Abstract
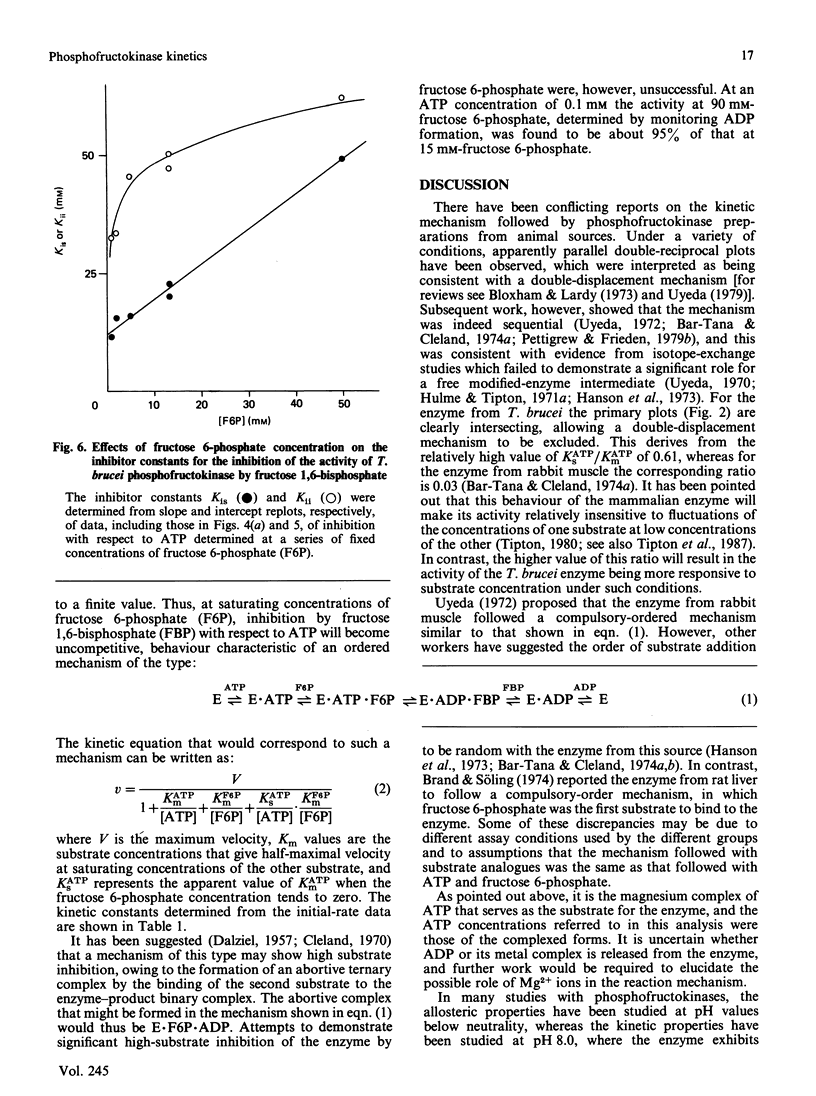
The steady-state kinetics of the reaction catalysed by the bloodstream form of Trypanosoma brucei were studied at pH 6.7. In the presence of 50 mM-potassium phosphate buffer, the apparent co-operativity with respect to fructose 6-phosphate and the non-linear relationship between initial velocity and enzyme concentration, which were found when the enzyme was assayed in 50 mM-imidazole buffer [Cronin & Tipton (1985) Biochem. J. 227, 113-124], are not evident. Studies on the variations of the initial rate with changing concentrations of MgATP and fructose 6-phosphate, the product inhibition by fructose 1,6-bisphosphate and the effects of the alternative substrate ITP were consistent with an ordered reaction pathway, in which MgATP binds to the enzyme before fructose 6-phosphate, and fructose 1,6-bisphosphate is the first product to dissociate from the ternary complex.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Tana J., Cleland W. W. Rabbit muscle phosphofructokinase. I. Anomeric specificity; initial velocity kinetics. J Biol Chem. 1974 Feb 25;249(4):1263–1270. [PubMed] [Google Scholar]
- Bar-Tana J., Cleland W. W. Rabbit muscle phosphofructokinase. II. Product and dead end inhibition. J Biol Chem. 1974 Feb 25;249(4):1271–1276. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brand I. A., Söling H. D. Rat liver phosphofructokinase. Purification and characterization of its reaction mechanism. J Biol Chem. 1974 Dec 25;249(24):7824–7831. [PubMed] [Google Scholar]
- Brohn F. H., Clarkson A. B., Jr Trypanosoma brucei brucei: patterns of glycolysis at 37 degrees C in vitro. Mol Biochem Parasitol. 1980 Sep;1(5):291–305. doi: 10.1016/0166-6851(80)90062-6. [DOI] [PubMed] [Google Scholar]
- COHN M. MAGNETIC RESONANCE STUDIES OF METAL ACTIVATION OF ENZYMIC REACTIONS OF NUCLEOTIDES AND OTHER PHOSPHATE SUBSTRATES. Biochemistry. 1963 Jul-Aug;2:623–629. doi: 10.1021/bi00904a001. [DOI] [PubMed] [Google Scholar]
- Cronin C. N., Tipton K. F. Purification and regulatory properties of phosphofructokinase from Trypanosoma (Trypanozoon) brucei brucei. Biochem J. 1985 Apr 1;227(1):113–124. doi: 10.1042/bj2270113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyer P., Hofer H. W., Krystek E., Pette D. Synthesis of glucose 1,6-biphosphate by the action of crystalline rabbit muscle phosphofructokinase. Eur J Biochem. 1971 May 28;20(2):153–159. doi: 10.1111/j.1432-1033.1971.tb01373.x. [DOI] [PubMed] [Google Scholar]
- FLORINI J. R., VESTLING C. S. Graphical determination of the dissociation constants for two-substrate enzyme systems. Biochim Biophys Acta. 1957 Sep;25(3):575–578. doi: 10.1016/0006-3002(57)90529-2. [DOI] [PubMed] [Google Scholar]
