Abstract
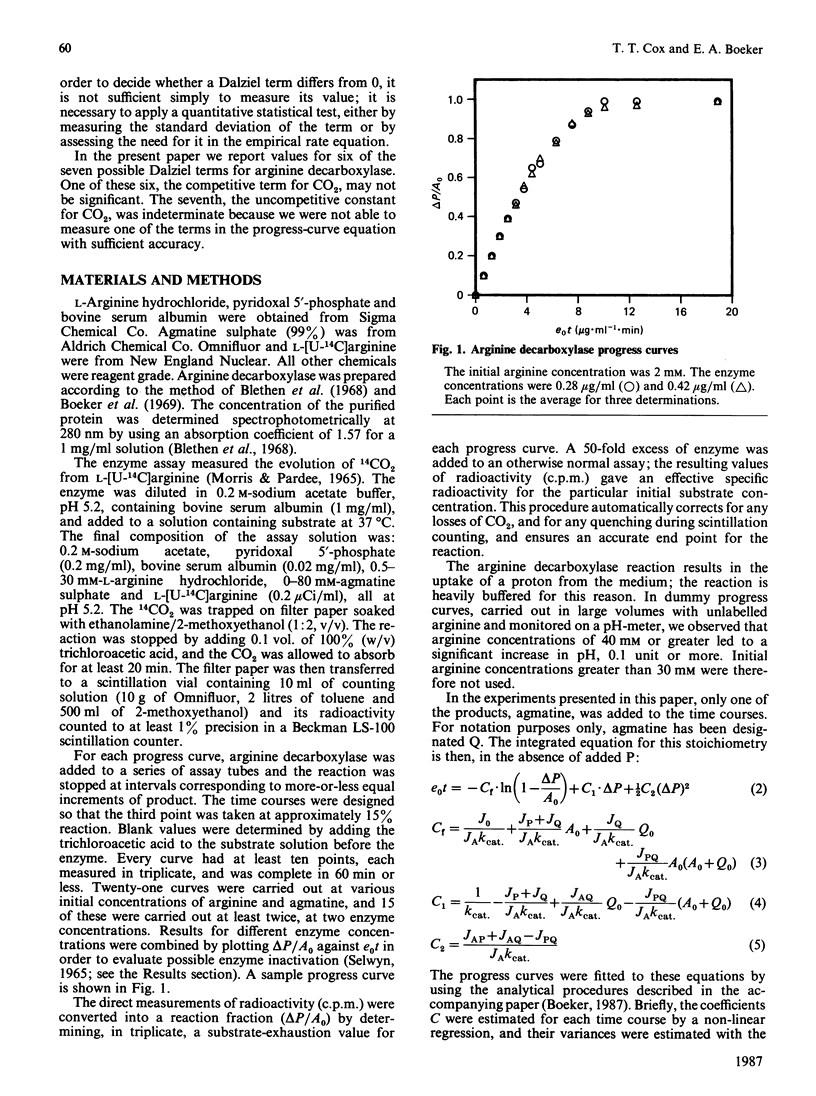
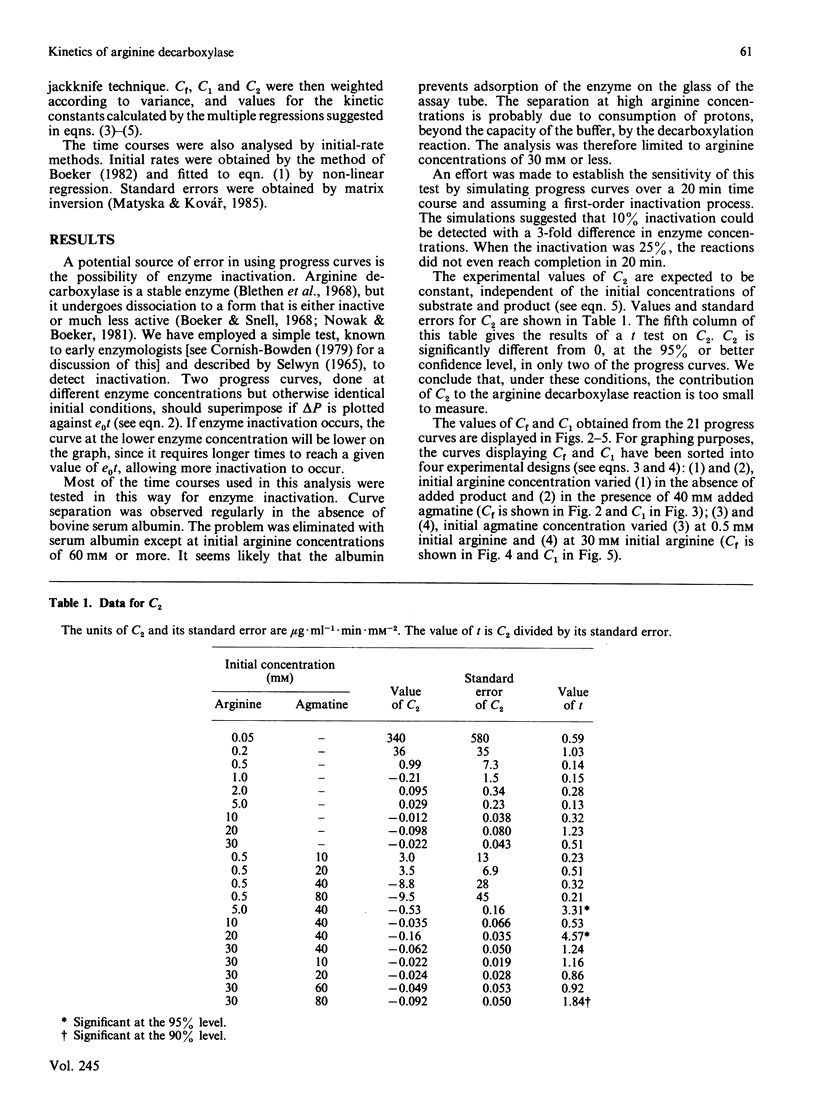
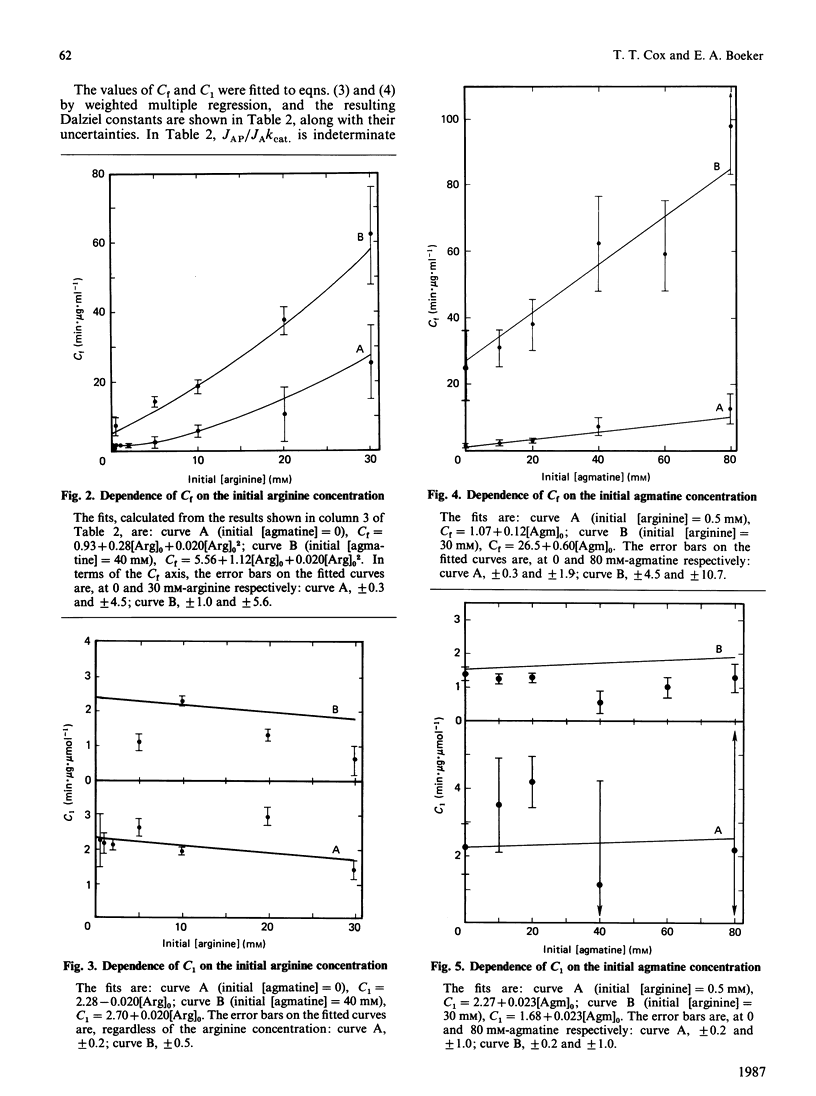
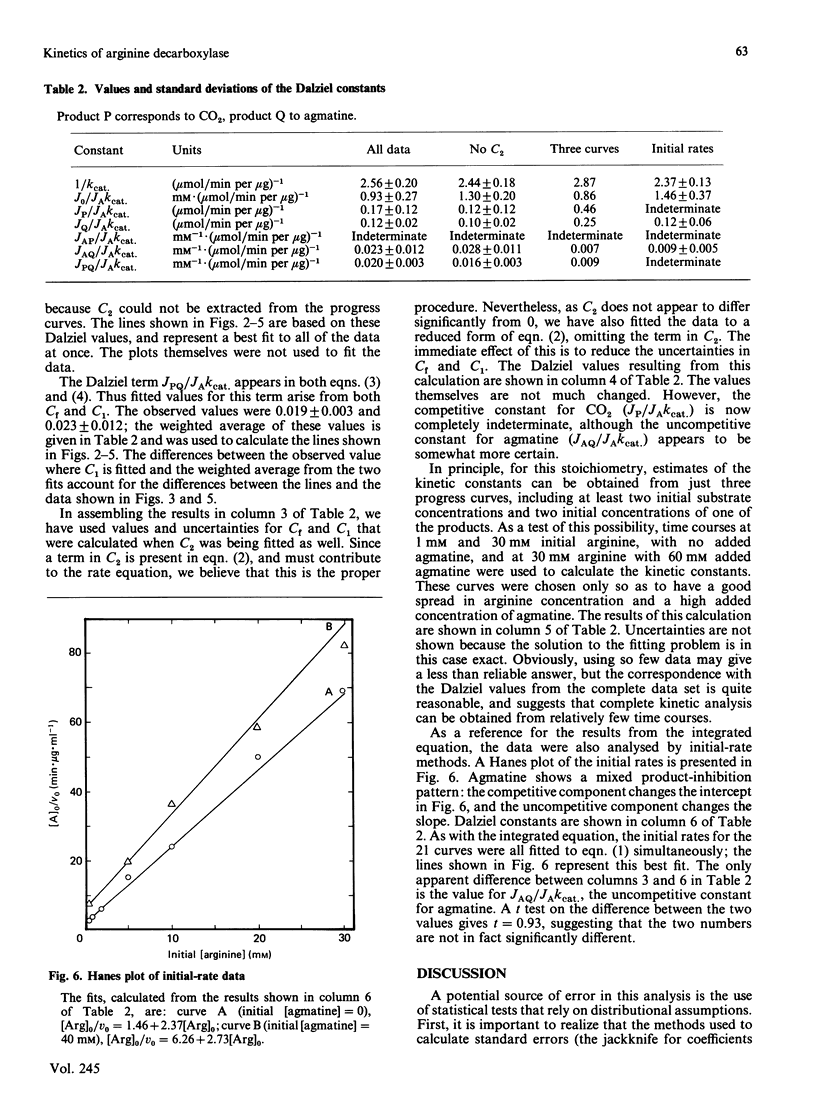
We have used an integrated rate equation to analyse the reaction catalysed by the inducible arginine decarboxylase from Escherichia coli B. The stoichiometry Arginine----agmatine + CO2 is the simplest of the multiple-substrate/multiple-product cases. Twenty-one time courses were carried out at various initial concentrations of arginine and agmatine, and were then fitted to the integrated equation by using appropriate analytical procedures. Values were obtained for six of the seven possible kinetic constants, corresponding to kcat, KArg, the terms for competitive inhibition by agmatine, by CO2 and by agmatine and CO2 together, and the term for uncompetitive inhibition by agmatine. The uncompetitive constant for CO2 was indeterminate. Our results indicate that it is both practical and experimentally economical to obtain kinetic constants from full time courses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blethen