Abstract
Purpose
To evaluate the effect of intraoperative ablation on the viability, distribution, phenotype, and potential for culture expansion of bursal cells harvested during arthroscopic rotator cuff surgery.
Methods
Tissue was collected during primary arthroscopic rotator cuff repair on 6 healthy, randomly selected patients from a fellowship-trained surgeon’s practice between September 2020 and January 2021. There were 3 women (aged 60 ± 8 years) and 3 men (aged 61 ± 10 years). At the time of surgery, subacromial bursal tissue was subjected to no ablation, 1 second of ablation, or 3 seconds of ablation. Tissues were collected by an autograft harvesting system connected to an arthroscopic shaver and a pituitary grasper. Tissue fragments from each condition were sampled for viability testing or cell isolation. A viability kit with confocal microscopy was used to assess live and dead cells. Cell isolation consisted of collagenase digestion or placing tissue fragments onto tissue culture–treated plates that induced migration of cells out of the tissue. Cell proliferation rates were monitored and surface markers for mesenchymal stem cells (MSC) and pericytes were analyzed via multicolor flow cytometry.
Results
Increased ablation time significantly reduced cell viability. The mean percentage of live cells was 55.2% ± 27.2% (range, 26%-90% live) in the control group, 46.8% ± 23.8% (range, 9.6%-69.6%, P = .045) in the short-ablation group, and 35.5% ± 19% (range, 11%-54%, P = .03) in the long-ablation group. No significant differences in population doubling level (1.6 ± 0.5 days) and population doubling time (6.7 ± 2.4 days) were observed in cells from any treatment. The surface marker profile indicated an MSC phenotype with absence of a pericyte population. Ablation or cell isolation procedure had no significant effect on the surface marker profile of isolated cells.
Conclusions
Radiofrequency ablation significantly reduced the overall tissue viability but had no significant effect on cell proliferation or expression of surface markers on isolated subacromial bursal cells harvested arthroscopically.
Clinical Relevance
Analysis of the viability and performance of cells harvested after the use of ablation devices using mechanical surgical collection during rotator cuff repair surgery could further our understanding of subacromial bursal tissue and its potential role in augmenting rotator cuff repair healing.
The prevalence of rotator cuff injuries in the general population is approximately 22% and increases with each decade of life.1, 2, 3, 4 Various biomechanical and technical aspects related to optimizing the outcomes of rotator cuff repair (RCR) have been studied extensively, but RCR continues to have a nontrivial tendon retear rate.3,5, 6, 7, 8, 9, 10
Enhancing rotator cuff healing through biologic means is another area of study.11, 12, 13 Some techniques include incorporating platelet-rich plasma and stem cell treatments to augment repairs at the time of surgery.14 Mesenchymal stem cells may help in healing RCRs.15,16 Because the subacromial bursa contains a greater number of mesenchymal stem cells (MSCs) over the tendon than over the muscle, it is an easily accessible local source for consideration to help RCR healing.16,17 It may be a source for biological augmentation of a repair without requiring additional invasive procedures such as bone marrow harvest.16, 17, 18 Some studies have advocated using stem cells from the bursa to augment healing of RCRs.19, 20, 21, 22
During arthroscopic RCR surgery, an ablation device is used frequently for subacromial bursectomy to aid visualization for RCR. High temperatures can be detrimental to cell viability. Temperatures in the shoulder when using cautery or ablation devices can vary from 21°C to 38°C on average but sometimes spiking to 45°C.23,24 This raises concerns about potential detrimental effects on MSC viability in the bursa tissue. Despite these high temperatures, studies have shown that radiofrequency ablation to remove bursa tissue is safe for cartilage and tendon during surgery.23, 24, 25 Morikawa et al.17 demonstrated that the greatest number of nucleated cells in the bursa were extracted through mechanical means. They found that subacromial bursa cells showed significantly higher proliferation compared with the cells derived from concentrated bone marrow aspirate. When compared with concentrated bone marrow aspirate, cells derived from subacromial bursa showed significantly higher differentiation ability and gene expression profiles indicative of chondrogenesis, osteogenesis, and adipogenesis. Analysis of the viability and performance of cells harvested after the use of ablation devices using mechanical surgical collection during surgery could further our understanding of subacromial bursal tissue and its potential role in augmenting RCR healing. The purpose of this study was to evaluate the effect of intraoperative ablation on the viability, distribution, phenotype, and potential for culture expansion of bursal cells harvested during arthroscopic rotator cuff surgery. We hypothesized that the use of ablation on subacromial bursal tissue will not reduce cell viability or expression of cell surface markers.