- FROMM H. J. THE USE OF ALTERNATIVE SUBSTRATES IN STUDYING ENZYMIC MECHANISMS INVOLVING TWO SUBSTRATES. Biochim Biophys Acta. 1964 Mar 9;81:413–417. doi: 10.1016/0926-6569(64)90126-9. [DOI] [PubMed] [Google Scholar]
- Flynn I. W., Bowman I. B. The metabolism of carbohydrate by pleomorphic African trypanosomes. Comp Biochem Physiol B. 1973 May 15;45(1):25–42. doi: 10.1016/0305-0491(73)90281-2. [DOI] [PubMed] [Google Scholar]
- GRANT P. T., SARGENT J. R. Properties of L-alpha-glycerophosphate oxidase and its role in the respiration of Trypanosoma rhodesiense. Biochem J. 1960 Aug;76:229–237. doi: 10.1042/bj0760229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D. J., Bowman I. B. Studies on glycerol kinase and its role in ATP synthesis in Trypanosoma brucei. Mol Biochem Parasitol. 1980 Dec;2(2):77–91. doi: 10.1016/0166-6851(80)90033-x. [DOI] [PubMed] [Google Scholar]
- Hanson R. L., Rudolph F. B., Lardy H. A. Rabbit muscle phosphofructokinase. The kinetic mechanism of action and the equilibrium constant. J Biol Chem. 1973 Nov 25;248(22):7852–7859. [PubMed] [Google Scholar]
- Huang C. Y. Use of alternative substrates to probe multisubstrate enzyme mechanisms. Methods Enzymol. 1979;63:486–500. doi: 10.1016/0076-6879(79)63021-5. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Tipton K. F. The dependence of phosphofructokinase kinetics upon protein concentration. FEBS Lett. 1971 Jan 25;12(4):197–200. doi: 10.1016/0014-5793(71)80019-4. [DOI] [PubMed] [Google Scholar]
- Hulme E. C., Tipton K. F. The isotope-exchange reactions of ox heart phosphofructokinase. Biochem J. 1971 Apr;122(2):181–187. doi: 10.1042/bj1220181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadsheh N. S., Tejwani G. A., Ramaiah A. Sedoheptulose-7-phosphate kinase activity of phosphofructokinase from the different tissues of rabbit. Biochim Biophys Acta. 1973 Nov 15;327(1):66–81. doi: 10.1016/0005-2744(73)90104-6. [DOI] [PubMed] [Google Scholar]
- Macfarlane N., Ainsworth S. A kinetic study of pig liver pyruvate kinase activated by fructose diphosphate. Biochem J. 1974 Jun;139(3):499–508. doi: 10.1042/bj1390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduro K. K., Bowman I. B., Flynn I. W. Trypanosoma brucei: preparation and some properties of a multienzyme complex catalysing part of the glycolytic pathway. Exp Parasitol. 1980 Oct;50(2):240–250. doi: 10.1016/0014-4894(80)90025-9. [DOI] [PubMed] [Google Scholar]
- Oduro K. K., Flynn I. W., Bowman I. B. Trypanosoma brucei: activities and subcellular distribution of glycolytic enzymes from differently disrupted cells. Exp Parasitol. 1980 Aug;50(1):123–135. doi: 10.1016/0014-4894(80)90014-4. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P., Bakker S., Leene W. Localization of glycerol-3-phosphate oxidase in the mitochondrion and particulate NAD+-linked glycerol-3-phosphate dehydrogenase in the microbodies of the bloodstream form to Trypanosoma brucei. Eur J Biochem. 