 100µg/ml of the compound and the same concentration of suramin but the percentage of blood vessels inhibition still high with suramin.
Figure 2.
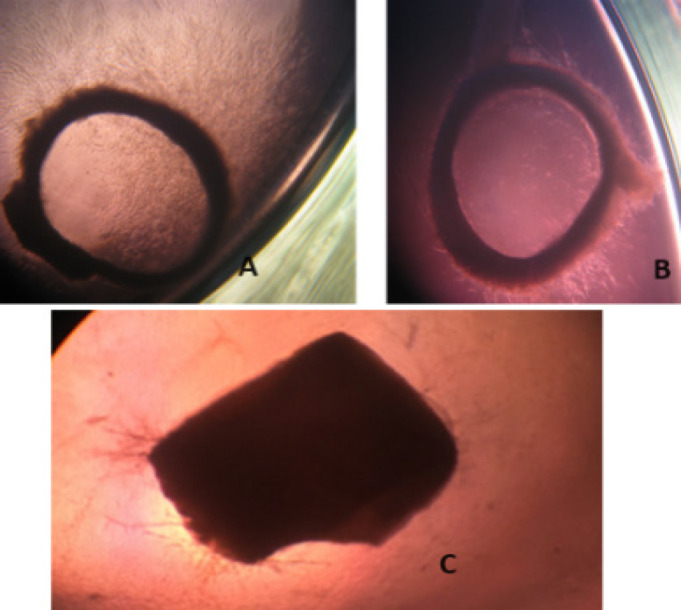
Rat Aorta Angiogenesis Assay. (A) Representative image of control 1% DMSO-treated rat aorta ring; (B) Representative image of rat aorta ring treated with 100µg/mL 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl)hydrazine-1-carbothioamide.(C) Representative image of rat aorta ring treated with 100µg/ml of suramin
Figure 3.
Dose Response Curve of 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide against Blood Vessels Growth.
Free radical scavenging activity of 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide
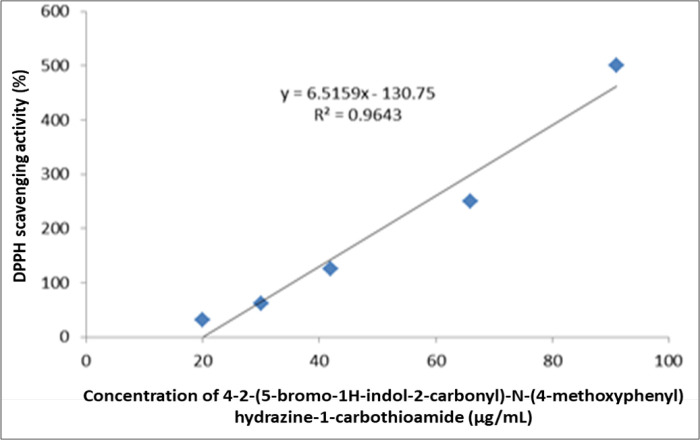
Figure 4 shows the free radical scavenging activity of 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide as 500, 250, 125, 62.5, and 31.25µg/ml showed 91, 66, 42, 30, 20% respectively. The IC50 was calculated to be 27.8µg/ml.
Figure 4.
Dose Response Curve of Free Radical Scavenging Activity of 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide.
Discussion
Angiogenesis is a very important physiological process during childhood and pregnancy, however, when this process occurs, it’s important to beg the concern and to inhibit in adult hood; otherwise, a serious disease may arise [12]. College of pharmacy in university of Baghdad had synthesized a 4-2-(5-bromo-1H-indol-2-carbonyl)-N-(4-methoxyphenyl) hydrazine-1-carbothioamide [13], The results of the study showed that the compound manage to inhibited vessels outgrowth in rat aorta model, this pharmacological effect was important to investigate, since endothelial cell is the cell of blood vessels and it’s the main cell that affected with the compound, anti-proliferative activity of the compound has been tested against HUVEC cell line and the results showed significant effect. These data may attribute to the capability of the compound to inhibit vascular endothelia growth factor VEGF. Because the compound is originally indol derivative and many studies conducted on indol had been revealed that indol significantly inhibit VEGF; the effect showed in this study may related to the same mechanism of action [14]. Since the compound showed robust blood vessels perturbation and anti-proliferation effect against HUVEC cell line it was important to investigate its capabilities in inhibiting the proliferation of cancer cell line in vitro, the compound showed significant anti-proliferation activity against lung cancer cell line with dose dependent manner. However, the IC50 was not within cytotoxic level and that indicate the compound had anti-angiogenic effect but not cytotoxic unless the concentrations increased to high level [14]. And this is agree with Hayder and coworkers in their study on 2009 [15]. Free radical scavenging activity was important to investigate as a mean to reach to the possible mechanism of action, the data showed in this study a significant dose dependent effect in scavenging the free radical by the compound; and this result may explain the possible mechanism as antioxidant capabilities [16]. Pathogenesis of diseases includes oxidative stress can lead to angiogenesis. Antioxidants manage to show a reduction of oxidative stress and may show a significant influence on neovascularization [17]. Moreover, the effect of exogenous antioxidants on angiogenesis and factors modulating this process is presented. Most synthetic antioxidants exert an inhibitory effect on neovascularization. Antioxidants compound demonstrated the inhibitory effect on angiogenesis and inflammation moreover reduced endothelial cells growth stimulated by FGF-2 and VEGF [18, 19]. Anti-angiogenic properties of antioxidant compound rely on the influence of VEGF signaling as well as tyrosine phosphorylation of vascular endothelial cadherin and β-catenin [20].
Acknowledgements
Authors would like to acknowledge college of pharmacy / Al-Nahrain University for funding this work.
Author Contribution Statement
All authors contributed equally in this study.
