Abstract
Objective:
One of the biggest therapy challenges for nasopharyngeal cancer (NPC) is still radioresistance. The radioresistance in NPC is thought to be caused by cyclin D1 overexpression. The purpose of this study was to determine how cyclin D1 contributes to radiation resistance in NPC.
Methods:
Adhering to the PRISMA guidelines, we systematically reviewed studies on cyclin D1-associated radioresistance in NPC from 2012 until 2023. From our search, 15 studies were included.
Results:
Cyclin D1’s role in radiotherapy resistance is elucidated through several mechanisms, notably SHP-1 and B-catenin. Overexpression of SHP-1 led to an increase in cyclin D1, a higher proportion of cells in the S-phase, and radioresistance. Conversely, inhibiting β-catenin and cyclin D1 expression enhances radiation sensitivity.
Conclusion:
In conclusion, Cyclin D1 has a strong correlation with radiation resistance; downregulation of the protein increases radiosensitivity, while overexpression of the protein promotes radioresistance.
Key Words: Cyclin D1, nasopharyngeal carcinoma, radiotherapy, radioresistant
Introduction
A malignant epithelial tumor that damages the lining of the lymphocyte-rich nasopharyngeal mucosa is known as nasopharyngeal carcinoma (NPC) [1]. This particular type of head and neck cancer has a distinct etiology that is influenced by a variety of factors, such as nutrition, genetic susceptibility, and Epstein-Barr virus (EBV) infection. NPC is distributed in a certain geographic area. Nasopharyngeal cancer (NPC) is a common kind of head and neck cancer in the southern provinces of China and Southeast Asia. This syndrome was initially observed in 1901 and was clinically recognized in 1922 [2]. In 2018, the number of NPC patients worldwide exceeded 130,000, with the highest incidence occurring in Southeast Asia. With a ratio of 2-3:1, men are more likely than women to acquire NPC [3]. Approximately 80% of NPC cases worldwide are reported to be in Asia, with China leading the way. Guangdong Province is home to the majority of NPCs in China, where there are 20–40 cases for every 100,000 people [2]. NPC is the fourth most prevalent type of cancer in the entire body and the most common type of head and neck tumor in Indonesia. Through recent decades more than 60% of head and neck malignancy data in Indonesia is NPC and the incidence keeps increasing [4]. Radiotherapy is the mainstay of NPC treatment, with chemotherapy added in the more severe cases [5]. Surgery is done only to treat very small initial lesions or minor recurrences because performing a large-scale resection generally results in much worse side effects than administering radiation to the affected area, but the obstacle is radioresistance [6]. It might be caused by a variety of factors, including the presence of cancer stem cells (CSCs), gene mutation or aberrant expression, epigenetic modification of genes, inappropriate activation of particular signaling pathways, alteration of the tumor microenvironment, production of stress granules (SGs), and more [7]. A protein called cyclin D1 is involved in the passage of the cell cycle from the G1 phase to the S phase. The CCNd1 gene, which can be found on chromosome 11q13, produces this protein. Cyclin D1 is well recognized for its function as a regulator of cell cycle progression in the nucleus. Through its function as an allosteric regulator of cyclin-dependent kinase 4 (CDK4) and CDK6, cyclin D1 influences the transition from G1 to S phase [8]. Numerous factors, including gene mutation or aberrant expression of genes, epigenetic modification of genes, abnormal activation of certain signaling pathways, transformation of the tumor microenvironment, creation of stress granules, etc., can lead to resistance to radiation. Cyclin D1 is relatively quickly triggered by growth factors in a variety of cells, and it then blocks a number of intracellular pathways that regulate cell proliferation. Uncontrolled cell proliferation is caused by dysregulation of Cyclin D1 transcription, accumulation, and ubiquitination, as well as assembly and hyperactivation of its associated CDK [8, 9]. Several journals have described the relationship between NPC-resistant radiotherapy and cyclin D1 protein, including nasopharyngeal cancer, breast cancer, lung cancer, and melanoma [10, 8, 11]. In this systematic review, we investigated the recent decades’ studies of the proportion of cyclin D1 protein in NPC patients and its relationship with radiotherapy response. The findings suggest cyclin D1 roles in radioresistance, with most mentioned pathways overexpressing SHP-1 and β-catenin.
Materials and Methods
The PRISMA (Preferred Reporting Items for Systematic Review and Meta-analysis) standards from 2021 were followed in doing the current systematic review [12]. The PICO (participants, interventions, comparisons, and outcomes) of the research that are included in this systematic review include cyclin D1 levels, radiotherapy, radiosensitive or radioresistant patients, and carcinoma pharynx patients. The PROSPERO database has this study listed (CRD42023397285).