Methods
Tissue Harvest
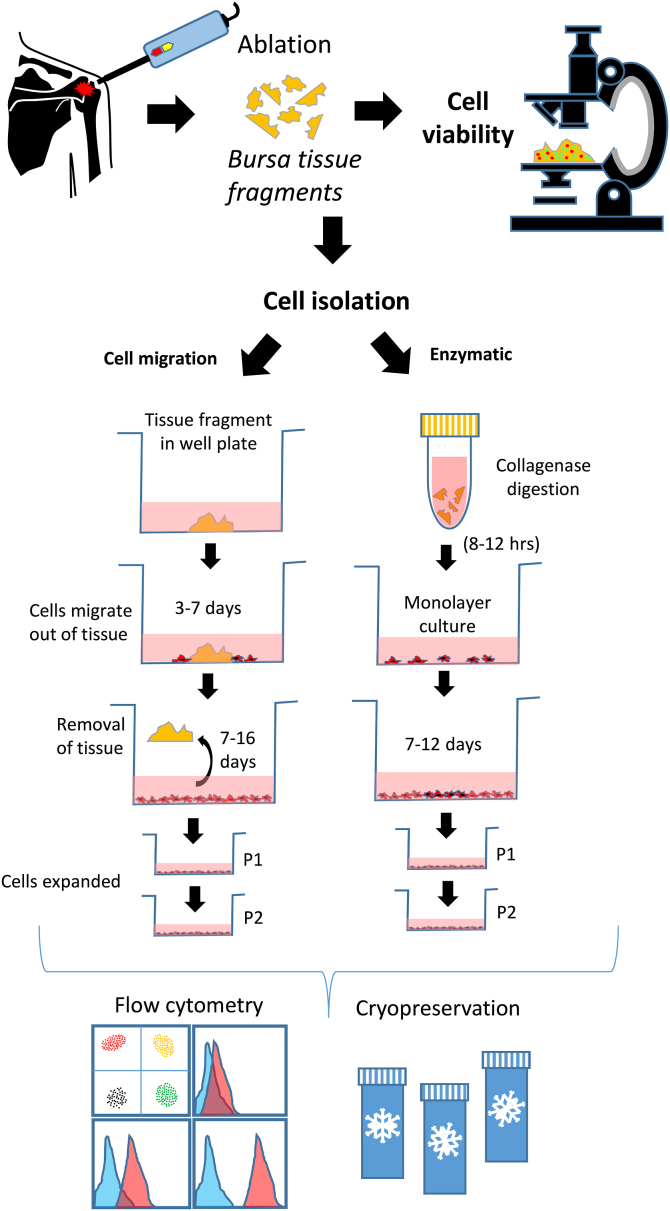
Approval was obtained from the Scripps Health Institutional Review Board for this study (14-6320). Tissue was collected during primary arthroscopic RCR on 6 randomly selected, healthy patients from a fellowship-trained surgeon’s practice (L.A.A.) between September 2020 and January 2021. There were 3 women (aged 60 ± 8 years) and 3 men (age 61 ± 10 years). Surgery was performed on days that the laboratory was immediately available for collecting and processing tissue. Patients with diabetes, cancer history, recent COVID-19 infection, and/or active smokers were excluded. Patients with concurrent procedures performed such as arthroscopic debridement, biceps surgery, or arthroscopic acromioplasty were not excluded. Bursal tissue was obtained prior to work performed in the subacromial space. At the time of the operation, subacromial bursal tissue was subjected to no ablation, a 1-second period of ablation, or a 3-second period of ablation at level 10 (radiofrequency ablation device SERFAS; Stryker). Tissue was collected by a commercially available autograft harvesting system connected to an arthroscopic shaver (GraftNet; Arthrex), as well as by a pituitary grasper. The grasper harvest was used to be able to readily identify an area of ablation for confocal microscopy evaluation. At the time of surgery, there were grossly obvious variations in the amount of bursa present. Attempts were made to get enough tissue to be able to perform all tests. Tissue was collected immediately in the area of ablation. The tissue fragments collected from the pituitary grasper and shaver for each ablation condition were combined and aseptically transferred to transport medium consisting of Dulbecco’s modified Eagle medium (DMEM) (Mediatech) and 1% penicillin/streptomycin/gentamycin (Gibco). Tissue fragments from each group were processed for assessment of cell viability or cells isolated for assessment of proliferation and expression of MSC markers.26 An overview of tissue collection and processing is outlined in Figure 1.
Fig 1.
