Abstract
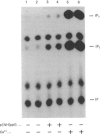
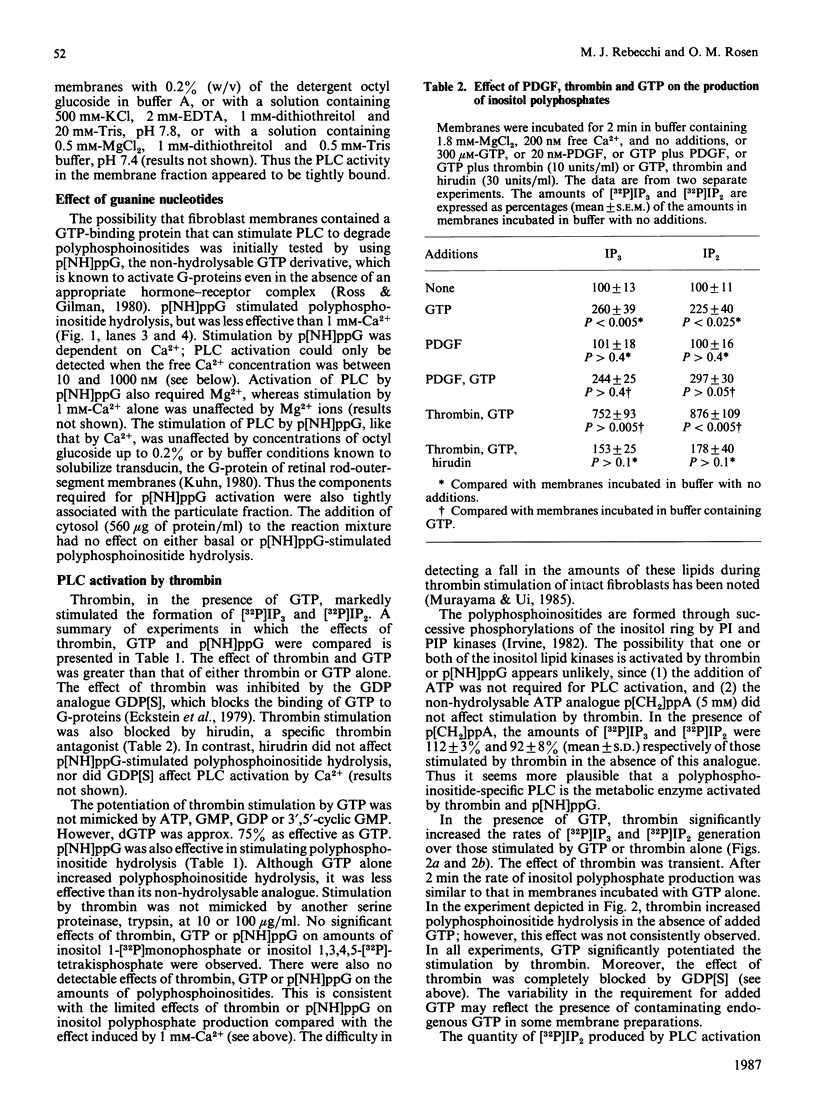
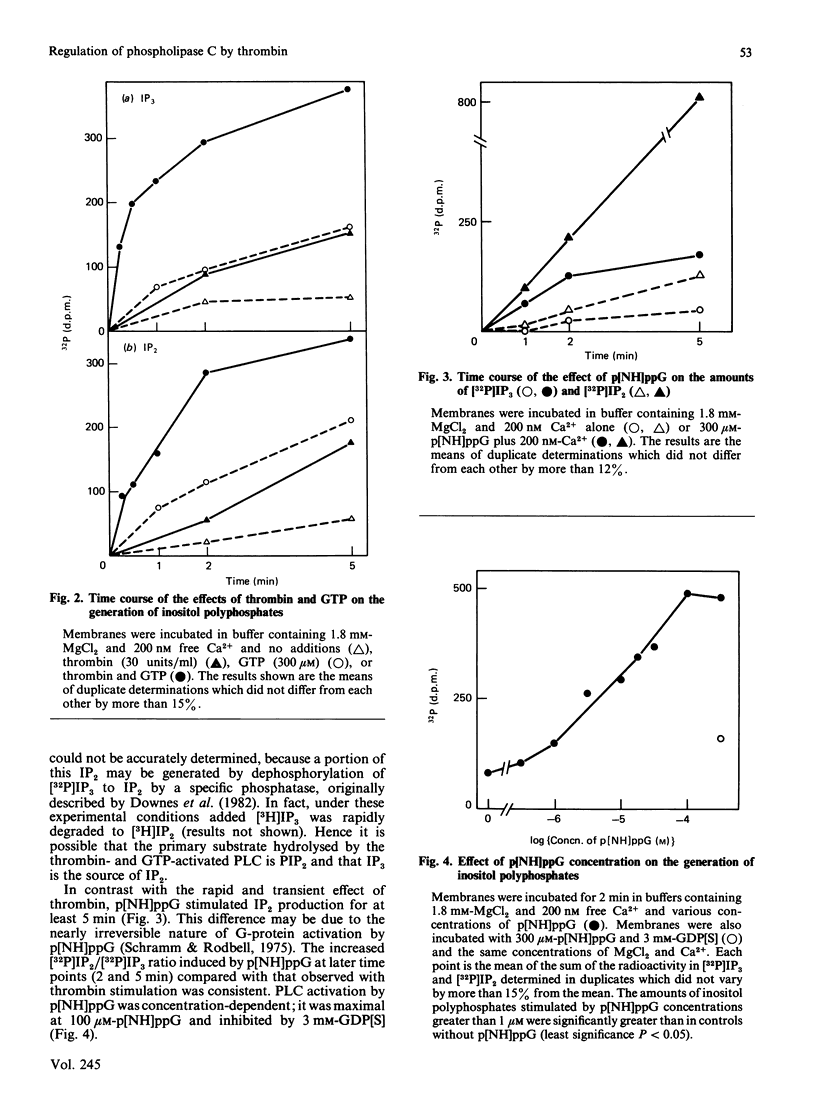
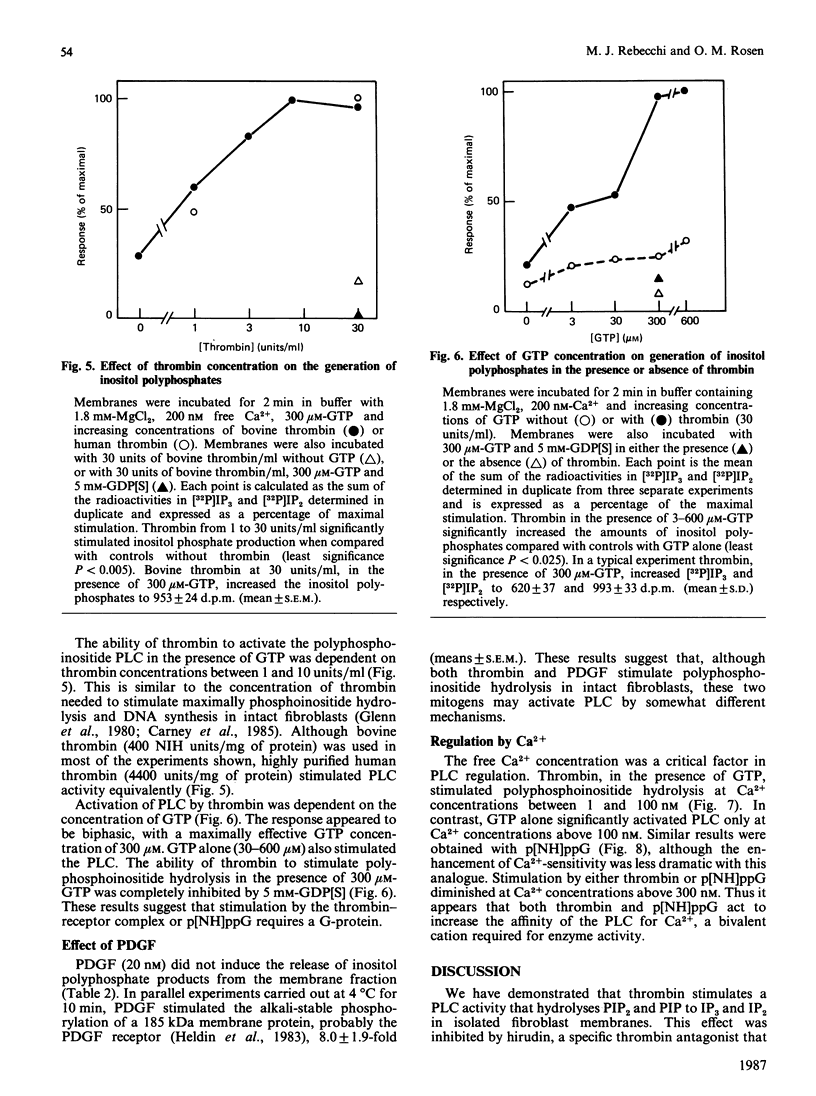
One of the earliest actions of thrombin in fibroblasts is stimulation of a phospholipase C (PLC) that hydrolyses phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. In membranes prepared from WI-38 human lung fibroblasts, thrombin activated an inositol-lipid-specific PLC that hydrolysed [32P]PIP2 and [32P]phosphatidylinositol 4-monophosphate (PIP) to [32P]IP3 and [32P]inositol 1,4-bisphosphate (IP2) respectively. Degradation of [32P]phosphatidylinositol was not detected. PLC activation by thrombin was dependent on GTP, and was completely inhibited by a 15-fold excess of the non-hydrolysable GDP analogue guanosine 5'-[beta-thio]diphosphate (GDP[S]). Neither ATP nor cytosol was required. Guanosine 5'-[beta gamma-imido]triphosphate (p[NH]ppG) also stimulated polyphosphoinositide hydrolysis, and this activation was inhibited by GDP[S]. Stimulation of PLC by either thrombin or p[NH]ppG was dependent on Ca2+. Activation by thrombin required Ca2+ concentrations between 1 and 100 nM, whereas stimulation of PLC activity by GTP required concentrations of Ca2+ above 100 nM. Thus the mitogen thrombin increased the sensitivity of PLC to concentrations of free Ca2+ similar to those found in quiescent fibroblasts. Under identical conditions, another mitogen, platelet-derived growth factor, did not stimulate polyphosphoinositide hydrolysis. It is concluded that an early post-receptor effect of thrombin is the activation of a Ca2+- and GTP-dependent membrane-associated PLC that specifically cleaves PIP2 and PIP. This result suggests that the cell-surface receptor for thrombin is coupled to a polyphosphoinositide-specific PLC by a GTP-binding protein that regulates PLC activity by increasing its sensitivity to Ca2+.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Akhtar R. A., Abdel-Latif A. A. Studies on the properties of triphosphoinositide phosphomonoesterase and phosphodiesterase of rabbit iris smooth muscle. Biochim Biophys Acta. 1978 Nov 10;527(1):159–170. doi: 10.1016/0005-2744(78)90265-6. [DOI] [PubMed] [Google Scholar]