Searching Strategy
The studies of the recent decade in the field of the Radioresistant related to cyclin D1 in nasopharyngeal carcinoma were identified through PubMed, Sciencedirect, Scopus, and Cochrane library databases using the search terms “Nasopharyngeal carcinoma”, “cyclin D1” and “radioresistant” along with the related MeSH terms, synonyms, and elaborations (Supp1). Manual searches for additional articles were also performed. The literature search was restricted to papers in the English language and published articles between 2012 and 2023. The last data collection was carried out in February 2023. The authors reviewed the abstract and full text. The authors were contacted for supplementary information if there were incomplete data from the full texts. Disagreements were resolved through debate. Duplicates were removed using Zotero.
Selection Criteria
Inclusion criteria (the guidelines to select the eligible studies that could be included in the process of the analysis) and exclusion criteria were chosen as follows: the inclusion criteria in this study were: (1) human and animal subjects; (2) the expression of Cyclin D1; (3) availability of data for both clinical samples and in vitro cell lines; (4) cyclin D1 involvement in Radioresistant or radiosensitivity in NPC; and (5) articles published in the last 10 years (2012 - 2022). The exclusion criteria in this study were: (1) non-English language published studies; (2) nonrelated studies to radiotherapy and NPC; (3) review, editorial, case reports, conference abstracts, meta-analysis, and systematic articles; and (4) inadequate/unavailable data and repeated studies.
Data extraction and quality assessment
After a careful review of the included studies, details were obtained from the articles that qualified for final inclusion. The following important headings were extracted from these studies: journal author, publication year, country, population study, information about cyclin D1 expression, sample size, immunohistochemistry analysis, western blot analysis, sample type, Radioresistant pathway, and outcome. The eligible articles were further reviewed and examined for data extraction.
The quality of the studies was assessed using Newcastle Ottawa Scale (NOS) (Table 1). Selection, comparability, and result assessment biases were among the assessment domains. Sensitivity analysis was used to determine the impact of bias on the effect estimates after the risk of bias assessment.
Table 1.
Newcastle Ottawa Scale Quality Assessment on Case Control Studies
| Study | Selection | Comparability | Exposure | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adequate definition | Representativeness of the cases | Selection of Controls | Definition of Controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-Response rate | ||
| Nurhidayat et al. [51] | * | * | * | * | * | 0 | * | 0 | 6 |
| Gang Peng et al. [13] | * | * | * | * | * | * | * | * | 8 |
| Zong et al. [52] | * | * | * | * | * | * | * | * | 8 |
| Han et al. [54] | * | * | * | * | * | * | * | * | 8 |
| Pu et al. [56] | * | * | * | * | * | * | * | * | 8 |
| Wu et al. [57] | * | * | * | * | * | * | * | * | 8 |
| Wu et al. [59] | * | * | * | * | * | * | * | * | 8 |
| Wang et al. [60] | * | * | * | * | * | * | * | * | 8 |
| Sun et al. [21] | * | * | * | * | * | 0 | * | * | 7 |
| Wang et al. [53] | * | * | * | * | * | * | * | * | 8 |
| He et al. [45] | * | * | * | * | * | 0 | * | * | 7 |
Results
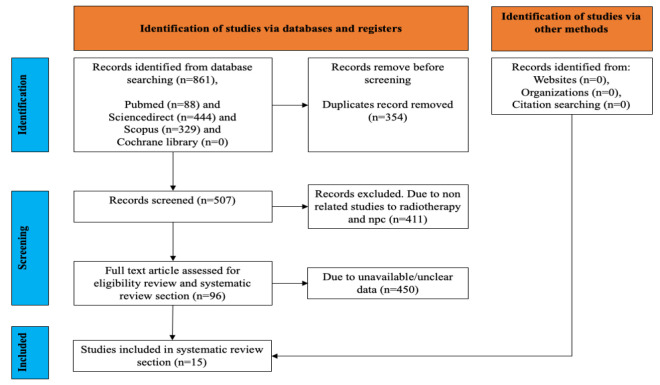
The initial search strategy identified 861 studies from Pubmed (n = 88), ScienceDirect (n = 444), Scopus (n=329), and Cochrane Library (n=0), as presented in Figure 1. After removing duplicated studies, 354 studies were considered relevant. After full-text screening and applying inclusion criteria, a total of 15 studies with cyclin D1 expression related to radioresistance or radiosensitivity in nasopharyngeal carcinoma were obtained for the present study (Table 2).
Figure 1.
Data Collection Flowchart Following PRISMA Guideline. The search initially found 861 studies from various databases. After removing duplicates, 2174 studies remained. Following full-text screening and applying inclusion criteria, 15 relevant studies on cyclin D1 expression in nasopharyngeal carcinoma and its relation to radioresistance or radiosensitivity were included.
Table 2.