References
- 1.Al-Qrimli A, B S, J K. Antiangiogenic activity and the isolation of five phenolic compounds from euphorbia milii ethyl acetate solvent extract. Res J Pharm Technol. 2023;3083 [Google Scholar]
- 2.Ali ZK, Sahib HB. Antiangiogenic activity of sweet almond (prunus dulcis) oil alone and in combination with aspirin in both in vivo and in vitro assays. Asian Pac J Cancer Prev. 2022;23(4):1405–13. doi: 10.31557/APJCP.2022.23.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abhinand CS, Raju R, Soumya SJ, Arya PS, Sudhakaran PR. Vegf-a/vegfr2 signaling network in endothelial cells relevant to angiogenesis. J Cell Commun Signal. 2016;10(4):347–54. doi: 10.1007/s12079-016-0352-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jabbar ZR, Sahib HB. The effects of abscisic acid on angiogenesis in both ex vivo and in vivo assays. Asian Pac J Cancer Prev. 2022;23(12):4193–203. doi: 10.31557/APJCP.2022.23.12.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel) 2017;9:5. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaseen Y, Kubba A, Shihab W, Tahtamouni L. Synthesis, docking study, and structure-activity relationship of novel niflumic acid derivatives acting as anticancer agents by inhibiting vegfr or egfr tyrosine kinase activities. Pharmacia. 2022:69. [Google Scholar]
- 7.Allawi MM, Mahmood AAR, Tahtamouni LH, AlSakhen MF, Kanaan SI, Saleh KM, et al. New indole-6-carboxylic acid derivatives as multi-target antiproliferative agents: Synthesis, in silico studies, and cytotoxicity evaluation. Chem Biodivers. 2024;21(2):e202301892. doi: 10.1002/cbdv.202301892. [DOI] [PubMed] [Google Scholar]
- 8.Abdulridha A, Mahmood A, Shihab W, Tahtamouni L. New tolfenamic acid derivatives with hydrazine-1-carbothioamide and 1,3,4-oxadiazole moieties targeting vegfr: Synthesis, in silico studies, and in vitro anticancer assessment. Med Chem Res. 2023:32. [Google Scholar]
- 9.Hassan OM, Kubba A, Tahtamouni LH. Novel 5-bromoindole-2-carboxylic acid derivatives as egfr inhibitors: Synthesis, docking study, and structure activity relationship. Anticancer Agents Med Chem. 2023;23(11):1336–48. doi: 10.2174/1871520623666230227153449. [DOI] [PubMed] [Google Scholar]
- 10.Yaseen YS, Mahmood AAR, Abbas AH, Shihab WA, Tahtamouni LH. New niflumic acid derivatives as egfr inhibitors: Design, synthesis, in silico studies, and anti-proliferative assessment. Med Chem. 2023;19(5):445–59. doi: 10.2174/1573406419666221219144804. [DOI] [PubMed] [Google Scholar]
- 11.Hassan om, razzak mahmood aa, hamzah ah, tahtamouni lh. Design, synthesis, and molecular docking studies of 5‐bromoindole‐2‐carboxylic acid hydrazone derivatives: In vitro anticancer and vegfr‐2 inhibitory effects. Chemistryselect. 2022;7(46):E202203726. [Google Scholar]
- 12.Abdulsahib W, Abd A, Qasim B, Sahib H. Antiangiogenesis and antioxidant effect of anabasis articulata stems extracts. Int J Pharm Sci Rev Res. 2016:41:88. [Google Scholar]
- 13.Ali ZK, Sahib HB. Anti-proliferative activity of prunus dulcis seed oil alone and in combination with aspirin on human umbilical vein endothelial (huvecs) and kaposi sarcoma (ks) cells. J popul ther clin pharmacol. 2023;30(3):220–31. [Google Scholar]
- 14.Sahib HB. The anti-angiogenic and anti-proliferative activity of methyl hydroxychalcone. Asian Pac J Cancer Prev. 2022;23(6):2071–7. doi: 10.31557/APJCP.2022.23.6.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zainab KA, Hayder B Sahib. Anti-proliferative activity of prunus dulcis seed oil alone and in combination with aspirin on human umbilical vein endothelial (huvecs) and kaposi sarcoma (ks) cells. J popl ther clin pharmacol. 2023;30(3):220–31. [Google Scholar]
- 16.Jasim G, Al-Zubaidy PDA, Hussain SM, Sahib H. The antiangiogenic activity of commiphora molmol oleo-gum-resin extracts. Int J Pharm Sci Rev Res. 2015;33:83–90. [Google Scholar]
- 17.Alshaya ZE, Kadhim EJ, Sahib HB. Antiproliferative activities of Althaea ludwigii L extract on Michigan Cancer Foundation-7 breast cancer cell line. J appl biol. 2019 ;7(3):9–11. [Google Scholar]
- 18.Gallardo-Fernández M, Cerezo AB, Hornedo-Ortega R, Troncoso AM, Garcia-Parrilla MC. Anti-vegf effect of bioactive indolic compounds and hydroxytyrosol metabolites. Foods. 2022;11:4. doi: 10.3390/foods11040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iacopetta D, Catalano A, Ceramella J, Barbarossa A, Carocci A, Fazio A, et al. Synthesis, anticancer and antioxidant properties of new indole and pyranoindole derivatives. Bioorg Chem. 2020;105:104440. doi: 10.1016/j.bioorg.2020.104440. [DOI] [PubMed] [Google Scholar]
- 20.Jalil ZH, Sahib HB. Antiangiogenic activity of quinine alone and in combination with vitamin c in both ex vivo and in vivo assays. Asian Pac J Cancer Prev. 2022;23(12):4185–92. doi: 10.31557/APJCP.2022.23.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]