Abstract
Background and aim
Cardiac arrhythmia diagnostic yield improves with increased duration of monitoring. We investigated patient comfort, diagnostic quality of ECG, and arrhythmia diagnostic yield using a single lead longer term external cardiac monitor (ECM).
Methods
The observational ECM feasibility study enrolled patients with increased risk of cardiac arrhythmia. The ECM investigational prototype was designed using a chest strap with dry electrodes connected to module capable of triggered loop recording of ECG, and automatic detection of arrhythmia. In group-A of study (24-h inpatient), patients wore ECM and Holter that recorded ECG from the ECM and adhesive electrodes. In group-B of study (12-weeks ambulatory), at monthly follow-ups patients filled out a comfort survey and device stored arrhythmia episodes were reviewed.
Results
The study enrolled 34 patients (38 % females, average age 57.5 years, 65 % had palpitations, 12 % had syncope). Diagnostic quality ECG was recorded on 76.5 % of the monitoring duration in 12 of 20 patients with reviewable data in group-A, with motion artifacts causing loss in ECG signal for 18.7 % of the time. In 14 patients in group-B, 94.9 % of the survey responses indicated that ECM was comfortable to wear. Cardiac arrhythmia was observed in 4 of 17 patients (24 %) in group-A and 9 of 14 patients (64 %) in group-B in device recorded episodes. All ECM detected pause and tachycardia were inappropriate detections due to motion artifacts and temporary device removal.
Conclusion
The chest strap-based ECM device was mostly comfortable to wear and recorded diagnostic quality ECG in three-fourth of monitoring period. Cardiac arrhythmia was observed in 64 % of patients over 3-month monitoring along with large number of motion artifact induced inappropriate detections.
Keywords: Cardiac arrhythmia, External cardiac monitor, long term ECG monitoring, Extended ECG monitoring
Clinical Trial Registration: CTRI/2020/02/023576
What's New.
-
•
The study shows that it is feasible to perform ambulatory 3-month cardiac arrhythmia monitoring using a chest strap and dry electrode based external cardiac monitoring system
-
•
The external cardiac monitor is mostly comfortable to wear continuously for 3 months with minimal irritation to the skin
-
•
Diagnostic quality ECG was observed over 75 % of the time during a 24-h period of monitoring
-
•
Cardiac arrhythmia diagnostic yield was 24 % over a 24-h period and increases to 64 % over a 3-month monitoring period
-
•
Motion artifacts caused a lot of inappropriate pause and tachycardia detection by the device, which can be reduced significantly using simple algorithm modifications
1. Introduction
Cardiac arrhythmia is primarily diagnosed with point of care electrocardiogram (ECG) recordings in the in-clinic or in-hospital setting on symptomatic presentation. If a diagnosis is not reached, then Holter monitors are prescribed for 24–48 h. Adhesive patches are also being used for ambulatory cardiac arrhythmia monitoring for 7–14 days and has shown to improve diagnostic yield. [1] Discomfort associated with adhesive electrodes, requirements for patient compliance, extensive overhead of a monitoring center for episode review limit such monitoring systems for a period of 7–10 days for single use. Many external devices [2] and wearable smartwatches [3,4], [5] are currently being used for longer term ambulatory monitoring to screen for cardiac arrhythmia, particularly atrial fibrillation (AF), worldwide [6], [7], [8], [9], [10].
Subcutaneous insertable cardiac monitors (ICM) have been used for continuous longer term automatic detection of cardiac arrhythmias and patient symptom triggered storage of loop recorded recent ECG. [11], [12] ICMs are minimally invasive and are implanted in the subcutaneous space. ICMs have been used for diagnosing the cause of unexplained syncope, [13], [14] monitoring of recurrent atrial fibrillation (AF) after ablation of AF, [15] and in patients with history of cryptogenic stroke. [16] In most of these cases the main objective is to deliver therapeutic interventions to patients in a timely manner to reduce clinical morbidity associated with these clinical conditions in a safe and cost efficient manner. [14], [17] While ICMs are considered the gold standard for cardiac arrhythmia monitoring it is often not considered an affordable option in multiple countries around the world due to device cost. The diagnostic yield for ICM based monitoring is highest due to the continuous long duration of monitoring requiring no patient compliance. [18], [19]
Chest strap employing dry electrodes have been used as ECG and heart rate monitors in the setting of fitness, athletic training, and military operations to monitor heart rate and respiratory rates during physically and mentally strenuous activity [[20], [21], [22], [23], [24]]. The objective of this study was to design an investigational external cardiac monitor (ECM) using chest strap with single lead dry electrodes and ICM electronics and evaluate the feasibility of using such a system for longer term (3–6 months) cardiac monitoring in an affordable manner. The overarching goal of such a system would be to bridge the gap in diagnostic yield, for intermittent and asymptomatic cardiac arrhythmia, between short-term monitoring using patches and long-term monitoring using ICMs in a manner that is comfortable to the patients. This study investigated (1) whether the diagnostic quality of the ECG is similar to ECG obtained using adhesive electrodes, (2) whether it is comfortable for patients to wear the designed ECM continuously for 3 months, and (3) the diagnostic yield of cardiac arrhythmia during short term and longer-term monitoring and the cardiac arrhythmia review burden for such a system.