Cyclin D1 Dysregulation Roles in NPC Treatment
| No. | Author, Year | Country | Types of Samples | Detection Technique | Cyclin D1 Dysregulation | Components That Affect | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | Gang Peng et al. [13] | China | Tissue | Western blot | Upregulated | SHP-1 | Radioresistant |
| 2 | Fu et al. [25] | China | Tissue | IHC | Upregulated | Cyclin d1 in Cycle Cell | Radioresistant |
| 3 | Nurhidayat et al. [51] | Indonesia | Tissue | IHC | Upregulated | Cyclin d1 in Cycle Cell | Radioresistant |
| 4 | Zong et al. [52] | China | Tissue | Western blot | Upregulated | ZNF488 | Radioresistant |
| 5 | Wang et al. [53] | China | Tissue | Western blot | Upregulated | c-MYB | Radioresistant |
| 6 | He et al. [45] | China | Tissue | Western blot | Upregulated | B-Catenin | Radioresistant |
| 7 | Han et al. [54] | China | Tissue | Western blot | Upregulated | PVT1 | Radioresistant |
| 8 | Song et al. [55] | China | Tissue | Western blot and IHC | Downregulated | INSM1 | Radiosensitive |
| 9 | Pu et al. [56] | China | Tissue | IHC | Downregulated | siFGFR2 | Radiosensitive |
| 10 | Irawan et al. [46] | Indonesia | Tissue | IHC | Upregulated | CCND1 Gene | Radiosensitive |
| 11 | Sun et al. [21] | China | Tissue | Western blot | Downregulated | SHP-1 | Radiosensitive |
| 12 | Wu et al. [57] | China | Tissue | Western blot | Downregulated | DNMT3B | Radiosensitive |
| 13 | Wang et al. [58] | China | Tissue | Western blot and IHC | Downregulated | B-Catenin | Radiosensitive |
| 14 | Wu et al. [59] | China | Tissue | Western blot | Downregulated | Notch2 | Radiosensitive |
| 15 | Wang et al. [58] | China | Tissue | Western blot | Downregulated | Rapamycin | Radiosensitive |
Of these studies, the expression of cyclin D1 showed that it could be a biomarker for radiotherapy resistance in nasopharyngeal carcinoma patients. In total, eight studies were upregulated, and seven studies were downregulated. Moreover, 14 studies were found to be related to Radioresistant, and one study stated there was no relationship. 13 studies were from China, and 2 studies were from Indonesia. China and Hong Kong are the countries with the highest NPC cases. All existing studies explain their respective radioresistant pathways. Studies conducted by Gang Peng et al. [13] and Sun et al. [14] both state Radioresistant pathways through SHP-1. At the same time, other studies mention different pathways, namely B-catenin, ZNF488, c-MYB, PVT1, INSM1, siFGFR2, CCND1 Gene, DNMT3B, Notch2, and Rapamycin. An increase in these components will trigger radioresistance (Table 3).
Table 3.
Newcastle Ottawa Scale Quality Assessment on Cohort Studies
| Study | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | ||
| Fu et al. [25] | * | * | * | * | * | * | * | * | 8 |
| Song et al. [55] | * | * | * | * | * | * | * | * | 8 |
| Irawan et al. [46] | * | * | * | * | 0 | * | * | * | 7 |
| Wang et al. [58] | * | * | * | * | * | * | * | * | 8 |
Discussion
Our study result found that Cyclin D1 is an essential part of oncogenic transformation by involving various components in its radioresistant pathway. Cyclin D1 overexpression is associated with the induction of genomic instability in irradiated cells. Cyclin D1 is frequently overexpressed in human cancers and has been reported to be a carcinogenic driver in most of these cancers; 263 and CDK inhibitors targeting cyclin D1 are considered a feasible method for cancer treatment. Studies have shown that cyclin D1 survival signalling pathway and autophagy are associated with radiological resistance in cancer [9, 15, 16].
Mechanisms of Radioresistance
Radioresistance in cancer is often caused by the repair response to radiation-induced DNA damage, cell cycle dysregulation, cancer stem cells (CSCs) resilience, and epithelial-mesenchymal transition (EMT). Radiation ionizes molecules and atoms to destroy DNA in cells in human tissue directly. Enhanced DNA repair, cell cycle redistribution, CSC resilience, EMT, and activation of prosurvival pathways are the main mechanisms by which radioresistance is induced in cancer. Many factors, such as ATM, p53, PARP, XRCC1, and Bim-1, greatly influence cancer radioresistance through these different mechanisms [17, 18].
Cyclin D1 and possible radioresistance pathways
Cyclin D1 is a protein involved in the cell cycle progression from the G1 phase to the S phase. This protein is encoded by the CCND1 gene located on chromosome 11q13 [19]. Cyclin D1 is mostly known for its role in the nucleus as a regulator of cell cycle progression. Cyclin D1 modulates the transition from G1 to S phase through its action as an allosteric regulator of the cyclin-dependent kinase 4 (CDK) and CDK6. Thus, Cyclin D1 is regarded as an oncogenic driver in different types of cancers, including breast cancer, lung cancer, and melanoma [20]. The radiation-induced apoptosis of tumor cells is one of the main mechanisms of radiotherapy, and the degree of tumor cell apoptosis is proportional to its radiosensitivity. Many studies have shown that targeting interference therapy in the apoptotic pathway could significantly improve the radiosensitivity of tumor cells.