S. L., Boeker E. A., Snell E. E. Argenine decarboxylase from Escherichia coli. I. Purification and specificity for substrates and coenzyme. J Biol Chem. 1968 Apr 25;243(8):1671–1677. [PubMed] [Google Scholar]
- Boeker E. A. Analytical methods for fitting integrated rate equations. A discontinuous assay. Biochem J. 1987 Jul 1;245(1):67–74. doi: 10.1042/bj2450067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeker E. A., Fischer E. H., Snell E. E. Arginine decarboxylase from Escherichia coli. 3. Subunit structure. J Biol Chem. 1969 Oct 10;244(19):5239–5245. [PubMed] [Google Scholar]
- Boeker E. A. Initial rates. A new plot. Biochem J. 1982 Apr 1;203(1):117–123. doi: 10.1042/bj2030117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeker E. A. Integrated rate equations for enzyme-catalysed first-order and second-order reactions. Biochem J. 1984 Oct 1;223(1):15–22. doi: 10.1042/bj2230015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeker E. A. Integrated rate equations for irreversible enzyme-catalysed first-order and second-order reactions. Biochem J. 1985 Feb 15;226(1):29–35. doi: 10.1042/bj2260029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeker E. A., Snell E. E. Arginine decarboxylase from Escherichia coli. II. Dissociation and reassociation of subunits. J Biol Chem. 1968 Apr 25;243(8):1678–1684. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. III. Prediction of initial velocity and inhibition patterns by inspection. Biochim Biophys Acta. 1963 Feb 12;67:188–196. doi: 10.1016/0006-3002(63)91816-x. [DOI] [PubMed] [Google Scholar]
- Gale E. F. The production of amines by bacteria: The decarboxylation of amino-acids by strains of Bacterium coli. Biochem J. 1940 Mar;34(3):392–413. doi: 10.1042/bj0340392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbinsky J. S., Cleland W. W. Kinetic studies of Escherichia coli galactokinase. Biochemistry. 1968 Feb;7(2):566–575. doi: 10.1021/bi00842a009. [DOI] [PubMed] [Google Scholar]
- Matyska L., Kovár J. Comparison of several non-linear-regression methods for fitting the Michaelis-Menten equation. Biochem J. 1985 Oct 1;231(1):171–177. doi: 10.1042/bj2310171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Pardee A. B. A biosynthetic ornithine decarboxylase in Escherichia coli. Biochem Biophys Res Commun. 