Overview of study. Bursa tissues were randomly treated with different ablation times and segregated for tissue viability analysis or for cell isolation. Tissue viability was assessed using the live/dead reagent to identify live cells (green) or dead cells (red). Images were captured using a confocal microscope. For cell isolation, tissue fragments were either subjected to collagenase digestion or placed onto tissue culture plastic in medium until cells migrated out. The cells were expanded for 1 to 2 passages and used for cell proliferation and flow cytometry analysis or cryopreserved.
Tissue Viability Assessment
Tissue viability was analyzed within 3 hours of harvest. Fragments of tissues (n = 3-4) from each treatment were immersed in the live/dead cell viability reagent (Live/Dead kit; Life Technologies) as previously described.26 Briefly, the live/dead reagent consists of Calcein-AM (1 mM) and Ethidium Homodimer-1 (8 mM) in phosphate-buffered saline (PBS). The tissues were maintained in the reagent for 40 minutes in an incubator (37°C, 5% CO2), washed with PBS solution, and then imaged using a laser confocal microscope (LSM-710; Zeiss). Live cells fluoresce green while the nuclei of dead cells are red.
Cell Isolations
Tissues reserved for cell isolations were subjected to 2 different methods: enzymatic (collagenase) digestion or isolation by cell migration. For enzymatic cell isolations, the tissues were further minced into smaller pieces in DMEM and suspended in 5 to 10 mL of 0.2% collagenase solution (DMEM and collagenase type II; Worthington). The sealed tube was placed into the 37°C shaker overnight (approximately 12-16 hours). The digested samples were passed through a 100-μm nylon cell strainer, centrifuged, and then washed at least 2 times in PBS. Cells were transferred into T75-cm2 flasks and cultured in MSC media (Lonza) with medium changes every 3 to 4 days.
For cell isolation via migration out of tissue, tissues were minced finely before placing fragments into several wells of a 6-well plate. The tissues were provided with 0.5 mL MSC medium overnight in a high-humidity box in a CO2 incubator. The next day, an additional 1.5 mL of medium was added to each well. Medium was changed every 3 to 4 days. Cells were seen emerging from the tissues between 3 and 5 days. The cells were cultured until most of the well was close to confluence. The tissue fragments were removed, and the cells were reseeded into T75-cm2 flasks at a density of 5,000 cells per cm2.
Cell Proliferation Assessment
Cells from each patient (N = 6) were used in the cell proliferation assays. The initial seeded number of cells for the first passage (P1) were recorded, and after 8 to 15 days, the final cell count was recorded. To determine the population doubling level (PDL) and population doubling time (PDT) in days, the following equations were used.
N0 = Initial number of cells seeded; N = Total number of cells harvested
Flow Cytometry
Cultured cells were detached, washed, and suspended in fluorescence-activated flow cytometry buffer at a concentration of 0.2 × 106 cells per 100 μL. Several antibodies were used to examine surface marker expression profiles. CD34 and CD45 are negative markers for MSC.27 The positive MSC surface markers tested were CD73, CD90, CD105, and CD271. CD140a and CD140b were used to detect the presence of pericytes.28 The antibodies were split into 2 panels with different fluorochromes for multicolor flow cytometry. Panel 1 included CD34-FITC, CD73- APC, CD271-BV421, CD140b-BV510, and CD105-BV650 (BioLegend) and CD90-PE-Cy7 (BD Biosciences). Panel 2 included CD140a-PE-Cy7 (BD Biosciences) and CD166-APC and CD45-BV510 (BioLegend). Matching isotype control IgGs with the same fluorochrome conjugates was used for background gating. To compensate for spectral overlap, unstained cells and individual tubes with each fluorochrome were incubated with Dynabeads, VersaComp Antibody Capture beads (Beckman Coulter, Inc., Brea, CA), to distinguish between positive and negative fluorochrome signals. Data generated from the flow cytometer (Novocyte; ACEA Biosciences) were analyzed using FlowJo software (version 10; FlowJo, LLC) to determine the positive signal for each surface marker relative to the nonspecific signal control (isotype background).
Statistical Analysis
Paired 1-tailed t tests were used to assess differences in cell viability and proliferation rates, as well as for comparisons between surface molecule expression of cells isolated from ablation treatments and cell isolation procedures (collagenase vs cell migration). Power analysis estimated that a sample size of N = 6 would detect significant differences in averages of 30% or greater between groups with a power greater than 80% at P values of .05 (R statistical package, R Foundation for Statistical Computing, Vienna, Austria).
