Abstract
Fruits are a rich source of nutrients, minerals, and dietary fibers for both humans and animals. While the gaseous phytohormone ethylene is well-known for its role in controlling fruit ripening, there is growing evidence that ethylene also plays crucial roles in regulating other developmental processes of fruits, such as sex determination, fruit set, and fruit growth. In this review, we aim to revisit these findings from various species like cucumber, melon, tomato, rice, maize, and more. These studies not only enhance our understanding of ethylene’s function in fruits but also highlight the potential for manipulating ethylene to improve crops. Furthermore, we discuss recent studies that show the ethylene precursor ACC (1-AMINOCYCLOPROPANE-1-CARBOXYLATE), and the ethylene signaling components EIN2 (ETHYLENE INSENSITIVE2) and EIN3 (ETHYLENE INSENSITIVE3) have ethylene-independent function in specific conditions. This phenomenon, combined with findings of dosage-dependent ethylene functions in certain conditions, highlights the importance of analyzing mutants with completely blocked ethylene pathways in different species at specific developmental stages and tissue types. Overall, this review offers a timely and essential summary of ethylene’s role in sex determination, fruit formation, and fruit growth, which could be beneficial for horticulture crop breeding.
Introduction
Flowering plants have evolved an elegant strategy for fruit development: during the growth stage, the unripe fruits are usually hard and unattractive to predators, providing seed protection. After seed development and fruit growth have been completed, a highly coordinated regulatory network involving phytohormones triggers alterations in fruit appearance, texture, flavor, and aroma, making fruits become attractive and edible to frugivores so that seed dispersal occurs as an accompaniment. Generally, the making of a fruit comprises three stages: fruit set, growth, and ripening. In the case of dioecious species, there is an additional but critical stage before fruit set: sex determination.
Literature has demonstrated that phytohormones plays a crucial role in the molecular regulation of all stages of fruit development. After fruit set, both dry fruits like Arabidopsis and fleshy fruits like tomato undergo cell division and expansion, which are essential for determining fruit size before maturation. Auxin and gibberellin (GA) have been identified as key regulators of cell division and expansion, respectively [1]. To initiate fruit set at the appropriate time, the preanthesis ovaries remain in a phase of dormancy, during which cell division activities are inhibited by the negative regulators of auxin signaling such as IAA9, ARF7, and the negative regulator of GA signaling DELLA [2–4]. Successful fertilization triggers an increase in auxin and GA content, leading to activated cell division and expansion in young fruits. Recently, the involvement of SlDELLA and the SlARF7/SlIAA9 complex in mediating crosstalk between auxin and GA pathways during fruit set and growth has been well characterized in tomato [5, 6]. There is a significant interaction between ethylene and auxin in various stages of the fruit set process [7]. Furthermore, the control of fruit maturation/ripening is influenced by various phytohormones in different species: auxin seems to be the primary regulator of fruit maturation in Arabidopsis, as a regulated auxin minimum is necessary for seed dispersal of Arabidopsis siliques [8]; fruit ripening in non-climacteric fruits like strawberry and in climacteric fruits like tomato appears to be primarily achieved by abscisic acid (ABA) and ethylene, respectively [9–11], although there are numerous interactions between these two hormones in both climacteric and non-climacteric fruits [12–14]. Through a genome-wide association study (GWAS) of fruit firmness in the 266 tomato core accessions, SlEIN4, an ethylene receptor gene, was identified as the key factor governing fruit firmness in tomato [15]. Additionally, other phytohormones including auxin, jasmonic acid (JA), gibberellin, and brassinosteroid (BR) have been implicated in the fine-tuning fruit ripening control by modulating ethylene biosynthesis in most cases. The ethylene response factor ERF.D7 activates the auxin response factor ARF2 to regulate fruit ripening in tomato [16, 17]. During fruit ripening, the accumulation of carotenoids is modulated by the auxin-ethylene balance [18]. The jasmonate-activated transcription factor MdMYC2 promotes fruit ripening by activating ethylene biosynthesis in apple [19]. In tomato, gibberellin participates in fruit ripening and softening by mediating multiple hormonal signals [20]. The BR biosynthetic gene SlCYP90B3 positively regulates fruit ripening via an ethylene-dependent pathway [21].
Owing to its fundamental role in ripening control of climacteric fruits, ethylene is the most widely explored phytohormone in fruit biology. Ancient Chinese people burned incense to ripen pears, and early Egyptians gashed figs to induce their ripening. These are the two earliest records of manipulating ethylene, despite the underlying mechanism not being elucidated until thousands of years later [22]. Over the past decades, the characterization of mutants and transgenic plants corresponding to ethylene biosynthesis, perception, and response pathways in Arabidopsis and tomato has greatly enhanced our understanding of the molecular basis of ethylene in fruit quality and ripening control [23]. Based on the autoinhibitory and autocatalytic features, McMurchie et al. [24] introduced two unique ethylene systems: System-1 represents the basal low level of ethylene production in preclimacteric fruits, vegetative tissues, and nonclimacteric fruits. System-2, on the other hand, is the high-level ethylene production observed during ripening in climacteric fruits and in certain senescent flowers [25]. Despite the extensively studied function of system-2 ethylene in fruit ripening control, emerging evidence shows that system-1 ethylene is essential for fruit set and growth, as well as sex determination in dioecious plants.
Ethylene in sex determination
Approximately 5–6% of flowering plant species produce unisexual flowers, representing a model system to unveil mechanisms of sex determination in plants. The successive cloning of genes underlying the genetic ‘FAM’ model in cucumber (Cucumis sativus) has made the ‘one-hormone hypothesis’ convincing and widely accepted [26, 27]. The hypothesis posits ethylene as the most important regulator of unisexual flower development in cucumber.