2. Methods
2.1. Design of the investigational ECM prototype
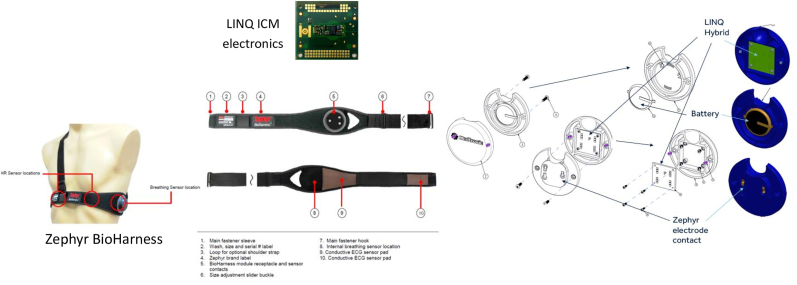
The considerations for the design of the investigation ECM prototype were that it needs to be comfortable and affordable for longer term use. Washable chest straps using dry electrodes (FDA-approved Zephyr™ BioHarness – Medtronic Inc., Minneapolis, USA) is used in the design to make the ECM comfortable for longer term and multiple use. The electronics used was what is available in the FDA-approved Reveal LINQ™ ICM (Medtronic Inc., Minneapolis, USA) to make it compatible with existing systems and infrastructure for wireless remote monitoring. The ICM is capable of loop recording patient triggered symptomatic events with up to 20 min of ECG recordable prior to the patient using a patient activator to trigger ECG storage after a symptomatic event. Further, the ICM has algorithms which are capable of automatically detecting pause, bradycardia, tachycardia, atrial fibrillation and atrial tachycardia from a single lead ECG. Replaceable off the shelf coin cell battery was used to power the electronics to make it compatible for multiple re-use of electronic components in multiple patients to make it affordable. Fig. 1 shows the various components of the designed investigational ECM.
Fig. 1.
Components of the external cardiac monitor investigational prototype.
2.2. Feasibility study design
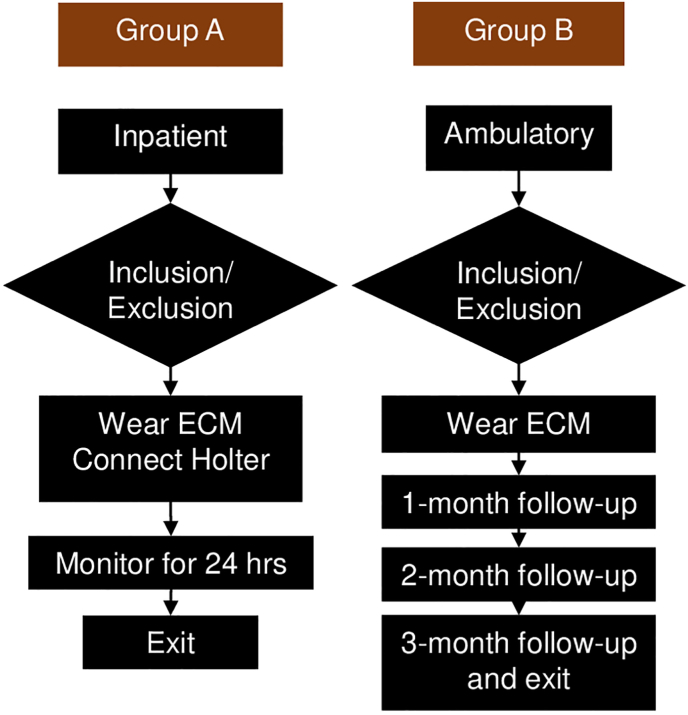
The ECM feasibility study was a multi-center prospective observational study using the investigational prototype. The purpose of study was to demonstrate feasibility of using the ECM prototype as a longer-term external loop recorder by characterizing the diagnostic quality of the acquired ECG signal in an acute 24-h Holter study and the feasibility (patient comfort and diagnostic yield) of doing continuous longer-term monitoring (up to 3 months) using the prototype. Patients with history of cardiovascular disease and suspected cardiac arrhythmia were enrolled in 2 sites in India.