Results
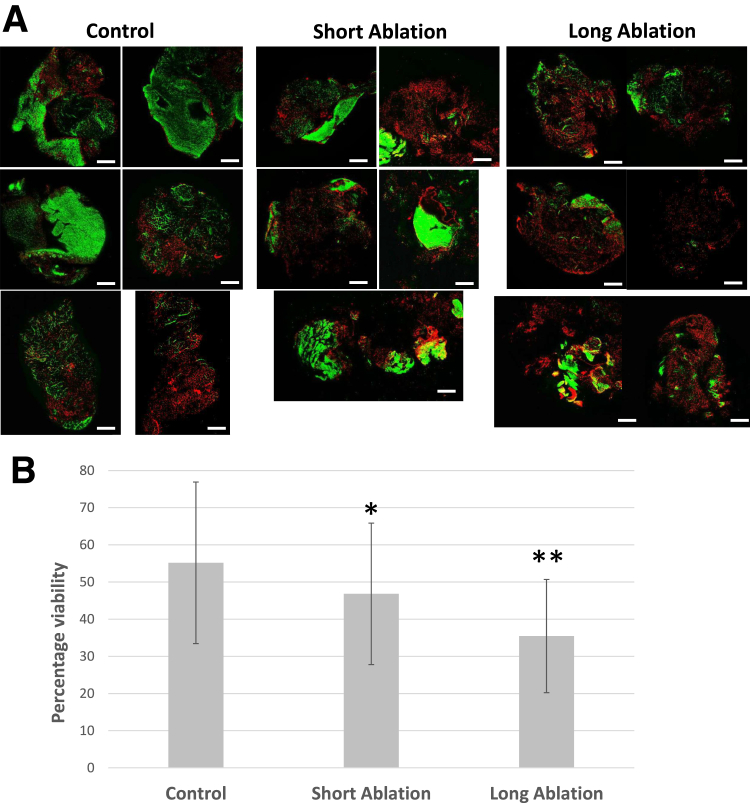
Tissue obtained showed some patient-to-patient variations in viability (Fig 2). The mean percentage of live cells in the control group with no radiofrequency ablation was 55.2% ± 27.2% (range, 26%-90% live), the short-ablation group was 46.8% ± 23.8% (range, 9.6%-69.6%, P = .045), and the long-ablation group was 35.5% ± 19% (range, 11%-54%, P = .03), indicating a dose-dependent decrease in viability with increasing time of ablation.
Fig 2.
Tissue viability following ablation. (A) Representative confocal live/dead viability images of bursa tissue (N = 6 patients) following no ablation (control), short ablation, or long ablation (green signal = live; red signal = dead). Scale bar: 200 mm. (B) Image-based assessment of percentage live cells showing a trend of reduced viability with increased ablation time. A range of viability (mean ± 95% confidence interval) was observed in all tissues collected. Control tissue, 55.2% ± 27.2% (range, 26%-90% live); short ablation: 46.8% ± 23.8% (range, 9.6%-69.6%; ∗P = .045); and long ablation, 35.5% ± 19% (range, 11%-54%; ∗∗P = .03).
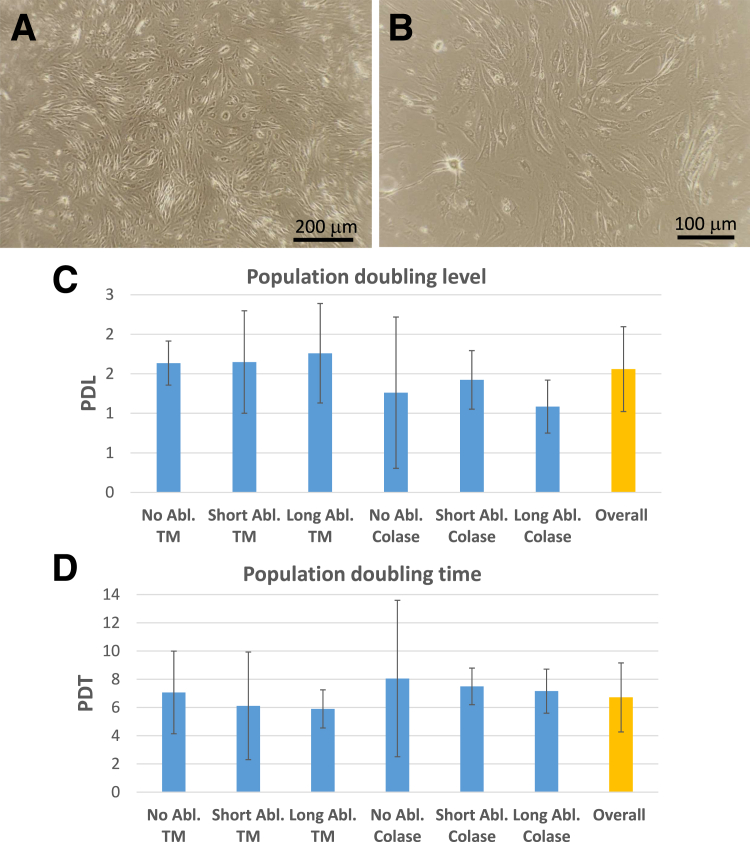
Viable cells isolated from all tissues had similar growth and surface molecule characteristics. Cell morphology following isolation appeared similar between patients and the different isolation approaches. Figure 3 presents the typical cell morphology following tissue migration. The growth kinetics were analyzed from cells isolated from collagenase digested and the cells that emerged from the tissue migration from all ablation treatments. Overall, the PDL was 1.6 ± 0.5 days, and the PDT was 6.7 ± 2.4 days (Fig 3 C and D). No differences in PDL or PDT was detected between ablation times or method of cell isolation.