With map-based cloning, it was found that all the ‘sex genes’ of cucumber, including F (Female, CsACS1G), M (Monoecious, CsACS2), and A (Androecious, CsACS11), encode 1-aminocyclopropane-1-carboxylate synthase (ACS), which catalyzes the rate-limiting step in ethylene biosynthesis. The nature of gynoecious cucumbers conferred by the F locus has been the most used accession for breeding high-yield varieties for half a century. The gain-of-function (GOF) of a duplicated copy of CsACS1G, but not CsACS1 per se or other genes, in the F locus is responsible for the development of all-female flowers [28, 29]. ACS1G in gynoecious cucumbers shows a distinct structural variation of the genome, demonstrating a different promoter and expression level than that of ACS1 in monoecious cucumbers. The higher CsACS1G transcripts increase ethylene content, leading to all-female flowers [30]. Regardless of the breeding value of CsACS1G in accessions containing the F locus, CsACS1 plays a less important role than M/CsACS2 and A/CsACS11 in sex determination. CsACS11 acts together with CsACO2 at an early stage of floral development, regulating carpel development of female flowers. Dysfunction of either CsACS11 or CsACO2 leads to the loss of female flowers [31, 32]. This is also the case for CmACS11 and CmACO3 in melon (Cucumis melon) and CpACO1A in zucchini (Cucurbita pepo) [33, 34]. Interestingly, M/CsACS2 mediates a positive feedback regulation of secondary ethylene biosynthesis. It is believed that the amplification of enough ethylene is crucial for the arrest of stamen primordia development, as CsACS2 knock-down plants display bisexual flowers [33, 34]. Recent studies have revealed that two ethylene responsive factors (ERF) in cucumber, CsERF110 and CsERF31, are involved in the positive feedback loop through directly activating the transcription of CsACS11 and CsACS2, respectively [35, 36]. Kock-down of CsERF31 in cucumber gynoecy line causes defective bisexual flowers, replacing the female flowers [36]. It is noteworthy that the A (Andromonoecy) locus in melon is encoded by an ACS family member designated as CmACS7, which inhibits the development of the male organs and is not required for carpel development [33]. The melon A/CmACS7 is a close homolog of the cucumber M/CsACS2 gene, and these two genes share a conserved function in regulating the andromonoecious phenotype of cucumber and melon [37]. Furthermore, two independent studies found that the watermelon (Citrullus lanatus) A locus gene A/CitACS4 is required for monoecy/andromonoecy regulation. Reduced ethylene production in the floral buds by a missense mutation of CitACS4 leads to the conversion of female into hermaphrodite flowers, and therefore of monoecy into andromonoecy [38, 39].
Notably, CsWIP1, which encodes a C2H2 zinc-finger-type transcription factor, plays an essential role in sex determination. Its ortholog CmWIP1 in melon indirectly represses the expression of the andromonoecious gene A/CmACS7 [40]. Gynoecious plants can be obtained via gene-editing of CsWIP1 and ClWIP1 in monoecious plants of cucumber and watermelon, respectively [41, 42]. In cucumber, CsWIP1 is suppressed by CsACS11 and controls the coexistence of male and female flowers in monoecious plants by acting as a carpel inhibitor [31]. CsWIP1 inhibits CsACO2 expression by binding to its promoter, leading to pistil abortion [31]. CsWIP1 further suppresses CsACS2 expression, resulting in the derepression of stamen development inhibition [31]. Interestingly, in melon, it was found that the female-promoting gene, CmACS11, represses the expression of the male-promoting gene CmWIP1 via deposition of H3K27me3 [43]. Further study revealed that CmWIP1 promotes male flower development by recruiting a corepressor TOPLESS to inhibit the carpel identity gene CRC (CRABS CLAW) expression through histone deacetylation [44].
Compared to ethylene biosynthesis genes, few mutants related to ethylene signaling components have been identified in dioecious species. Two semi-dominant ethylene-insensitive mutants in C. pepo, Cpetr1a and Cpetr2b, were recently discovered through ethyl methanesulfonate (EMS)-generated mutant screening. A comprehensive characterization of single and double mutants revealed that CpETR genes collaborate in controlling female flower determination. The proportion of male flowers is positively correlated with the degree of ethylene insensitivity, which depends on the dosage of CpETR-gene mutant alleles [45, 46]. It has been suggested that different levels of ethylene responses in stamen or carpel primordia are associated with the selective arrest during sex determination [47]. Ethylene produced in melon carpel is perceived in the stamen primordia through spatially differentially expressed ethylene receptors. Subsequently, the CmEIN3/CmEIL1 genes in stamen primordia activate the expression of CmHB40 to inhibit stamen development [48]. In contrast, ethylene in cucumber could induce female flower development through an anther-specific DNA damage process caused by the stamen preferential downregulation of the ethylene receptor gene CsETR1 [49]. CsAP3, encoding a MADS-box transcription factor, is responsible for the organ preferential downregulation of CsETR1 [50]. Finally, a calcium-dependent DNase gene, CsCaN, is activated by ethylene and is associated with the anther-specific DNA damage [51]. These findings reveal that ethylene is essential for sex determination in Cucurbitaceae species, involving ethylene biosynthesis and signal transduction genes at both the transcriptional regulation and the epigenetic regulation levels (Fig. 1).
Figure 1.
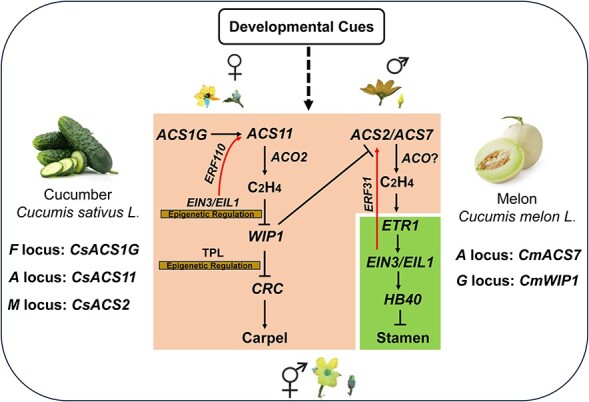
A working model demonstrating the involvement of ethylene pathway in sex determination in Cucurbitaceae species. At different flowering stages, ACS1G, which only presented in cucumber, and ACS11 synergistically with ACO2 to produce endogenous ethylene, which suppresses WIP1 expression. WIP1 can inhibit carpel development by repressing the ACS2/ACS7 expression, which in turn inhibits stamen development. To amplify enough ethylene for the arrest of stamen primordia development, ERF31 and ERF110 mediate the positive feedback regulation via directly activating the transcription of ACS2/ACS7 and ACS11, respectively. The transcription factor genes CRC and HB40 act as carpel initiator and stamen inhibitor at the downstream of WIP1 and EIN3/EIL1, respectively. The accumulation of WIP1 and CRC transcripts are associated with the ethylene-mediated histone methylation and deacetylation, respectively. To date, there are three locus (F/CsACS1G, M/CsACS12, A/CsACS2) and two locus (A/CmACS7, G/CmWIP1) controlling sex determination that have been map-cloned in cucumber and melon, respectively. The regular arrow indicates positive regulation, and the ‘T’ represents negative regulation. The red arrow indicates positive feedback regulation.