Group An underwent short-term inpatient cardiac monitoring with prototype and DR220 Holter for up to 24 h while admitted in the hospital (Fig. 2). Clinical history, demographics, clinical events before enrollment, save to disk from prototype, Holter flash card, and patient survey at exit was collected. Group B followed a longer-term cardiac ambulatory monitoring of 3 Months with ECM (Fig. 2). At baseline clinical history, demographics, and symptoms were collected. At 1-,2-,3-month follow-up and unscheduled visits, clinical actions taken, clinical events, device save to disk file, symptoms, patient survey, and patient diary were collected. The device is capable of remote monitoring but was not used as part of the study.
Fig. 2.
The external cardiac monitor feasibility study workflow.
2.3. Data analysis
The diagnostic quality of the ECG recorded by the investigational device was evaluated in group-A (short-term) of the study, where patients were continuously monitored for 24 h in an inpatient setting. Enrolled patients wore the ECM prototype in parallel with the DR220 Holter monitoring device to acquire raw ECG signal from the ECM device and surface ECG electrodes using adhesive electrodes. Segments in the Holter recording was marked for periods of diagnostic quality ECG, periods of baseline wandering, periods of motion artifacts, and periods of loss of Holter telemetry. Proportion of duration of diagnostic quality ECG was quantified for periods when there was no loss of telemetry from device to the Holter system.
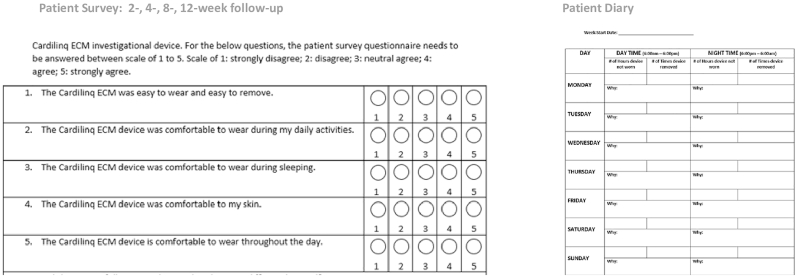
Patient comfort level of wearing a chest strap based dry electrode system for continuous monitoring for a period of 3 months was investigated in group-B of the study, where patients were continuously monitored for 3-months in an ambulatory setting. Enrolled patients wore the investigational ECM prototype at home continuously except when they were having a bath or having discomfort. Patient filled out a survey, designed for this study, at 2-, 4-, 8-, and 12-week follow-ups which answered five questions related to their comfort level in using the device continuously (Fig. 3). Patients also maintained a diary noting down the number of times they temporarily removed the device and the reason for the removal (Fig. 3). The responses in the survey and diary were summarized.
Fig. 3.
Patient survey questionnaire and the patient diary used during the study to collect information on patient comfort in using the investigational prototype for longer term cardiac arrhythmia monitoring in an ambulatory setting.
Diagnostic yield evaluation was performed both in group-A (short-term) of the study, where patients were continuously monitored for 24 h in an inpatient setting, and in Group-B (longer-term) of the study, where patients were continuously monitored for 3-months in an ambulatory setting. The prototype electronics was capable of loop recording ECG initiated by patient, and automatic detection of pause, bradycardia, tachycardia, and atrial tachycardia/fibrillation (AT/AF). Patient completed 4-, 8-, and 12-week clinic follow-ups during which device stored episodes were downloaded using a programmer. The ECM device recorded cardiac arrhythmia episode data was reviewed for presence of true arrhythmia.
3. Results
The study enrolled a total of 34 patients (average age 57.5 years, 38 % females, 65 % had history of palpitations, 12 % had history of syncope) of which 20 patients enrolled in Group-A for 24-h inpatient monitoring (average age 63.4 years, 53 % females, average BMI 24.8 kg/m2) and the other 14 patients enrolled in Group-B for 12 week ambulatory monitoring (average age 49.7 years, 21 % females, average BMI 26.1 kg/m2).