Fig 3.
Cell morphology and cell proliferation rates. (A, B) Representative morphology of cells isolated from bursa tissues using the migration approach (49 female, passage 2). (C) Population doubling levels (PDLs) for cells derived from tissues following each ablation treatment and isolation method in days. (D) Population doubling time (PDT) for cells derived from tissues following each ablation treatment and isolation method in days. (No significant differences were found for either PDL or PDT between all treatments.) (TM, Tissue migration.)
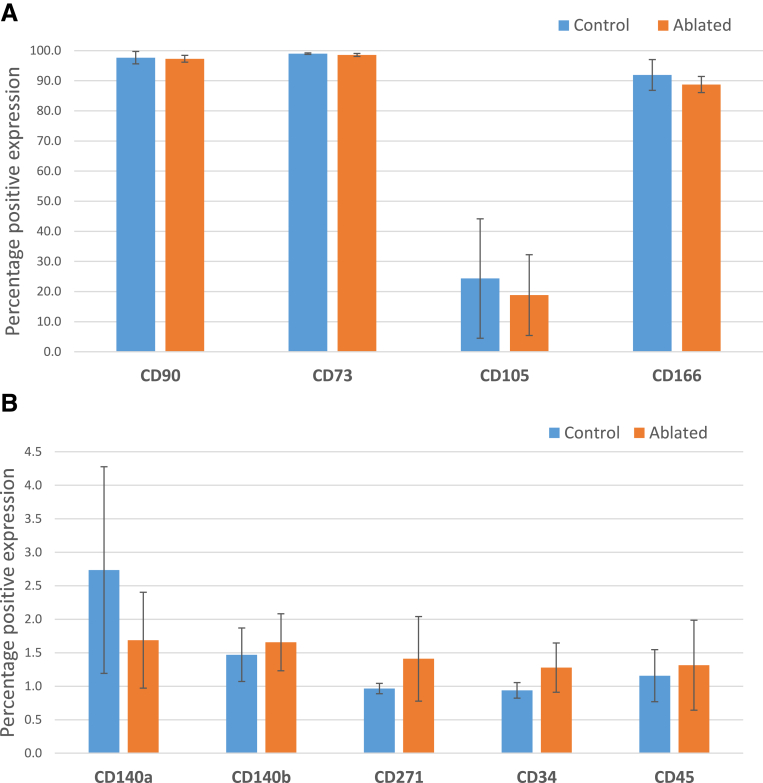
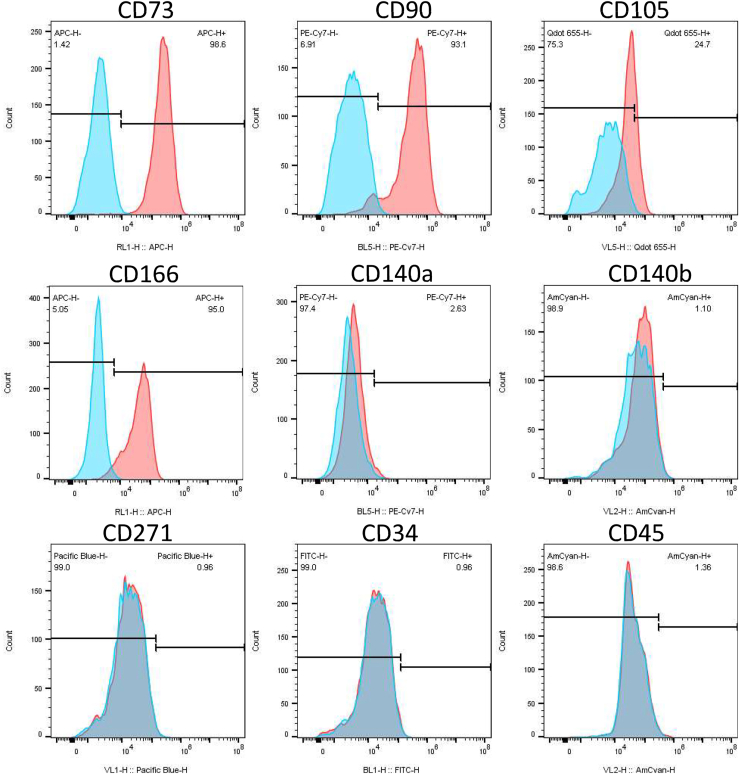
The surface marker signature of the isolated cells was not significantly altered due to treatment or cell isolation procedure (Fig 4). The surface marker profile indicates an MSC phenotype displaying the absence of hematopoietic stem cell markers (CD34 and CD45); high levels of CD73, CD90, and CD166 (>90%); and moderate levels of CD105 (∼20%) but with very low to negative levels of CD271. The very low to negative expression of platelet-derived growth factor receptors α and β (CD140a and CD140b, respectively) were expressed in very few cells, indicating the absence of a pericyte population. Expression of CD90, CD73, CD105, and CD166 was not affected by radiofrequency ablation. Representative flow cytometry histograms showing specific surface marker profiles are presented in Figure 5.
Fig 4.
Overview of surface molecules (percentage positive) present on isolated bursa cells. No significant difference in surface molecule expression was noted due to ablation treatment. (A) Typical mesenchymal stem cell (MSC) surface molecules expressed in all cells examined. (B) Additional surface molecules examined show background to negative levels of expression for platelet-derived growth factor receptors α and β (CD140a and CD140b) indicating the lack of pericytes from this cell population. Low-affinity nerve growth factor receptor (CD271), an MSC marker, was observed as negative, along with the classic hematopoietic stem cell markers CD34 and CD45, which are negative for MSC. (No significant differences in surface molecule percentage positive signal were found between control and ablated tissues.)
Fig 5.
Representative flow cytometry histograms of cells isolated from subacromial tissue. Red = positive signal for target surface molecule; blue = isotype control for nonspecific binding.
Discussion