It is noteworthy that some other hormones also play a role in sex determination in Cucurbitaceae species. The treatment of BR to cucumber results in both earlier and increased levels of female flower production. This effect also appears to depend on ethylene production and sensitivity, suggesting that BR acts upstream of ethylene during floral sex determination [52]. In contrast, GA induces the formation of male flowers in gynoecious cucumber, possibly by down-regulating ethylene biosynthesis and signaling genes in the apical shoot [53]. Intriguingly, the predominant role of ethylene in sex determination seems to be unique to Cucurbitaceae species. For instance, in maize, JA plays a predominant role in sex determination. Several maize tasselseed (ts) mutants affecting JA biosynthesis or catabolism, including ts1, ts2, ts5, exhibit a reversal in sex determination, resulting in generation of seeds in tassels [54]. Additionally, maize BR biosynthesis mutants demonstrate feminized tassels [55, 56]. These findings indicate that the function of ethylene in plant reproductive stages has extensive crosstalk with other phytohormones, and elucidating the interplay conferred by different hormones in sex determination merits further investigation.
Ethylene in fruit set
The involvement of ethylene in pollination and fertilization processes has been a long-term topic in plant reproductive biology. There is a transient ethylene burst (<12 h) in tomato ovaries upon pollination, which is proposed to facilitate senescence of certain floral organs [57, 58]. The pollination process stimulates the expression of the ethylene biosynthetic genes in a developmentally regulated and tissue-specific manner, which is important for ovary/ovule and gametophyte development in orchid flowers [59, 60]. In Nicotiana attenuata, a self-compatible wild tobacco accession, the post-pollination ethylene burst is recognized as the harbinger of non-random mate selection [61]. In fact, ethylene acts as a suppressor of the self-incompatibility response. Treating stigmas with ethylene or suppressing the expression of a negative regulator of ethylene signaling, CTR1 (CONSTITUTIVE TRIPLE RESPONSE 1), broke down the self-incompatibility in Brassica rapa [62]. During pollination, ethylene signaling modulates pollen tube growth through modifications of cell wall remodeling and calcium gradient in tomato [63]. Tomato pollen tubes in etr3-KO (knock out), a loss-of-function (LOF) mutant, and Nr (Never ripe), a GOF mutant, grow faster and slower than in WT, respectively [63]. In Arabidopsis, it was shown that ethylene promotes pollen tube growth by affecting actin filament organization via the cGMP-dependent pathway [64]. Upon successful fertilization, ethylene emissions and transcripts of ethylene biosynthesis/signaling genes decrease in tomato ovaries/young fruits [65, 66], suggesting a negative role of ethylene in fruit set. In accordance with that, the application of 1-MCP, an ethylene perception inhibitor, results in parthenocarpic tomato fruits [67]. As a typical self-pollination flowering plant, tomato stigmas wrapped by stamens at the anthesis stage ensure precise pollen spread. Sletr1–1, a GOF mutant of the tomato ethylene receptor gene, exhibits protruded stigmas and parthenocarpic fruits [67]. It was further demonstrated that ethylene prevents excess growth of tomato ovaries/stigmas at the anthesis stage by modulating GA biosynthesis/metabolism [67]. Consistently, the LOF mutant slein2 also yields a large proportion of facultative parthenocarpic fruits in tomatoes [68]. Together, this evidence shows that upon pollination, ethylene might confer distinct functions before and after fertilization. Because the time of pollination and the subsequent fertilization are linear, it is suggested that we should make a distinction when dissecting the role of ethylene at different periods during fruit set.
To ensure successful fertilization, the ethylene signal is crucial for both attracting the pollen tube and preventing polytubey in the female gametophyte/ovule. The over-accumulation of EIN3 in ebf1 ebf2 synergid cells leads to the failure of pollen tube guidance, possibly by activating a sugar transporter gene, SENESCENCE-ASSOCIATED GENE29 (SAG29/SWEET15) [69]. When the pollen tube reaches the ovule, it delivers sperm cells to execute the double fertilization of two female gametophytes: the egg cell and the central cell. Fertilization of the central cell induces the synergid-endosperm fusion, which causes rapid dilution of pre-secreted pollen tube attractant in the persistent synergid cell. This process also leads to selective disorganization of the synergid nucleus during endosperm proliferation, preventing the attractions of an excess number of pollen tubes [70]. Meanwhile, fertilization of the egg cell predominantly activates ethylene signaling, which induces the disorganization of the synergid nucleus [70]. Arabidopsis ein2 and ein3 eil1 mutants show persistent synergid presence and attraction of extra pollen tubes [71], suggesting that ethylene signaling is essential for linking fertilization to synergid breakdown and the formation of a pollen tube barrier. Analysing these three findings suggests that there is a subtle dosage effect of EIN3 protein in controlling plant fertility, as mutants with either enhanced accumulation of EIN3 or deletion of EIN2-EIN3/EIL1 show compromised fertilization in Arabidopsis [69–71].
Very recently, however, by generating a CRISPR/Cas9 LOF quintuple mutation of all five 1-AMINOCYCLOPROPANE-1-CARBOXYLATE OXIDASE (ACO) coding genes, which are responsible for converting ACC to ethylene gas in vivo, Li et al. [72] found that the lack of ethylene does not affect reproductive success and synergid cell death in Arabidopsis. The finding, together with observations in ein2 and ein3eil1 mutants, suggests that certain components of ethylene signaling, including EIN2 and EIN3/EIL1, but not ethylene per se, are involved in the degeneration of the persistent synergid cell [70–72]. In fact, both EIN2 and EIN3 have recently been proposed to mediate ethylene-independent signaling pathways. EIN2 was reported to participate in glucose-mTOR signaling to mediate growth in Arabidopsis [73]. EIN3 confers an ethylene-independent function of synergid cell death upon fertilization in Arabidopsis [74]. Moreover, the ethylene precursor ACC also exhibits an ethylene-independent function in controlling pollen tube attraction, as ACS octuplet mutant plants demonstrate defects in pollen tube attraction and fertility, which could not be rescued by ethylene gas treatment [75].
For a better understanding of the ethylene function during fruit set, we have integrated all these findings in Figs 2 and 3 in the present review. Given that all these ethylene-independent functions are deciphered in the model plant Arabidopsis, our knowledge of the role of ethylene pathway genes in fleshy fruit might need to be re-evaluated by more comprehensive genetic evidence. In fact, a recent study in tomato demonstrates the embryo lethality of the high-order sleil LOF mutant sleil1 sleil2 sleil3 sleil4 but the viability of the upstream slein2 LOF mutant, suggesting that SlEIL proteins must carry out, to some extent, SlEIN2-independent functions in tomato [68].
Figure 2.