- Baldassare J. J., Fisher G. J. GTP and cytosol stimulate phosphoinositide hydrolysis in isolated platelet membranes. Biochem Biophys Res Commun. 1986 Jun 13;137(2):801–805. doi: 10.1016/0006-291x(86)91150-2. [DOI] [PubMed] [Google Scholar]
- Banno Y., Nakashima S., Nozawa Y. Partial purification of phosphoinositide phospholipase C from human platelet cytosol; characterization of its three forms. Biochem Biophys Res Commun. 1986 Apr 29;136(2):713–721. doi: 10.1016/0006-291x(86)90498-5. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Heslop J. P., Irvine R. F., Brown K. D. Inositol trisphosphate formation and calcium mobilization in Swiss 3T3 cells in response to platelet-derived growth factor. Biochem J. 1984 Aug 15;222(1):195–201. doi: 10.1042/bj2220195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. H., Cunningham D. D. Role of specific cell surface receptors in thrombin-stimulated cell division. Cell. 1978 Dec;15(4):1341–1349. doi: 10.1016/0092-8674(78)90059-4. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Scott D. L., Gordon E. A., LaBelle E. F. Phosphoinositides in mitogenesis: neomycin inhibits thrombin-stimulated phosphoinositide turnover and initiation of cell proliferation. Cell. 1985 Sep;42(2):479–488. doi: 10.1016/0092-8674(85)90105-9. [DOI] [PubMed] [Google Scholar]
- Carney D. H., Stiernberg J., Fenton J. W., 2nd Initiation of proliferative events by human alpha-thrombin requires both receptor binding and enzymic activity. J Cell Biochem. 1984;26(3):181–195. doi: 10.1002/jcb.240260306. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Buchanan J. M. Mitogenic activity of blood components. I. Thrombin and prothrombin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):131–135. doi: 10.1073/pnas.72.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S. H., Hoban C. J., Owen A. J., Geyer R. P. Platelet-derived growth factor stimulates rapid polyphosphoinositide breakdown in fetal human fibroblasts. J Cell Physiol. 1985 Sep;124(3):391–396. doi: 10.1002/jcp.1041240306. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Michell R. H. The control by Ca2+ of the polyphosphoinositide phosphodiesterase and the Ca2+-pump ATPase in human erythrocytes. Biochem J. 1982 Jan 15;202(1):53–58. doi: 10.1042/bj2020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Glenn K. C., Carney D. H., Fenton J. W., 2nd, Cunningham D. D. Thrombin active site regions required for fibroblast receptor binding and initiation of cell division. J Biol Chem. 1980 Jul 25;255(14):6609–6616. [PubMed] [Google Scholar]