We found a significant decrease in cell viability of bursal tissue with longer durations of ablation. The ablation-treated harvested tissues did show areas of damage, but the residual viable cells proliferated in culture and appeared to be MSC-like. Our study showed that stem cell surface marker characteristics were present on the bursal cells collected after the use of the ablation. These MSC-like cells were present in all samples with no detectable difference in PDL and PDT.
We noted a high patient-to-patient variability in cell viability in the subacromial bursal tissue harvested at the time of RCR surgery. At the time of surgery, patients visually had varying amounts of bursal tissue in the subacromial space. In an effort to harvest enough tissue for evaluation, more medial tissue was likely harvested. The subacromial space has more stem cell colony-forming units in the bursa over the tendon in comparison to cells derived from medially tissue located over the muscle.17 This could have been a factor in the variability of cell viability in harvest tissues. Our harvested bursal cell populations displayed similar characteristics to those previously reported by Levy, Mazzocca29 and others.17,18,20,29, 30, 31 Subacromial bursal cells proliferated and displayed an MSC-like profile. The presence of cell surface markers CD73, CD90, CD166, and CD105 on the bursal cells and the absence of hematopoietic stem cell markers CD31 and CD45 are consistent with their results. Similarly, the surface marker signature was not significantly altered due to a cell isolation procedure. This study provides additional support for the viability of MSCs obtained from subacromial bursa tissue during arthroscopic RCR surgery. Our study demonstrates that subacromial bursal tissue collected under conditions during arthroscopic RCR surgery using ablators and shavers contains cells resembling the phenotype of MSCs. These results suggest that harvesting bursal tissue during the procedure is a viable option for biological augmentation. To maximize cell viability, we recommend harvesting bursal tissue prior to ablation. This study focused primarily on assessing the viability of harvested cells and their surface markers but did not investigate the functional properties of these cells or evaluate their impact on the long-term healing potential of rotator cuff tendon repair.
In conclusion, our study provides insights into the overall cell viability, cell proliferation, and the expression of MSC surface markers of MSCs harvested from bursa tissue during RCR surgery. Despite areas of ablation-induced damage, many harvested cells remained viable and were able to proliferate in culture. The tissue collected still contained MSCs despite fewer living cells present with longer ablation times.