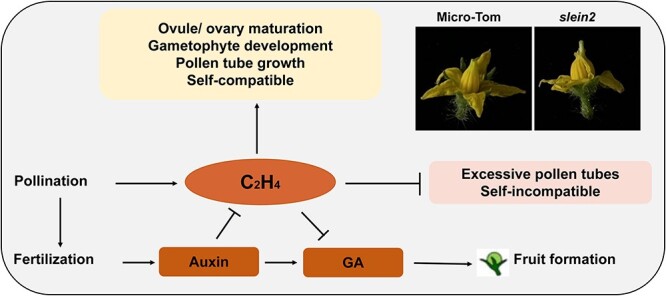
Model of the ethylene-mediated fruit set. In flowering plant, ethylene promotes selfing (self-compatible) while inhibiting outcrossing (self-incompatible). The pollination process stimulates a transient ethylene burst, which plays a positive role in the maturation of the reproductive organs and pollen tube growth for the following fertilization. Successful fertilization induces the auxin pathway that can downregulate ethylene biosynthesis, leading to derepressed GA pathway and thereby initiates fruit formation. The upper right corner photos show the extruded stigma in tomato slein2 LOF mutant. The regular arrow indicates positive regulation, and the ‘T’ represents negative regulation.
Figure 3.
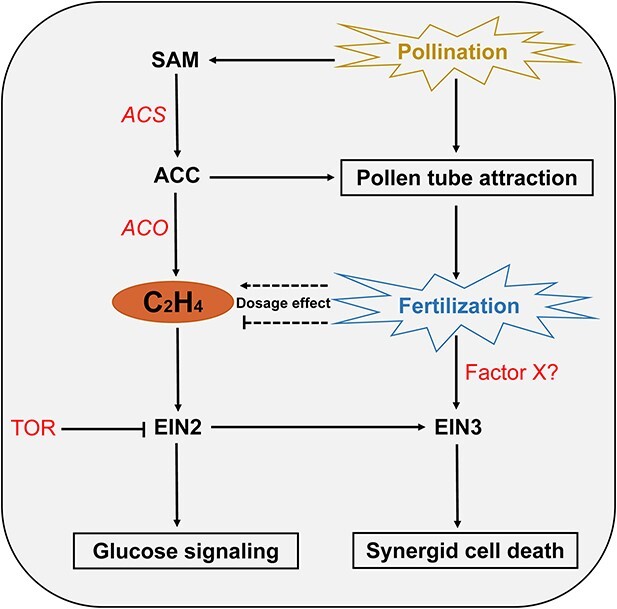
Schematic diagram of ethylene-independent function of ACC, EIN2, and EIN3 in Arabidopsis. Upon pollination, ACC functions in pollen tube attraction in an ethylene-independent manner, as the failure of pollen tube attraction in ACS octuplet mutant could not be rescued by ethylene gas treatment [75]. The successful fertilization depends on the dosage effect of the ethylene signaling, as either enhanced accumulation of EIN3 or deletion of EIN2-EIN3/EIL1 shows compromised fertilization [70–72]. In parallel, fertilization stimulates EIN3 accumulation to trigger synergid cell death by an unknown factor [74]. Besides, EIN2, the central component of the ethylene pathway, participates in the mTOR-glucose signaling pathway in an ethylene-independent manner [73], while it needs further investigation to see whether the mTOR-EIN2-glucose signaling module gets involved in fruit set. The regular arrow indicates positive regulation, and the ‘T’ represents negative regulation.
Ethylene in fruit growth
Successful fertilization is followed by rapid cell division and expansion of the newly formed young fruits. While auxin and GA play predominant roles during cell division and expansion in developing fruits, other hormones also have the capacity to influence fruit growth, possibly through crosstalk with auxin and GA [12]. Among them, the role of ethylene in controlling fruit size in different species appears to be controversial.
Based on the larger rosettes, leaves, and petals of Arabidopsis mutants with impaired positive regulators of ethylene signaling, ethylene has long been recognized as a growth inhibitor [76]. The application of a high level of ACC to fertilized ovaries results in smaller tomatoes [67], while treatment with ethylene inhibitors leads to increased fruit size in apples [77], suggesting a negative role of ethylene in fruit growth control, similar to the discovery in Arabidopsis. In support of this notion, tomatoes that overproduce ethylene and those with enhanced ethylene signal also exhibit smaller fruit size [78, 79]. Surprisingly, when ethylene signaling was completely blocked, it was found that tomato slein2-KO mutant plants produced much smaller fruits. The tomato SlEIL high-order mutant sleil1 sleil2 sleil3/SlEIL3 sleil4 also resulted in smaller fruits, indicating that ethylene also positively regulates fruit growth in tomatoes [68]. The suppressed fruit growth in slein2 is mediated, at least partially, by the impaired auxin accumulation derived from developing seeds. This implies the existence of a regulatory axis involving ethylene-auxin crosstalk and fruit growth regulation [68]. To explain these seemingly inconsistent conclusions regarding ethylene’s function in controlling fruit growth, the authors proposed that an appropriate basal concentration of ethylene is optimal for fruit growth, while the absence or excess of ethylene production is inhibitory. Consistent with this proposal, it was found that cucumber mutants with either higher (short fruit 1, sf1) or reduced (acs2) ethylene production exhibit fewer cell divisions and shorter fruits than the WT [80]. Another study in melon suggests that ethylene acts as an inhibitor of cell division but as an activator of cell expansion during fruit elongation [81].
The inconsistency also extends to dry fruits. The Arabidopsis mutant with enhanced ethylene signaling, eer5 (ENHANCED ETHYLENE RESPONSE 5), shows hypersensitivity to ethylene and shorter siliques [82]. In contrast, ACC-deaminase transgenic canola lines exhibit decreased ethylene content, resulting in smaller siliques and seeds [83]. Compared to other species with dry fruits, mounting evidence shows that ethylene promotes grain size in rice. The 1000-grain weights of OsETR2 silencing lines are dramatically higher than those of the control, while OsETR2 overexpressing plants show the opposite or unaltered effect [84]. In line with this, both the length and width of well-filled grains significantly decrease in Osein2/mhz7 mutants, while overexpression of OsEIN2/MHZ7 leads to increased grain length [85]. Similarly, the Oseil1/mhz6 LOF mutant shows a remarkable decrease in grain length and width, while overexpression of OsEIL1/MHZ6 increases grain size [86]. Further investigation has identified the rice OsEIL1-OsERF115-target gene regulatory module that controls grain size and weight by promoting longitudinal elongation and transverse division of spikelet hull cells, as well as enhancing grain-filling activity [87]. Interestingly, overexpression of OsEIL2, homologous to OsEIL1, results in smaller grain size [86]. Moreover, the characterization of a quantitative trait locus in maize, qEL7 encoding ZmACO2, reveals a negative role of ethylene in controlling ear length [88]. Together, it seems that the role of ethylene in organ size control is much more complicated, implying that its functional characterization is highly dependent on the specificity of species as well as the tissue types (Fig. 4).