1977 Jun 1;76(1):29–39. doi: 10.1111/j.1432-1033.1977.tb11567.x. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P., Fonck K. The potential use of inhibitors of glycerol-3-phosphate oxidase for chemotherapy of African trypanosomiasis. FEBS Lett. 1976 Feb 15;62(2):169–172. doi: 10.1016/0014-5793(76)80045-2. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: the glycosome. FEBS Lett. 1977 Aug 15;80(2):360–364. doi: 10.1016/0014-5793(77)80476-6. [DOI] [PubMed] [Google Scholar]
- Pettersson G. Asymptotic properties of enzymatic rate equations of the Wong-Hanes type. Acta Chem Scand. 1970;24(4):1271–1274. doi: 10.3891/acta.chem.scand.24-1271. [DOI] [PubMed] [Google Scholar]
- Pettigrew D. W., Frieden C. Binding of regulatory ligands to rabbit muscle phosphofructokinase. A model for nucleotide binding as a function of temperature and pH. J Biol Chem. 1979 Mar 25;254(6):1887–1895. [PubMed] [Google Scholar]
- Pettigrew D. W., Frieden C. Rabbit muscle phosphofructokinase. A model for regulatory kinetic behavior. J Biol Chem. 1979 Mar 25;254(6):1896–1901. [PubMed] [Google Scholar]
- Ricard J., Noat G., Got C., Borel M. The theory of alternative substrates in enzyme kinetics and its application to yeast hexokinase. Eur J Biochem. 1972 Nov 21;31(1):14–24. doi: 10.1111/j.1432-1033.1972.tb02494.x. [DOI] [PubMed] [Google Scholar]
- Storer A. C., Cornish-Bowden A. Concentration of MgATP2- and other ions in solution. Calculation of the true concentrations of species present in mixtures of associating ions. Biochem J. 1976 Oct 1;159(1):1–5. doi: 10.1042/bj1590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton K. F. Kinetic mechanism and enzyme function. Biochem Soc Trans. 1980 Jun;8(3):242–245. doi: 10.1042/bst0080242. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K. Phosphofructokinase. Adv Enzymol Relat Areas Mol Biol. 1979;48:193–244. doi: 10.1002/9780470122938.ch4. [DOI] [PubMed] [Google Scholar]
- Uyeda K. Studies on the fructose 1-phosphate kinase activity of rabbit muscle phosphofructokinase. J Biol Chem. 1972 Mar 25;247(6):1692–1698. [PubMed] [Google Scholar]
- Uyeda K. Studies on the reaction mechanism of skeletal muscle phosphofructokinase. J Biol Chem. 1970 May 10;245(9):2268–2275. [PubMed] [Google Scholar]
- Visser N., Opperdoes F. R., Borst P. Subcellular compartmentation of glycolytic intermediates in Trypanosoma brucei. Eur J Biochem. 1981 Sep 1;118(3):521–526. doi: 10.1111/j.1432-1033.1981.tb05550.x. [DOI] [PubMed] [Google Scholar]
- Voorheis H. P., Gale J. S., Owen M. J., Edwards W. The isolation and partial characterization of the plasma membrane from Trypanosoma brucei. Biochem J. 1979 Apr 15;180(1):11–24. doi: 10.1042/bj1800011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