- Guillon G., Balestre M. N., Mouillac B., Devilliers G. Activation of membrane phospholipase C by vasopressin. A requirement for guanyl nucleotides. FEBS Lett. 1986 Feb 3;196(1):155–159. doi: 10.1016/0014-5793(86)80232-0. [DOI] [PubMed] [Google Scholar]
- Habenicht A. J., Glomset J. A., King W. C., Nist C., Mitchell C. D., Ross R. Early changes in phosphatidylinositol and arachidonic acid metabolism in quiescent swiss 3T3 cells stimulated to divide by platelet-derived growth factor. J Biol Chem. 1981 Dec 10;256(23):12329–12335. [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Potentiation by thrombin of the secretion of serotonin from permeabilized platelets equilibrated with Ca2+ buffers. Relationship to protein phosphorylation and diacylglycerol formation. Biochem J. 1984 Sep 1;222(2):351–361. doi: 10.1042/bj2220351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Receptor-induced diacylglycerol formation in permeabilized platelets; possible role for a GTP-binding protein. J Recept Res. 1984;4(1-6):605–629. doi: 10.3109/10799898409042576. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Ek B., Rönnstrand L. Characterization of the receptor for platelet-derived growth factor on human fibroblasts. Demonstration of an intimate relationship with a 185,000-Dalton substrate for the platelet-derived growth factor-stimulated kinase. J Biol Chem. 1983 Aug 25;258(16):10054–10061. [PubMed] [Google Scholar]
- Houslay M. D., Bojanic D., Gawler D., O'Hagan S., Wilson A. Thrombin, unlike vasopressin, appears to stimulate two distinct guanine nucleotide regulatory proteins in human platelets. Biochem J. 1986 Aug 15;238(1):109–113. doi: 10.1042/bj2380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Irvine R. F. The enzymology of stimulated inositol lipid turnover. Cell Calcium. 1982 Oct;3(4-5):295–309. doi: 10.1016/0143-4160(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Keough K. M., Thompson W. Soluble and particulate forms of phosphoinositide phosphodiesterase in ox brain. Biochim Biophys Acta. 1972 Jul 7;270(3):324–336. doi: 10.1016/0005-2760(72)90197-x. [DOI] [PubMed] [Google Scholar]