Cyclin D1 expression was increased by 6-fold in the breast cancer cell line MCF7 in the absence of tetracycline culture, and cyclin D1 overexpressed cells could induce apoptosis more efficiently and were more sensitive to radiation than control cells [10]. Ding and cyclin D1 expression levels were positively correlated with nasopharyngeal carcinoma cell radiosensitivity. However, after the depletion of the human tumor cyclin D1 levels in mice missing pRb, the cells became more sensitive to radiation and DNA damage factors. The expression levels of cyclin D1 were negatively correlated with tumor radiosensitivity [9].
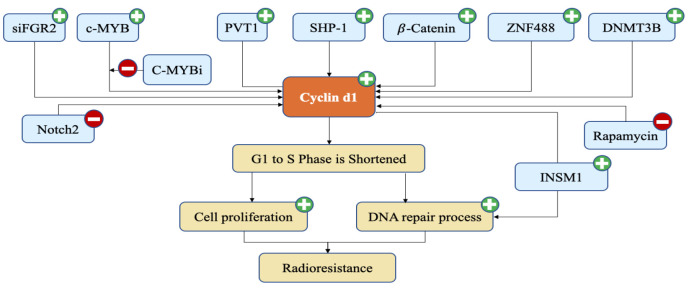
In the diagram above, it is explained that the expression of SHP-1, B-Catenin, ZNF488, DNMT3B, and cMYB proteins that were inhibited by cMYBi caused an increase in cyclin D1 expression (Figure 2). Decreased expression of the Notch2 protein also led to an increase in cyclin D1. An increase in cyclin D1 will cause a shortening of the cell cycle phase from G1 to the S phase. This increases the number of cells in the S phase and increases cell proliferation. In addition, the increase in cyclin D1 also makes the DNA repair process increase. This causes Radioresistance to NPC therapy.
Figure 2.
Radioresistant Pathway Related to Cyclin d1 Protein. The plus mark represents the increase process, the minus mark represents the reduction process. The expression of SHP-1, B-Catenin, ZNF488, DNMT3B, PVT1, siFGR2, and cMYB proteins that were inhibited by cMYBi led to an increase in cyclin d1 expression
Several mechanisms can be used to explain cyclin D1’s function as a radiation resistance marker. SHP-1 is the pathway that is frequently addressed in various publications. SHP-1 may regulate the cell cycle and contribute to NPC cells’ radioresistance. Radioresistance was caused by an increase in cyclin D1, a larger percentage of cells in S-phase, and overexpression of SHP-1 [3]. SHP-1 has recently emerged as a useful diagnostic marker and potential therapeutic target in a number of malignancies due to its function in controlling cell proliferation and the dispersion of the tumor cell cycle [21]. SHP-1 has been identified as being aberrantly expressed in a number of malignancies, including NPC, although no functional studies on NPC have been reported to yet [22-25]. SHP-1 is expressed in hematopoietic cells, various cell types, and malignant cells, most notably in malignant epithelial cells [26]. SHP-1 regulates cell proliferation by catalyzing tyrosine dephosphorylation, which decreases or eliminates kinase activity, and by regulating essential cell cycle proteins, including cyclin D1. Acquired radioresistance was discovered to be correlated with the overexpression of cyclin D1 [27]. Cells are typically radioresistant during the S phase, radiosensitive during the G0/1 phase, and most radiosensitive during the G2-M phase [28]. The results showed that the parental cells and the CNE 2S1 group had the same proportion of G2/M cells but that the parental cells had a much higher proportion of S-phase cells and a significantly lower proportion of G1-phase cells. Notably, there was little change in the ratios of cells at different stages of the cell cycle in the CNE 2S2 cells. These results gave rise to the theory that Radioresistance in NPC cells may be significantly influenced by cell cycle disruption [13].
β-catenin overexpression may lessen the sensitivity of NPC CNE-2 cells to radiation through the activation of transcriptional factors that are downstream from -catenin and the reduction of G2/M arrest and cell death. The Wnt/-catenin signaling pathway emerged and propagated early in the development of many human cancers [29-34]. A crucial component of the Wnt/ β-catenin signaling pathway, β-catenin, has been identified aids in the radiosensitivity of malignancies [35-37]. Because activation of the β-catenin pathway effectively promoted the repair of DNA double strand breaks (DSBs) in radioirradiated osteoblasts and up-regulation of β-catenin expression increased the capacity of DSB repair in cancer cells, it is thought that the role of β-catenin in DNA damage repair may be a factor affecting cellular radiation sensitivity [38, 39]. Inhibition of β-catenin expression resulted in a considerable rise in the proportion of hepatocellular cancer stem cells in the G2/M phase [40]. When treated with polyphyllin I (PPI), a crucial active component derived from Paris polyphyllin, caused cell cycle arrest at the G2/M phase and down-regulation of β-catenin expression in multiple myeloma cells [41]. Cyclin D1, a critical cell cycle-related protein, controls cell cycle checkpoints [42]. Radiation sensitivity was found to rise with inhibition of β-catenin and Cyclin D1 expression. Cancer radiosensitivity has been observed to significantly correlate with Cyclin D1 expression [43, 44]. Cyclin D1 expression is increased in CNE-2 cells that are overexpressing β-catenin [45].