1965 Sep 22;20(6):697–702. doi: 10.1016/0006-291x(65)90072-0. [DOI] [PubMed] [Google Scholar]
- Nowak S., Boeker E. A. The inducible arginine decarboxylase of Escherichia coli B: activity of the dimer and the decamer. Arch Biochem Biophys. 1981 Mar;207(1):110–116. doi: 10.1016/0003-9861(81)90015-1. [DOI] [PubMed] [Google Scholar]
- O'Leary M. H., Piazza G. J. Specificity in enzymatic decarboxylation. J Am Chem Soc. 1978 Jan 18;100(2):632–633. doi: 10.1021/ja00470a049. [DOI] [PubMed] [Google Scholar]
- Recsei P. A., Snell E. E. Pyruvoyl enzymes. Annu Rev Biochem. 1984;53:357–387. doi: 10.1146/annurev.bi.53.070184.002041. [DOI] [PubMed] [Google Scholar]
- Selwyn M. J. A simple test for inactivation of an enzyme during assay. Biochim Biophys Acta. 1965 Jul 29;105(1):193–195. doi: 10.1016/s0926-6593(65)80190-4. [DOI] [PubMed] [Google Scholar]
- WONG J. T., HANES C. S. Kinetic formulations for enzymic reactions involving two substrates. Can J Biochem Physiol. 1962 Jun;40:763–804. [PubMed] [Google Scholar]