- Kikuchi A., Kozawa O., Kaibuchi K., Katada T., Ui M., Takai Y. Direct evidence for involvement of a guanine nucleotide-binding protein in chemotactic peptide-stimulated formation of inositol bisphosphate and trisphosphate in differentiated human leukemic (HL-60) cells. Reconstitution with Gi or Go of the plasma membranes ADP-ribosylated by pertussis toxin. J Biol Chem. 1986 Sep 5;261(25):11558–11562. [PubMed] [Google Scholar]
- Knight D. E., Hallam T. J., Scrutton M. C. Agonist selectivity and second messenger concentration in Ca2+-mediated secretion. Nature. 1982 Mar 18;296(5854):256–257. doi: 10.1038/296256a0. [DOI] [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Gaining access to the cytosol: the technique and some applications of electropermeabilization. Biochem J. 1986 Mar 15;234(3):497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litosch I., Fain J. N. 5-Methyltryptamine stimulates phospholipase C-mediated breakdown of exogenous phosphoinositides by blowfly salivary gland membranes. J Biol Chem. 1985 Dec 25;260(30):16052–16055. [PubMed] [Google Scholar]
- Litosch I., Wallis C., Fain J. N. 5-Hydroxytryptamine stimulates inositol phosphate production in a cell-free system from blowfly salivary glands. Evidence for a role of GTP in coupling receptor activation to phosphoinositide breakdown. J Biol Chem. 1985 May 10;260(9):5464–5471. [PubMed] [Google Scholar]
- Low M. G., Weglicki W. B. Resolution of myocardial phospholipase C into several forms with distinct properties. Biochem J. 1983 Nov 1;215(2):325–334. doi: 10.1042/bj2150325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. F., Lucas D. O., Bajjalieh S. M., Kowalchyk J. A. Thyrotropin-releasing hormone activates a Ca2+-dependent polyphosphoinositide phosphodiesterase in permeable GH3 cells. GTP gamma S potentiation by a cholera and pertussis toxin-insensitive mechanism. J Biol Chem. 1986 Feb 25;261(6):2918–2927. [PubMed] [Google Scholar]
- Moolenaar W. H., Tertoolen L. G., de Laat S. W. Growth factors immediately raise cytoplasmic free Ca2+ in human fibroblasts. J Biol Chem. 1984 Jul 10;259(13):8066–8069. [PubMed] [Google Scholar]