3.1. Signal quality
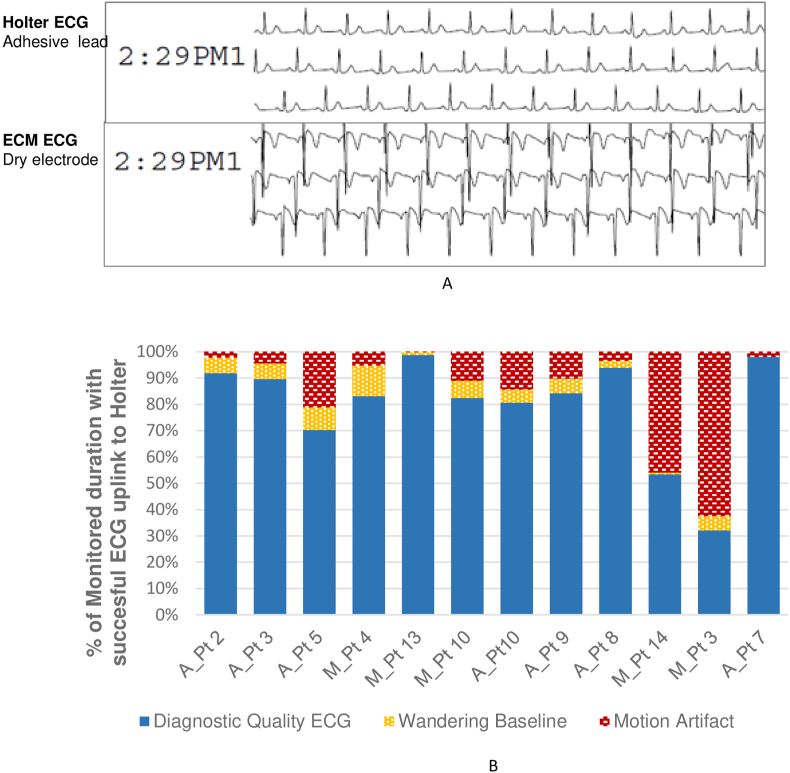
Of the 20 patients enrolled in group-A of the study, reviewable data for evaluating signal quality was successfully telemetered to DR220 Holter in 12 patients. Two patients exited the study prior to start of Holter recording and the other 6 patients had telemetry error in the uplink of the raw device measured ECG to the DR220 Holter. This telemetry uplink functionality of the device is specially designed for use in clinical studies and is not intended for routine clinical use. The ECM signal looks comparable (Fig. 4A) to surface ECG with adhesive ECG electrodes, with respect to visibility of p-waves, QRS complex, and T-waves, when prototype dry electrodes are in contact with the skin. The ICM polarity is dependent on the electrode position relative to the two inputs to the ICM electronics and the QRS polarity may be reversed based on the electrode configuration in specific devices. The diagnostic quality ECG was recorded on an average 76.5 % of the monitoring duration with successful ECG recording uplink to the Holter device (Fig. 4B). Motion artifacts, which included durations with complete displacement of the chest strap, caused loss in ECG signal for 18.7 % of the time leading to false pause or tachycardia detection by the device. Cardiac arrhythmia in 3 of 12 patients with Holter recordings (two patients with 3 episodes of AF and one patient with junctional arrhythmia). All episodes were detected by ECM - the device automatically detected true AT/AF in 2 patients, junctional rhythm in 1 patient; however, duration was underestimated due to motion artifacts.
Fig. 4.
(A) ECG quality comparison between adhesive lead based Holter recording and ECG recorded simultaneously using dry electrode based investigational ECM system. (B) Proportion of diagnostic quality ECG recorded in each group A patient.
3.2. Patient comfort
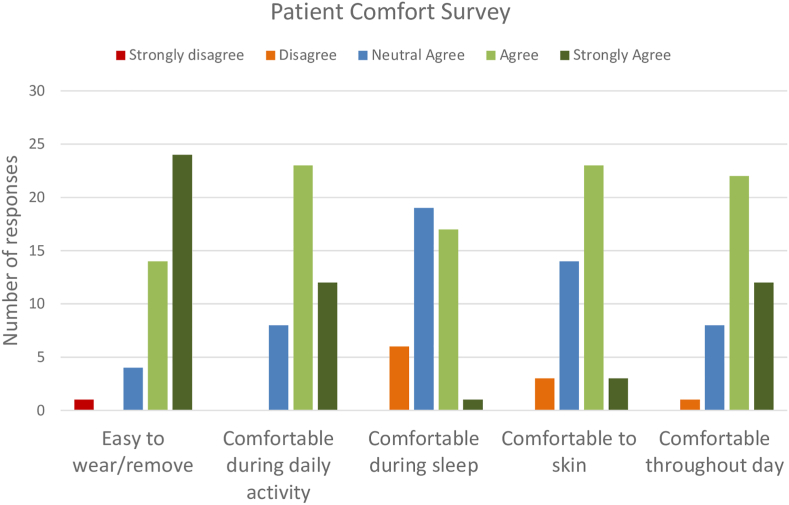
In the group-B patients, 43 patient comfort survey responses were completed with 14, 10, 10, and 9 patients filling out the survey at 2-, 4-, 8-, and 12- week follow-ups respectively. Overall, only 5.1 % of the responses indicated that patients disagreed that the ECM system was comfortable to wear, 2.8 % indicating ECM device was uncomfortable during sleep and 1.4 % indicating that it was uncomfortable for the skin (Fig. 5).