Contradictory findings
There is one study that says the reverse of the majority of studies, which claim that higher expression of cyclin D1 is a sign of radiation resistance. The study found that patients with NPC who expressed more cyclin D1 responded better to therapy. The group of participants who responded to treatment had a higher proportion of cyclin D1 expression compared to the group of participants who did not [46]. This is in contrast to earlier research by Noel et al. [47] and Biliran et al. [48], which discovered higher levels of cyclin D1 expression in NPC patients who do not respond to treatment than in those who do. Numerous studies explain the cyclin D1 effect’s ambiguous/paradoxical nature under the reaction to cytostatic drugs in various cancer types [14].
According to certain studies, the cyclin D1 reaction may differ depending on the degree of DNA damage. After considerable DNA damage, such as that caused by chemotherapy or high-dose radiation, cyclin D1 levels will drop precipitously, preventing cells from entering the S phase. As a result, cells will stop reproducing and finally die. The modest DNA damage is inadequate to significantly reduce cyclin D1 levels; thus, the cell continues to divide. In cases of low-grade DNA damage, cyclin D1, which is involved in DNA repair processes, will be present in the cell nucleus. Moreover, cyclin D1 will cause the DNA repair protein RAD51 to become active [43].
Cyclin D1 expression has been associated with chromosomal instability in various cancers (breast, neck, and head cancer) because the cells are pushed to enter the S phase immediately away, preventing DNA repair [15]. The overexpression of cyclin D1 can also result in the overexpression of a protein during the DNA repair process. As a result of excessive RAD51 activation, which also disrupts the DNA repair process and causes DNA instability, DNA will become toxic. An increase in RAD51 expression would lead to abnormalities in chromosomal structure and genome instability caused by the formation of aneuploid chromosomes [49]. An increase in cyclin D1a and D1b isoforms caused various responses of colorectal patients to 5-fluorouracil, which is more favourable towards the expression of cyclin D1a. This can be due to an imbalance between DNA production and repair ability [50].
NPC is a type of cancer that is highly dependent on radiotherapy in its management. It is important for the clinician to understand that a patient is already radioresistant. Preventive efforts are needed so that the patient remains in a radiosensitive condition. The potential limitation of our systematic review is the restriction to studies published in English, which may imply a loss of information published in other languages, which would have been missed.
The optimal treatment of NPC involves a multidisciplinary approach involving ENT specialists, radiation oncologists, and medical oncologists. Radiotherapy is the main therapy in the treatment of this disease, so it is important for clinicians to know when radioresistance occurs. Understanding the molecular alterations that lead to radioresistance may provide new diagnostic markers and therapeutic targets to improve radiotherapy efficacy. Cyclin d1 may be useful for determining prognostic indicators and targets for the molecular treatment of nasopharyngeal cancer.
Acknowledgements
The authors would like to thank them for the invaluable assistance provided to them by Mentors, Seniors Dr. Soetomo General Hospital, and the Medical Faculty of Universitas Airlangga Indonesia in the preparation of this paper for publication.
Additional Information
This review is not being undertaken as a part of planning for other research.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflict of Interest Statement for all authors
Nothing to declare.
Author Contribution Statement
A.C.R., M.R.M.Y. arranged study design, data interpretation, and supervision. A.S.R., C.F.A., L.A.W., I.N.W., arranged study design, data interpretation, data collection, data analysis, literature analysis, and preparation of manuscript. All authors read and approved the final manuscript.