Figure 4.
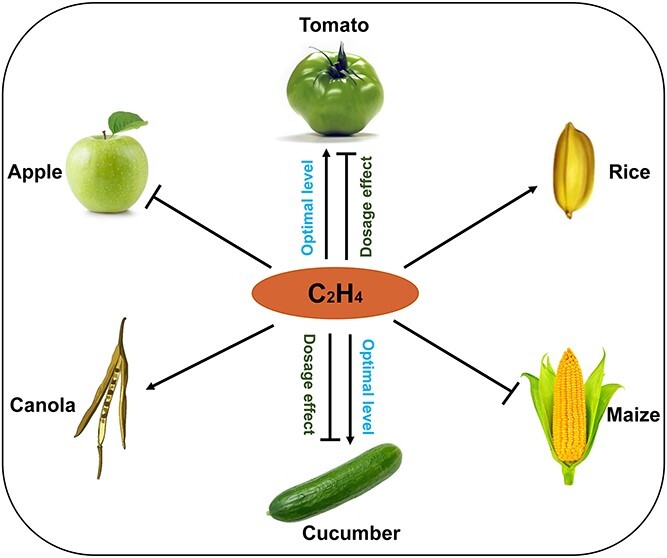
Schematic diagram demonstrating ethylene-mediated positive and/or negative regulation of fruit growth in six crop species. In particularl, the ethylene-mediated tomato and cucumber fruits’ growth requires an optimal ethylene level in a dosage-dependent manner. The regular arrow indicates positive regulation, and the ‘T’ represents negative regulation.
It has been shown that tomato and cucumber fruits with either higher or lower ethylene content display suppressed fruit growth [68, 80], suggesting that ethylene might function in a dosage-dependent manner. To maintain the optimal status, ethylene has evolved both autoinhibitory and autocatalytic mechanisms in climacteric fruits like tomato [9]. To operate the two contrasting ethylene generation systems at different developmental stages, some intrinsic molecular modules have been disclosed. For instance, several NAC and MADS-box transcription factors, which are specifically activated by tomato EIN3-like proteins at the ripening stage, are responsible for the ethylene burst required for the timely achievement of fully ripe fruits [68, 89]. Meanwhile, to avoid over-ripeness, other transcription factors, including SlAP2a, SlERF6, and SlMADS1, act as negative regulators of ethylene biosynthesis during tomato ripening [90–92]. The positive feedback regulation mediated by CsERF33-CsACS2 and CsERF110-CsACS11 modules has also been documented as a key molecular basis for initiating female flower development in cucumber [35, 36]. The transcription factor WIP1 plays a crucial role in regulating the level of ethylene during flower development in Cucurbits [93]. In order to produce female flowers, the expression of WIP1 is suppressed by a high level of ethylene signaling in the carpel. Conversely, WIP1 hinders the development of male flowers by suppressing ethylene biosynthesis in the stamen [44]. Therefore, it is imperative to explore additional factors that contribute to the ethylene dosage effect in various plant species.
Future perspectives
As a gaseous phytohormone, it remains poorly understood about how ethylene is confined to specific areas at an optimal level. The switching on or off of ethylene pathway genes in specific tissues dictates the formation of female or male flowers in cucurbits [26, 27]. Tomato fruit growth requires a relatively low level of ethylene, but a complete block of ethylene signaling leads to growth inhibition [68]. With the development of omics technology, particularly spatiotemporal transcriptome analysis, we can now differentiate transcripts not only of ethylene pathway genes but also their putative upstream regulators and downstream genes at the single-cell level. For instance, exploring the initiation site of floral sex determination in cucurbits, deciphering the switch between cell division and cell expansion at the early fruit growth stage, and uncovering the differences in ethylene perception in different cell types of fruits would become possible and informative in the near future.
The involvement of epigenetic regulation in fruit development and ripening is another important topic, as RNA modifications (e.g., m6A) and DNA modifications (e.g., 5mC) have been proven to be essential for fruit ripening in strawberry and tomato [94–96]. Moreover, it has been shown that transgenic expression of the human RNA demethylase FTO (fat mass and obesity-associated gene) in rice and potato results in a remarkable increase in yield [97]. During tomato fruit growth, a large number of fruit expansion-related genes are m6A modified and expressed more actively than the non-m6A-modified genes, suggesting a potential role of m6A modification in tomato fruit expansion [98]. Because ethylene plays a role in the entire life cycle of fruit development, the relationship between ethylene and epigenetic modifications remains to be explored. Additionally, the regulatory network of ethylene regulation in fruits responding to environmental factors such as light, temperature, and adverse conditions is far from understood, which would have high economic and agronomic value.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32200263 to W.H.), the Shenzhen Science and Technology Program (KQTD20190929173906742 to H.G. and KQTD20230301092839007 to C.T.), the Science and Technology Major Special Project of Shenzhen (KJZD20230923114607016 to W.H.), and the Shenzhen Branch, Guangdong Laboratory for Lingnan Modern Agriculture (AGIS-ZDXM202201 to H.G.).
Contributor Information
Wei Huang, State Key Laboratory of Agricultural Genomics, Key Laboratory of Genomics, Ministry of Agricultural, BGI Research, Shenzhen 518083, China; BGI Bioverse, Shenzhen 518083, China.
Cong Tan, BGI Bioverse, Shenzhen 518083, China.
Hongwei Guo, New Cornerstone Science Laboratory, Institute of Plant and Food Science, Department of Biology, School of Life Sciences, Southern University of Science and Technology (SUSTech), Shenzhen, Guangdong 518055, China; Shenzhen Branch, Guangdong Laboratory for Lingnan Modern Agriculture, Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences, Shenzhen 518120, China.
Author contributions
W. H. conceived the review. W. H. and C.T. wrote the review. H. G. critically revised and edited the review.
Conflict of interest statement
All authors declare no conflict of interest.