Limitations
This study is not without limitations. One limitation is the substantial variability observed in the viability of harvested tissue samples, which may be attributable to variability in the quantity and quality of bursa present in each patient’s samples from the subacromial space. The location of harvested tissue (e.g., bursal tissue over the tendon vs over the muscle) could also introduce potential confounding factors. In addition, we did not standardize the amount of tissue collected by weight or volume. Despite this variability, we found a statistically significant reduction in cell viability with the duration of tissue ablation. Our sample size may limit the generalizations of the findings to all RCRs.
Conclusions
Radiofrequency ablation significantly reduced the overall tissue viability but had no significant effect on cell proliferation or expression of surface markers on isolated subacromial bursal cells harvested arthroscopically.
Disclosures
The authors report the following potential conflicts of interest or sources of funding: The work was supported by a Scripps Clinic Medical Group Research Award and by Donald and Darlene Shiley. A.H.D. has received personal fees from Arthrex. All other authors (S.P.G, V.B., B.N., D.D.D., L.A.A.) declare they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Acknowledgments
Technical assistance was provided by Nicholas E. Glembotski, B.S. (tissue viability imaging and flow cytometry), and April Damon, B.S. (cell culturing and population doubling analysis). We thank Emily Martin, M.Bt., for her assistance in manuscript preparation, formatting, and copy editing.
Supplementary Data
References
- 1.Fehringer E.V., Sun J., VanOeveren L.S., Keller B.K., Matsen F.A., III Full-thickness rotator cuff tear prevalence and correlation with function and co-morbidities in patients sixty-five years and older. J Shoulder Elbow Surg. 2008;17:881–885. doi: 10.1016/j.jse.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto A., Takagishi K., Osawa T., et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. doi: 10.1016/j.jse.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Duong J.K.H., Lam P.H., Murrell G.A.C. Anteroposterior tear size, age, hospital, and case number are important predictors of repair integrity: An analysis of 1962 consecutive arthroscopic single-row rotator cuff repairs. J Shoulder Elbow Surg. 2021;30:1907–1914. doi: 10.1016/j.jse.2020.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Hwang A., Zhang L., Ramirez G., Maloney M., Voloshin I., Thirukumaran C. Black race, Hispanic ethnicity, and Medicaid insurance are associated with lower rates of rotator cuff repair in New York State. Arthroscopy. 2022;38:3001–3010.e3002. doi: 10.1016/j.arthro.2022.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Tokish J.M., Hawkins R.J. Current concepts in the evolution of arthroscopic rotator cuff repair. JSES Rev Rep Tech. 2021;1:75–83. doi: 10.1016/j.xrrt.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cha S.W., Lee C.K., Sugaya H., Kim T., Lee S.C. Retraction pattern of delaminated rotator cuff tears: Dual-layer rotator cuff repair. J Orthop Surg Res. 2016;11:75. doi: 10.1186/s13018-016-0407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boileau P., Brassart N., Watkinson D.J., Carles M., Hatzidakis A.M., Krishnan S.G. Arthroscopic repair of full-thickness tears of the supraspinatus: Does the tendon really heal? J Bone Joint Surg Am. 2005;87:1229–1240. doi: 10.2106/JBJS.D.02035. [DOI] [PubMed] [Google Scholar]
- 8.Sugaya H., Maeda K., Matsuki K., Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair. A prospective outcome study. J Bone Joint Surg Am. 2007;89:953–960. doi: 10.2106/JBJS.F.00512. [DOI] [PubMed] [Google Scholar]
- 9.Galatz L.M., Ball C.M., Teefey S.A., Middleton W.D., Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004;86:219–224. doi: 10.2106/00004623-200402000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Khazzam M., Sager B., Box H.N., Wallace S.B. The effect of age on risk of retear after rotator cuff repair: A systematic review and meta-analysis. JSES Int. 2020;4:625–631. doi: 10.1016/j.jseint.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenspoon J.A., Petri M., Warth R.J., Millett P.J. Massive rotator cuff tears: Pathomechanics, current treatment options, and clinical outcomes. J Shoulder Elbow Surg. 2015;24:1493–1505. doi: 10.1016/j.jse.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 12.van der Meijden O.A., Wijdicks C.A., Gaskill T.R., Jansson K.S., Millett P.J. Biomechanical analysis of two-tendon posterosuperior rotator cuff tear repairs: Extended linked repairs and augmented repairs. Arthroscopy. 2013;29:37–45. doi: 10.1016/j.arthro.