References
- 1.Hau PM, Lung HL, Wu M, Tsang CM, Wong KL, Mak NK, et al. Targeting epstein-barr virus in nasopharyngeal carcinoma. Front Oncol. 2020;10:600. doi: 10.3389/fonc.2020.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou X, Cui J, Macias V, Kajdacsy-Balla AA, Ye H, Wang J, et al. The progress on genetic analysis of nasopharyngeal carcinoma. Comp Funct Genomics. 2007;2007:57513. doi: 10.1155/2007/57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM. Cancer incidence in five continents. Lyon: International Agency for Research on Cancer. 1992;6:960–1. [Google Scholar]
- 4.Chang ET, Ye W, Zeng YX, Adami HO. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1035–47. doi: 10.1158/1055-9965.EPI-20-1702. [DOI] [PubMed] [Google Scholar]
- 5.Shah AB, Nagalli S. Nasopharyngeal carcinoma. StatPearls. 2022. Available from: http://www.ncbi.nlm.nih.gov/books/NBK554588 (accessed 29 May 2023)
- 6.Ding X, Liu Y-P, Hua Y-J, Zou X, Wang Z-Q, Xie Y-L, et al. Clinical advances in nasopharyngeal carcinoma surgery and a video demonstration. Visualized Cancer Medicine. 2021;2:2. [Google Scholar]
- 7.Zhan Y, Fan S. Multiple mechanisms involving in radioresistance of nasopharyngeal carcinoma. J Cancer. 2020;11(14):4193–204. doi: 10.7150/jca.39354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krieger S, Gauduchon J, Roussel M, Troussard X, Sola B. Relevance of cyclin d1b expression and ccnd1 polymorphism in the pathogenesis of multiple myeloma and mantle cell lymphoma. BMC Cancer. 2006;6:238 . doi: 10.1186/1471-2407-6-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alao JP. The regulation of cyclin d1 degradation: Roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24 . doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coco Martin JM, Balkenende A, Verschoor T, Lallemand F, Michalides R. Cyclin d1 overexpression enhances radiation-induced apoptosis and radiosensitivity in a breast tumor cell line. Cancer Res. 1999;59(5):1134–40. [PubMed] [Google Scholar]
- 11.Gautschi O, Ratschiller D, Gugger M, Betticher DC, Heighway J. Cyclin d1 in non-small cell lung cancer: A key driver of malignant transformation. Lung Cancer. 2007;55(1):1–14. doi: 10.1016/j.lungcan.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng G, Cao RB, Li YH, Zou ZW, Huang J, Ding Q. Alterations of cell cycle control proteins shp1/2, p16, cdk4 and cyclin d1 in radioresistant nasopharyngeal carcinoma cells. Mol Med Rep. 2014;10(4):1709–16. doi: 10.3892/mmr.2014.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun Y, Luo D, Liao DJ. Cyclind1 protein plays different roles in modulating chemoresponses in mcf7 and mda-mb231 cells. J Carcinog. 2012;11:12 . doi: 10.4103/1477-3163.100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jirawatnotai S, Sittithumcharee G. Paradoxical roles of cyclin d1 in DNA stability. DNA Repair (Amst). 2016;42:56–62. doi: 10.1016/j.dnarep.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Di Sante G, Pagé J, Jiao X, Nawab O, Cristofanilli M, Skordalakes E, et al. Recent advances with cyclin-dependent kinase inhibitors: Therapeutic agents for breast cancer and their role in immuno-oncology. Expert Rev Anticancer Ther. 2019;19(7):569–87. doi: 10.1080/14737140.2019.1615889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivares-Urbano MA, Griñán-Lisón C, Marchal JA, Núñez MI. Csc radioresistance: A therapeutic challenge to improve radiotherapy effectiveness in cancer. Cells. 2020;9:7. [Google Scholar]
- 18.Liu YP, Zheng CC, Huang YN, He ML, Xu WW, Li B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm. 2021;2(3):315–40. doi: 10.1002/mco2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inaba T, Matsushime H, Valentine M, Roussel MF, Sherr CJ, Look AT. Genomic organization, chromosomal localization, and independent expression of human cyclin d genes. Genomics. 1992;13(3):565–74. doi: 10.1016/0888-7543(92)90126-d. [DOI] [PubMed] [Google Scholar]
- 20.Tchakarska G, Sola B. The double dealing of cyclin d1. Cell Cycle. 2020;19(2):163–78. doi: 10.1080/15384101.2019.1706903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun Z, Pan X, Zou Z, Ding Q, Wu G, Peng G. Increased shp-1 expression results in radioresistance, inhibition of cellular senescence, and cell cycle redistribution in nasopharyngeal carcinoma cells. Radiat Oncol. 2015;10:152 . doi: 10.1186/s13014-015-0445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, Sun M, Liu L, Zhou GW. The function of the protein tyrosine phosphatase shp-1 in cancer. Gene. 2003;306:1–12. doi: 10.1016/s0378-1119(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 23.Amin S, Kumar A, Nilchi L, Wright K, Kozlowski M. Breast cancer cells proliferation is regulated by tyrosine phosphatase shp1 through c-jun n-terminal kinase and cooperative induction of rfx-1 and ap-4 transcription factors. Mol Cancer Res. 2011;9(8):1112–25. doi: 10.1158/1541-7786.MCR-11-0097. [DOI] [PubMed] [Google Scholar]