References
- 1. de Jong M, Mariani C, Vriezen WH. The role of auxin and gibberellin in tomato fruit set. J Exp Bot. 2009;60:1523–32 [DOI] [PubMed] [Google Scholar]
- 2. Wang H, Jones B, Li Z. et al. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell. 2005;17:2676–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Jong M, Wolters-Arts M, Feron R. et al. The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 2009;57:160–70 [DOI] [PubMed] [Google Scholar]
- 4. Carrera E, Ruiz-Rivero O, Peres LEP. et al. Characterization of the procera tomato mutant shows novel functions of the SlDELLA protein in the control of flower morphology, cell division and expansion, and the auxin-signaling pathway during fruit-set and development. Plant Physiol. 2012;160:1581–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruan YL, Patrick JW, Bouzayen M. et al. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012;17:656–65 [DOI] [PubMed] [Google Scholar]
- 6. Hu J, Israeli A, Ori N. et al. The interaction between DELLA and ARF/IAA mediates crosstalk between gibberellin and auxin signaling to control fruit initiation in tomato. Plant Cell. 2018;30:1710–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. An J, Almasaud RA, Bouzayen M. et al. Auxin and ethylene regulation of fruit set. Plant Sci. 2020;292:110381 [DOI] [PubMed] [Google Scholar]
- 8. Sorefan K, Girin T, Liljegren SJ. et al. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature. 2009;459:583–6 [DOI] [PubMed] [Google Scholar]
- 9. Liu M, Pirrello J, Chervin C. et al. Ethylene control of fruit ripening: revisiting the complex network of transcriptional regulation. Plant Physiol. 2015;169:2380–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li BJ, Grierson D, Shi Y. et al. Roles of abscisic acid in regulating ripening and quality of strawberry, a model non-climacteric fruit. Hortic Res. 2022;9:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McAtee P, Karim S, Schaffer R. et al. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front Plant Sci. 2013;4:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fenn MA, Giovannoni JJ. Phytohormones in fruit development and maturation. Plant J. 2021;105:446–58 [DOI] [PubMed] [Google Scholar]
- 13. Sharif R, Su L, Chen X. et al. Hormonal interactions underlying parthenocarpic fruit formation in horticultural crops. Hortic Res. 2022;9:uhab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zou J, Li N, Hu N. et al. Co-silencing of ABA receptors (SlRCAR) reveals interactions between ABA and ethylene signaling during tomato fruit ripening. Hortic Res. 2022;9:uhac057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang S, Wu S, Jia Z. et al. Exploring the influence of a single-nucleotide mutation in EIN4 on tomato fruit firmness diversity through fruit pericarp microstructure. Plant Biotechnol J. 2024;22:2379–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gambhir P, Singh V, Parida A. et al. Ethylene response factor ERF. D7 activates auxin response factor 2 paralogs to regulate tomato fruit ripening. Plant Physiol. 2022;190:2775–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hao Y, Hu G, Breitel D. et al. Auxin response factor SlARF2 is an essential component of the regulatory mechanism controlling fruit ripening in tomato. PLoS Genet. 2015;11:e1005649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Su L, Diretto G, Purgatto E. et al. Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol. 2015;15:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li T, Xu Y, Zhang L. et al. The jasmonate-activated transcription factor MdMYC2 regulates ETHYLENE RESPONSE FACTOR and ethylene biosynthetic genes to promote ethylene biosynthesis during apple fruit ripening. Plant Cell. 2017;29:1316–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu M, Liu K, Li H. et al. Gibberellins involved in fruit ripening and softening by mediating multiple hormonal signals in tomato. Hortic Res. 2024;11:uhad275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hu S, Liu L, Li S. et al. Regulation of fruit ripening by the brassinosteroid biosynthetic gene SlCYP90B3 via an ethylene-dependent pathway in tomato. Hortic Res. 2020;7:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chang C. Q&A: How do plants respond to ethylene and what is its importance? BMC Biol. 2016;14:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tucker GA, Grierson D. Fruit ripening. In: The Biochemistry of Plants. 2013,265–318
- 24. McMurchie EJ, McGlasson WB, Eaks IL. Treatment of fruit with propylene gives information about the biogenesis of ethylene. Nature. 1972;237:235–6 [DOI] [PubMed] [Google Scholar]
- 25. Yang SF, Oetiker JH. The role of ethylene in fruit ripening. Postharvest Physiol Fruits. 1994;398:167–78 [Google Scholar]
- 26. Martínez C, Jamilena M. To be a male or a female flower, a question of ethylene in cucurbits. Curr Opin Plant Biol. 2021;59:101981 [DOI] [PubMed] [Google Scholar]
- 27. Zheng J, Xia R. Flower development and sex determination in horticultural crops. Fruit Res. 2022;2:1–9 [Google Scholar]
- 28. Zhang Z, Mao L, Chen H. et al. Genome-wide mapping of structural variations reveals a copy number variant that determines reproductive morphology in cucumber. Plant Cell. 2015;27:1595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang H, Li S, Yang L. et al. Gain-of-function of the 1-aminocyclopropane-1-carboxylate synthase gene ACS1G induces female flower development in cucumber gynoecy. Plant Cell. 2021;33:306–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boualem A, Troadec C, Camps C. et al. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science. 2015;350:688–91 [DOI] [PubMed] [Google Scholar]
- 31. Chen H, Sun J, Li S. et al. An ACC oxidase gene essential for cucumber carpel development. Mol Plant. 2016;9:1315–27 [DOI] [PubMed] [Google Scholar]
- 32. Cebrián G, Iglesias-Moya J, Romero J. et al. The ethylene biosynthesis gene CpACO1A: a new player in the regulation of sex determination and female flower development in Cucurbita pepo. Front Plant Sci. 2021;12:817922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boualem A, Fergany M, Fernandez R. et al. A conserved mutation in an ethylene biosynthesis enzyme leads to andromonoecy in melons. Science. 2008;321:836–8 [DOI] [PubMed] [Google Scholar]
- 34. Li Z, Huang S, Liu S. et al. Molecular isolation of the M gene suggests that a conserved-residue conversion induces the formation of bisexual flowers in cucumber plants. Genetics. 2009;182:1381–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tao Q, Niu H, Wang Z. et al. Ethylene responsive factor ERF110 mediates ethylene-regulated transcription of a sex determination-related orthologous gene in two Cucumis species. J Exp Bot. 2018;69:2953–65 [DOI] [PubMed] [Google Scholar]