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Oh J.H., Kim D.H., Jeong H.J., Park J.H., Rhee S.M. Effect of recombinant human parathyroid hormone on rotator cuff healing after arthroscopic repair. Arthroscopy. 2019;35:1064–1071. doi: 10.1016/j.arthro.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Goldenberg B.T., Lacheta L., Dekker T.J., Spratt J.D., Nolte P.C., Millett P.J. Biologics to improve healing in large and massive rotator cuff tears: A critical review. Orthop Res Rev. 2020;12:151–160. doi: 10.2147/ORR.S260657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernigou P., Flouzat Lachaniette C.H., Delambre J., et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: A case-controlled study. Int Orthop. 2014;38:1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 16.Song N., Armstrong A.D., Li F., Ouyang H., Niyibizi C. Multipotent mesenchymal stem cells from human subacromial bursa: Potential for cell based tendon tissue engineering. Tissue Eng Part A. 2014;20:239–249. doi: 10.1089/ten.tea.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morikawa D., Johnson J.D., Kia C., et al. Examining the potency of subacromial bursal cells as a potential augmentation for rotator cuff healing: An in vitro study. Arthroscopy. 2019;35:2978–2988. doi: 10.1016/j.arthro.2019.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Muench L.N., Baldino J.B., Berthold D.P., et al. Subacromial bursa-derived cells demonstrate high proliferation potential regardless of patient demographics and rotator cuff tear characteristics. Arthroscopy. 2020;36:2794–2802. doi: 10.1016/j.arthro.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Lanham N.S., Swindell H.W., Levine W.N. The subacromial bursa: Current concepts review. JBJS Rev. 2021;9:e21.00110. doi: 10.2106/JBJS.RVW.21.00110. [DOI] [PubMed] [Google Scholar]
- 20.Otto A., LeVasseur M.R., Baldino J.B., et al. Arthroscopic rotator cuff repair with a fibrin scaffold containing growth factors and autologous progenitor cells derived from humeral cBMA improves clinical outcomes in high risk patients. Arthrosc Sports Med Rehabil. 2022;4:e1629–e1637. doi: 10.1016/j.asmr.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pancholi N., Gregory J.M. Biologic augmentation of arthroscopic rotator cuff repair using minced autologous subacromial bursa. Arthrosc Tech. 2020;9:e1519–e1524. doi: 10.1016/j.eats.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Utsunomiya H., Uchida S., Sekiya I., Sakai A., Moridera K., Nakamura T. Isolation and characterization of human mesenchymal stem cells derived from shoulder tissues involved in rotator cuff tears. Am J Sports Med. 2013;41:657–668. doi: 10.1177/0363546512473269. [DOI] [PubMed] [Google Scholar]
- 23.Barker S.L., Johnstone A.J., Kumar K. In vivo temperature measurement in the subacromial bursa during arthroscopic subacromial decompression. J Shoulder Elbow Surg. 2012;21:804–807. doi: 10.1016/j.jse.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Faruque R., Matthews B., Bahho Z., et al. Comparison between 2 types of radiofrequency ablation systems in arthroscopic rotator cuff repair: A randomized controlled trial. Orthop J Sports Med. 2019;7 doi: 10.1177/2325967119835224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKeon B., Baltz M.S., Curtis A., Scheller A. Fluid temperatures during radiofrequency use in shoulder arthroscopy: A cadaveric study. J Shoulder Elbow Surg. 2007;16:107–111. doi: 10.1016/j.jse.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Grogan S.P., Aklin B., Frenz M., Brunner T., Schaffner T., Mainil-Varlet P. In vitro model for the study of necrosis and apoptosis in native cartilage. J Pathol. 2002;198:5–13. doi: 10.1002/path.1169. [DOI] [PubMed] [Google Scholar]
- 27.Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 28.Traktuev D.O., Merfeld-Clauss S., Li J., et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 29.Levy B.J., McCarthy M.B., Lebaschi A., Sanders M.M., Cote M.P., Mazzocca A.D. Subacromial bursal tissue and surrounding matrix of patients undergoing rotator cuff repair contains progenitor cells. Arthroscopy. 2022;38:1115–1123. doi: 10.1016/j.arthro.2021.10.029. [DOI] [PubMed] [Google Scholar]
- 30.Morikawa D., Muench L.N., Baldino J.B., et al. Comparison of preparation techniques for isolating subacromial bursa-derived cells as a potential augment for rotator cuff repair. Arthroscopy. 2020;36:80–85. doi: 10.1016/j.arthro.2019.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Muench L.N., Kia C., Jerliu A., et al. Clinical outcomes following biologically enhanced patch augmentation repair as a salvage procedure for revision massive rotator cuff tears. Arthroscopy. 2020;36:1542–1551. doi: 10.1016/j.arthro.2020.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.