- 24.Evren S, Wan S, Ma XZ, Fahim S, Mody N, Sakac D, et al. Characterization of shp-1 protein tyrosine phosphatase transcripts, protein isoforms and phosphatase activity in epithelial cancer cells. Genomics. 2013;102(5-6):491–9. doi: 10.1016/j.ygeno.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Fu SM, Xu MX, Lin SM, Liang Z, Cai JH. Association of cyclin d1 and survivin expression with sensitivity to radiotherapy in patients with nasopharyngeal carcinoma. Genet Mol Res. 2014;13(2):3502–9. doi: 10.4238/2014.February.14.6. [DOI] [PubMed] [Google Scholar]
- 26.López-Ruiz P, Rodriguez-Ubreva J, Cariaga AE, Cortes MA, Colás B. Shp-1 in cell-cycle regulation. Anticancer Agents Med Chem. 2011;11(1):89–98. doi: 10.2174/187152011794941154. [DOI] [PubMed] [Google Scholar]
- 27.Shimura T. Acquired radioresistance of cancer and the akt/gsk3β/cyclin d1 overexpression cycle. J Radiat Res. 2011;52(5):539–44. doi: 10.1269/jrr.11098. [DOI] [PubMed] [Google Scholar]
- 28.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Philadelphia: Lippincott Williams & Wilkins; p. 572. [Google Scholar]
- 29.Monga SP. Role of wnt/β-catenin signaling in liver metabolism and cancer. Int J Biochem Cell Biol. 2011;43(7):1021–9. doi: 10.1016/j.biocel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White BD, Chien AJ, Dawson DW. Dysregulation of wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142(2):219–32. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krausova M, Korinek V. Wnt signaling in adult intestinal stem cells and cancer. Cell Signal. 2014;26(3):570–9. doi: 10.1016/j.cellsig.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Deitrick J, Pruitt WM. Wnt/β catenin-mediated signaling commonly altered in colorectal cancer. Prog Mol Biol Transl Sci. 2016;144:49–68. doi: 10.1016/bs.pmbts.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Santos JC, Carrasco-Garcia E, Garcia-Puga M, Aldaz P, Montes M, Fernandez-Reyes M, et al. Sox9 elevation acts with canonical wnt signaling to drive gastric cancer progression. Cancer Res. 2016;76(22):6735–46. doi: 10.1158/0008-5472.CAN-16-1120. [DOI] [PubMed] [Google Scholar]
- 34.Rahmani F, Avan A, Hashemy SI, Hassanian SM. Role of wnt/β-catenin signaling regulatory micrornas in the pathogenesis of colorectal cancer. J Cell Physiol. 2018;233(2):811–7. doi: 10.1002/jcp.25897. [DOI] [PubMed] [Google Scholar]
- 35.Chang HW, Roh JL, Jeong EJ, Lee SW, Kim SW, Choi SH, et al. Wnt signaling controls radiosensitivity via cyclooxygenase-2-mediated ku expression in head and neck cancer. Int J Cancer. 2008;122(1):100–7. doi: 10.1002/ijc.23069. [DOI] [PubMed] [Google Scholar]
- 36.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang G, Wang W, Yao C, Zhang S, Liang L, Han M, et al. Radiation-resistant cancer stem-like cell properties are regulated by pten through the activity of nuclear β-catenin in nasopharyngeal carcinoma. Oncotarget. 2017;8(43):74661–72. doi: 10.18632/oncotarget.20339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng R, Tang J, Ma JG, Chen SP, Xia LP, Zhou WJ, et al. Pkb/akt promotes dsb repair in cancer cells through upregulating mre11 expression following ionizing radiation. Oncogene. 2011;30(8):944–55. doi: 10.1038/onc.2010.467. [DOI] [PubMed] [Google Scholar]
- 39.Chandra A, Lin T, Zhu J, Tong W, Huo Y, Jia H, et al. Pth1-34 blocks radiation-induced osteoblast apoptosis by enhancing DNA repair through canonical wnt pathway. J Biol Chem. 2015;290(1):157–67. doi: 10.1074/jbc.M114.608158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le AP, Zhang LL, Liu W, Shi YF. Cantharidin inhibits cell proliferation and induces apoptosis through g2/m phase cell cycle arrest in hepatocellular carcinoma stem cells. Oncol Rep. 2016;35(5):2970–6. doi: 10.3892/or.2016.4684. [DOI] [PubMed] [Google Scholar]
- 41.Liang Y, Li X, He X, Qiu X, Jin XL, Zhao XY, et al. Polyphyllin i induces cell cycle arrest and apoptosis in human myeloma cells via modulating β-catenin signaling pathway. Eur J Haematol. 2016;97(4):371–8. doi: 10.1111/ejh.12741. [DOI] [PubMed] [Google Scholar]
- 42.Li Z, Wang C, Prendergast GC, Pestell RG. Cyclin d1 functions in cell migration. Cell Cycle. 2006;5(21):2440–2. doi: 10.4161/cc.5.21.3428. [DOI] [PubMed] [Google Scholar]