- 36. Pan J, Wen H, Chen G. et al. A positive feedback loop mediated by CsERF31 initiates female cucumber flower development. Plant Physiol. 2021;186:1088–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boualem A, Troadec C, Kovalski I. et al. A conserved ethylene biosynthesis enzyme leads to andromonoecy in two Cucumis species. PLoS One. 2009;4:e6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ji G, Zhang J, Zhang H. et al. Mutation in the gene encoding 1-aminocyclopropane-1-carboxylate synthase 4 (CitACS4) led to andromonoecy in watermelon. J Integr Plant Biol. 2016;58:762–5 [DOI] [PubMed] [Google Scholar]
- 39. Manzano S, Aguado E, Martínez C. et al. The ethylene biosynthesis gene CitACS4 regulates monoecy/andromonoecy in watermelon (Citrullus lanatus). PLoS One. 2016;11:e0154362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin A, Troadec C, Boualem A. et al. A transponson-induced epigenetic change leads to sex determination in melon. Nature. 2009;461:1135–8 [DOI] [PubMed] [Google Scholar]
- 41. Hu B, Li D, Liu X. et al. Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol Plant. 2017;10:1575–8 [DOI] [PubMed] [Google Scholar]
- 42. Zhang J, Guo S, Ji G. et al. A unique chromosome translocation disrupting ClWIP1 leads to gynoecy in watermelon. Plant J. 2020;101:265–77 [DOI] [PubMed] [Google Scholar]
- 43. Latrasse D, Rodriguez-Granados NY, Veluchamy A. et al. The quest for epigenetic regulation underlying unisexual flower development in Cucumis melo. Epigenetics Chromatin. 2017;10:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang S, Tan FQ, Chung CH. et al. The control of carpel determinacy pathway leads to sex determination in cucurbits. Science. 2022;378:543–9 [DOI] [PubMed] [Google Scholar]
- 45. García A, Aguado E, Martínez C. et al. The ethylene receptors CpETR1A and CpETR2B cooperate in the control of sex determination in Cucurbita pepo. J Exp Bot. 2020;71:154–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. García A, Aguado E, Garrido D. et al. Two androecious mutations reveal the crucial role of ethylene receptors in the initiation of female flower development in Cucurbita pepo. Plant J. 2020;103:1548–60 [DOI] [PubMed] [Google Scholar]
- 47. Yin T, Quinn JA. Tests of a mechanistic model of one hormone regulating both sexes in Cucumis sativus (Cucurbitaceae). Am J Bot. 1995;82:1537–46 [Google Scholar]
- 48. Rashid D, Devani RS, Rodriguez-Granados NY. et al. Ethylene produced in carpel primordia controls CmHB40 expression to inhibit stamen development. Nat Plants. 2023;9:1675–87 [DOI] [PubMed] [Google Scholar]
- 49. Wang DH, Li F, Duan QH. et al. Ethylene perception is involved in female cucumber flower development. Plant J. 2010;61:862–72 [DOI] [PubMed] [Google Scholar]
- 50. Sun JJ, Li F, Wang DH. et al. CsAP3: a cucumber homolog to Arabidopsis APETALA3 with novel characteristics. Front Plant Sci. 2016;07:204293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gu HT, Wang DH, Li X. et al. Characterization of an ethylene-inducible, calcium-dependent nuclease that is differentially expressed in cucumber flower development. New Phytol. 2011;192:590–600 [DOI] [PubMed] [Google Scholar]
- 52. Manzano S, Martínez C, Megías Z. et al. The role of ethylene and brassinosteroids in the control of sex expression and flower development in Cucurbita pepo. Plant Growth Regul. 2011;65:213–21 [Google Scholar]
- 53. Zhang Y, Zhao G, Li Y. et al. Transcriptomic analysis implies that GA regulates sex expression via ethylene-dependent and ethylene-independent pathways in cucumber (Cucumis sativus L.). Front Plant Sci. 2017;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang F, Yuan Z, Zhao Z. et al. Tasselseed5 encodes a cytochrome C oxidase that functions in sex determination by affecting jasmonate catabolism in maize. J Integr Plant Biol. 2020;62:247–55 [DOI] [PubMed] [Google Scholar]
- 55. Hartwig T, Chuck GS, Fujioka S. et al. Brassinosteroid control of sex determination in maize. Proc Natl Acad Sci USA. 2011;108:19814–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Best NB, Hartwig T, Budka J. et al. nana plant2 encodes a maize ortholog of the Arabidopsis brassinosteroid biosynthesis gene DWARF1, identifying developmental interactions between brassinosteroids and gibberellins. Plant Physiol. 2016;171:2633–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Llop-Tous I, Barry CS, Grierson D. Regulation of ethylene biosynthesis in response to pollination in tomato flowers. Plant Physiol. 2000;123:971–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pattison RJ, Csukasi F, Zheng Y. et al. Comprehensive tissue-specific transcriptome analysis reveals distinct regulatory programs during early tomato fruit development. Plant Physiol. 2015;168:1684–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang XS, O'Neill SD. Ovary and gametophyte development are coordinately regulated by auxin and ethylene following pollination. Plant Cell. 1993;5:403–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. O'Neill SD, Nadeau JA, Zhang XS. et al. Interorgan regulation of ethylene biosynthetic genes by pollination. Plant Cell. 1993;5:419–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bhattacharya S, Baldwin IT. The post-pollination ethylene burst and the continuation of floral advertisement are harbingers of non-random mate selection in Nicotiana attenuata. Plant J. 2012;71:587–601 [DOI] [PubMed] [Google Scholar]
- 62. Su S, Dai H, Wang X. et al. Ethylene negatively mediates self-incompatibility response in Brassica rapa. Biochem Biophys Res Commun. 2020;525:600–6 [DOI] [PubMed] [Google Scholar]
- 63. Althiab-Almasaud R, Chen Y, Maza E. et al. Ethylene signaling modulates tomato pollen tube growth through modifications of cell wall remodeling and calcium gradient. Plant J. 2021;107:893–908 [DOI] [PubMed] [Google Scholar]
- 64. Jia H, Yang J, Liesche J. et al. Ethylene promotes pollen tube growth by affecting actin filament organization via the cGMP-dependent pathway in Arabidopsis thaliana. Protoplasma. 2018;255:273–84 [DOI] [PubMed] [Google Scholar]
- 65. Wang H, Schauer N, Usadel B. et al. Regulatory features underlying pollination-dependent and-independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell. 2009;21:1428–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hu G, Huang B, Wang K. et al. Histone posttranslational modifications rather than DNA methylation underlie gene reprogramming in pollination-dependent and pollination-independent fruit set in tomato. New Phytol. 2021;229:902–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shinozaki Y, Hao S, Kojima M. et al. Ethylene suppresses tomato (Solanum lycopersicum) fruit set through modification of gibberellin metabolism. Plant J. 2015;83:237–51 [DOI] [PubMed] [Google Scholar]