- Murayama T., Ui M. Receptor-mediated inhibition of adenylate cyclase and stimulation of arachidonic acid release in 3T3 fibroblasts. Selective susceptibility to islet-activating protein, pertussis toxin. J Biol Chem. 1985 Jun 25;260(12):7226–7233. [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Pertussis toxin inhibits thrombin-induced activation of phosphoinositide hydrolysis and Na+/H+ exchange in hamster fibroblasts. EMBO J. 1986 Jan;5(1):55–60. doi: 10.1002/j.1460-2075.1986.tb04177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Rodriquez R., Franchi A., Pouysségur J. Growth factor requirements of Chinese hamster lung fibroblasts in serum free media: high mitogenic action of thrombin. Cell Biol Int Rep. 1981 Apr;5(4):347–357. doi: 10.1016/0309-1651(81)90004-7. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Schramm M., Rodbell M. A persistent active state of the adenylate cyclase system produced by the combined actions of isoproterenol and guanylyl imidodiphosphate in frog erythrocyte membranes. J Biol Chem. 1975 Mar 25;250(6):2232–2237. [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Straub R. E., Gershengorn M. C. Thyrotropin-releasing hormone and GTP activate inositol trisphosphate formation in membranes isolated from rat pituitary cells. J Biol Chem. 1986 Feb 25;261(6):2712–2717. [PubMed] [Google Scholar]
- Takamatsu J., Horne M. K., 3rd, Gralnick H. R. Identification of the thrombin receptor on human platelets by chemical crosslinking. J Clin Invest. 1986 Feb;77(2):362–368. doi: 10.1172/JCI112313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefsen D. M., Majerus P. W. Evidence for a single class of thrombin-binding sites of human platelets. Biochemistry. 1976 May 18;15(10):2144–2149. doi: 10.1021/bi00655a018. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Van Obberghen-Schilling E., Chambard J. C., Paris S., L'Allemain G., Pouysségur J. alpha-Thrombin-induced early mitogenic signalling events and G0 to S-phase transition of fibroblasts require continual external stimulation. EMBO J. 1985 Nov;4(11):2927–2932. doi: 10.1002/j.1460-2075.1985.tb04025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. P., McConnell R. T., Lapetina E. G. The rapid formation of inositol phosphates in human platelets by thrombin is inhibited by prostacyclin. J Biol Chem. 1984 Nov 10;259(21):13199–13203. [PubMed] [Google Scholar]
- Wilson D. B., Bross T. E., Hofmann S. L., Majerus P. W. Hydrolysis of polyphosphoinositides by purified sheep seminal vesicle phospholipase C enzymes. J Biol Chem. 1984 Oct 10;259(19):11718–11724. [PubMed] [Google Scholar]