- 43.Jirawatnotai S, Hu Y, Michowski W, Elias JE, Becks L, Bienvenu F, et al. A function for cyclin d1 in DNA repair uncovered by protein interactome analyses in human cancers. Nature. 2011;474(7350):230–4. doi: 10.1038/nature10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su H, Jin X, Shen L, Fang Y, Fei Z, Zhang X, et al. Inhibition of cyclin d1 enhances sensitivity to radiotherapy and reverses epithelial to mesenchymal transition for esophageal cancer cells. Tumour Biol. 2016;37(4):5355–63. doi: 10.1007/s13277-015-4393-z. [DOI] [PubMed] [Google Scholar]
- 45.He H, Lin K, Su Y, Lin S, Zou C, Pan J, et al. Overexpression of β-catenin decreases the radiosensitivity of human nasopharyngeal carcinoma cne-2 cells. Cell Physiol Biochem. 2018;50(5):1929–44. doi: 10.1159/000494873. [DOI] [PubMed] [Google Scholar]
- 46.Irawan C, Cahyanur R, Lisnawati L, Abdullah M, Yunus RE. The difference in the cyclin d1 expression in advanced stage nasopharyngeal cancer based on treatment response: A retrospective cohort study. Acta Med Indones. 2020;52(2):147–54. [PubMed] [Google Scholar]
- 47.Noel EE, Yeste-Velasco M, Mao X, Perry J, Kudahetti SC, Li NF, et al. The association of ccnd1 overexpression and cisplatin resistance in testicular germ cell tumors and other cancers. Am J Pathol. 2010;176(6):2607–15. doi: 10.2353/ajpath.2010.090780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biliran H Jr, Wang Y, Banerjee S, Xu H, Heng H, Thakur A, et al. Overexpression of cyclin d1 promotes tumor cell growth and confers resistance to cisplatin-mediated apoptosis in an elastase-myc transgene-expressing pancreatic tumor cell line. Clin Cancer Res. 2005;11(16):6075–86. doi: 10.1158/1078-0432.CCR-04-2419. [DOI] [PubMed] [Google Scholar]
- 49.Richardson C, Stark JM, Ommundsen M, Jasin M. Rad51 overexpression promotes alternative double-strand break repair pathways and genome instability. Oncogene. 2004;23(2):546–53. doi: 10.1038/sj.onc.1207098. [DOI] [PubMed] [Google Scholar]
- 50.Qie S, Diehl JA. Cyclin d1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl) 2016;94(12):1313–26. doi: 10.1007/s00109-016-1475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nurhidayat A, Afiati A, Usman H, Hernowo B. The role of cyclin d1 and vegf in radiotherapy response of advance stage undifferentiated nasopharyngeal carcinoma. Folia Medica Indonesiana. 2020;56:248. [Google Scholar]
- 52.Zong D, Jiang N, Xu JH, Wang DJ, Zhu HF, Wu LR, et al. Znf488 is an independent prognostic indicator in nasopharyngeal carcinoma and promotes cell adhesion and proliferation via collagen iv/fak/akt/cyclin d1 pathway. Cancer Manag Res. 2019;11:5871–82. doi: 10.2147/CMAR.S200001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W, Wu S, Shi Y, Miao Y, Luo X, Ji M, et al. C-myb regulates cell growth and DNA damage repair through modulating mir-143. FEBS Lett. 2015;589(5):555–64. doi: 10.1016/j.febslet.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 54.Han Y, Li F, Xie J, Wang Y, Zhang H. Pvt1 mediates cell proliferation, apoptosis and radioresistance in nasopharyngeal carcinoma through regulating mir-515-5p/pik3ca axis. Cancer Manag Res. 2020;12:10077–90. doi: 10.2147/CMAR.S257583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song R, Wei X, Wang Y, Hu S, Ba Y, Xiao X, et al. Insulinoma-associated protein 1 controls nasopharyngeal carcinoma to radiotherapy by modulating cyclin d1-dependent DNA repair machinery. Carcinogenesis. 2020;41(3):326–33. doi: 10.1093/carcin/bgz101. [DOI] [PubMed] [Google Scholar]
- 56.Pu L, Su L, Kang X. The efficacy of cisplatin on nasopharyngeal carcinoma cells may be increased via the downregulation of fibroblast growth factor receptor 2. Int J Mol Med. 2019;44(1):57–66. doi: 10.3892/ijmm.2019.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu C, Guo E, Ming J, Sun W, Nie X, Sun L, et al. Radiation-induced dnmt3b promotes radioresistance in nasopharyngeal carcinoma through methylation of p53 and p21. Mol Ther Oncolytics. 2020;17:306–19. doi: 10.1016/j.omto.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang W, Wen Q, Luo J, Chu S, Chen L, Xu L, et al. Suppression of β-catenin nuclear translocation by cgp57380 decelerates poor progression and potentiates radiation-induced apoptosis in nasopharyngeal carcinoma. Theranostics. 2017;7(7):2134–49. doi: 10.7150/thno.17665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu LZ, Huang ML, Qi CL, Shen LJ, Zou Y, Yang R, et al. Overexpression of notch2 enhances radiosensitivity via inhibition of the akt/mtor signaling pathway in nasopharyngeal carcinoma. Bioengineered. 2021;12(1):3398–409. doi: 10.1080/21655979.2021.1949236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang D, Gao L, Liu X, Yuan C, Wang G. Improved antitumor effect of ionizing radiation in combination with rapamycin for treating nasopharyngeal carcinoma. Oncol Lett. 2017;14(1):1105–8. doi: 10.3892/ol.2017.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.