- 68. Huang W, Hu N, Xiao Z. et al. A molecular framework of ethylene-mediated fruit growth and ripening processes in tomato. Plant Cell. 2022;34:3280–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang C, Teng XD, Zheng QQ. et al. Ethylene signaling is critical for synergid cell functional specification and pollen tube attraction. Plant J. 2018;96:176–87 [DOI] [PubMed] [Google Scholar]
- 70. Maruyama D, Völz R, Takeuchi H. et al. Rapid elimination of the persistent synergid through a cell fusion mechanism. Cell. 2015;161:907–18 [DOI] [PubMed] [Google Scholar]
- 71. Völz R, Heydlauff J, Ripper D. et al. Ethylene signaling is required for synergid degeneration and the establishment of a pollen tube block. Dev Cell. 2013;25:310–6 [DOI] [PubMed] [Google Scholar]
- 72. Li W, Li Q, Lyu M. et al. Lack of ethylene does not affect reproductive success and synergid cell death in Arabidopsis. Mol Plant. 2022;15:354–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fu L, Liu Y, Qin G. et al. The TOR–EIN2 axis mediates nuclear signalling to modulate plant growth. Nature. 2021;591:288–92 [DOI] [PubMed] [Google Scholar]
- 74. Heydlauff J, Serbes IE, Vo D. et al. Dual and opposing roles of EIN3 reveal a generation conflict during seed growth. Mol Plant. 2022;15:363–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mou W, Kao YT, Michard E. et al. Ethylene-independent signaling by the ethylene precursor ACC in Arabidopsis ovular pollen tube attraction. Nat Commun. 2020;11:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dubois M, Van den Broeck L, Inzé D. The pivotal role of ethylene in plant growth. Trends Plant Sci. 2018;23:311–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Byers RE, Carbaugh DH, Combs LD. Ethylene inhibitors delay fruit drop, maturity, and increase fruit size of 'Arlet' apples. HortScience. 2005;40:2061–5 [Google Scholar]
- 78. Liu M, Diretto G, Pirrello J. et al. The chimeric repressor version of an ethylene response factor (ERF) family member, Sl-ERF.B3, shows contrasting effects on tomato fruit ripening. New Phytol. 2014;203:206–18 [DOI] [PubMed] [Google Scholar]
- 79. Sharma K, Gupta S, Sarma S. et al. Mutations in tomato 1-aminocyclopropane carboxylic acid synthase2 uncover its role in development beside fruit ripening. Plant J. 2021;106:95–112 [DOI] [PubMed] [Google Scholar]
- 80. Xin T, Zhang Z, Li S. et al. Genetic regulation of ethylene dosage for cucumber fruit elongation. Plant Cell. 2019;31:1063–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Boualem A, Berthet S, Devani RS. et al. Ethylene plays a dual role in sex determination and fruit shape in cucurbits. Curr Biol. 2022;32:2390–2401.e4 [DOI] [PubMed] [Google Scholar]
- 82. Christians MJ, Robles LM, Zeller SM. et al. The eer5 mutation, which affects a novel proteasome-related subunit, indicates a prominent role for the COP9 signalosome in resetting the ethylene-signaling pathway in Arabidopsis. Plant J. 2008;55:467–77 [DOI] [PubMed] [Google Scholar]
- 83. Walton LJ, Kurepin LV, Yeung EC. et al. Ethylene involvement in silique and seed development of canola, Brassica napus L. Plant Physiol Biochem. 2012;58:142–50 [DOI] [PubMed] [Google Scholar]
- 84. Wuriyanghan H, Zhang B, Cao WH. et al. The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell. 2009;21:1473–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ma B, He SJ, Duan KX. et al. Identification of rice ethylene-response mutants and characterization of MHZ7/OsEIN2 in distinct ethylene response and yield trait regulation. Mol Plant. 2013;6:1830–48 [DOI] [PubMed] [Google Scholar]
- 86. Yang C, Ma B, He SJ. et al. MAOHUZI6/ETHYLENE INSENSITIVE3-LIKE1 and ETHYLENE INSENSITIVE3-LIKE2 regulate ethylene response of roots and coleoptiles and negatively affect salt tolerance in rice. Plant Physiol. 2015;169:148–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu C, Ma T, Yuan D. et al. The OsEIL1-OsERF115-target gene regulatory module controls grain size and weight in rice. Plant Biotechnol J. 2022;20:1470–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ning Q, Jian Y, Du Y. et al. An ethylene biosynthesis enzyme controls quantitative variation in maize ear length and kernel yield. Nat Commun. 2021;12:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lü P, Yu S, Zhu N. et al. Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nat Plants. 2018;4:784–91 [DOI] [PubMed] [Google Scholar]
- 90. Karlova R, Rosin FM, Busscher-Lange J. et al. Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell. 2011;23:923–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee JM, Joung JG, McQuinn R. et al. Combined transcriptome, genetic diversity and metabolite profiling in tomato fruit reveals that the ethylene response factor SlERF6 plays an important role in ripening and carotenoid accumulation. Plant J. 2012;70:191–204 [DOI] [PubMed] [Google Scholar]
- 92. Dong T, Hu Z, Deng L. et al. A tomato MADS-box transcription factor, SlMADS1, acts as a negative regulator of fruit ripening. Plant Physiol. 2013;163:1026–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li D, Sheng Y, Niu H. et al. Gene interactions regulating sex determination in cucurbits. Front Plant Sci. 2019;10:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lang Z, Wang Y, Tang K. et al. Critical roles of DNA demethylation in the activation of ripening-induced genes and inhibition of ripening-repressed genes in tomato fruit. Proc Natl Acad Sci USA. 2017;114:4511–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Zhou L, Tian S, Qin G. RNA methylomes reveal the m6A-mediated regulation of DNA demethylase gene SlDML2 in tomato fruit ripening. Genome Biol. 2019;20:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhou L, Tang R, Li X. et al. N6-methyladenosine RNA modification regulates strawberry fruit ripening in an ABA-dependent manner. Genome Biol. 2021;22:1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yu Q, Liu S, Yu L. et al. RNA demethylation increases the yield and biomass of rice and potato plants in field trials. Nat Biotechnol. 2021;39:1581–8 [DOI] [PubMed] [Google Scholar]
- 98. Hu J, Cai J, Umme A. et al. Unique features of mRNA m6A methylomes during expansion of tomato (Solanum lycopersicum) fruits. Plant Physiol. 2022;188:2215–27 [DOI] [PMC free article] [PubMed] [Google Scholar]