Fig. 5.
Summary of results from the patient survey related to the evaluation of patient comfort in wearing the investigational prototype daily for a period of up to 12 weeks.
There was a total of 726 days with temporary removal of device as reported by the patients in the patient diary. There was a total of 613, 102, and 11 days with 1, 2, or 3 removals per day respectively. Most of the removals (675 removals, 93 %) was for ≤2 h in duration in a day of which a fair share (591 removals, 81 %) was ≤2 h in duration. The main reason for removal was for bathing (677 instances, 93 %) as instructed per protocol of the study. The other reasons for removal included sleep disturbance (31 instances in 1 patient), discomfort (7 instances, 2 patients) and skin irritation or rashes or itching on 11 instances in 2 patients.
3.3. Diagnostic yield
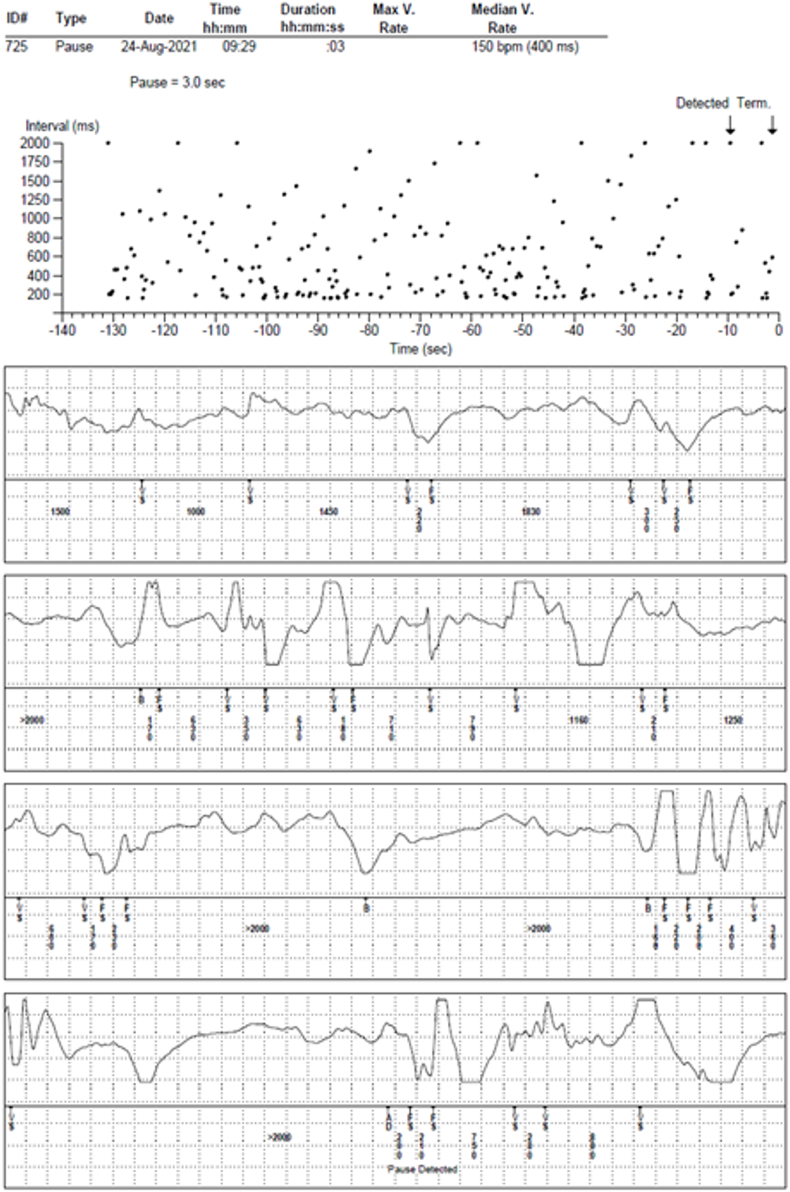
Device stored episodes available for review in 31 of the 34 patients enrolled in the study. Table 1 shows the total number episodes stored by the device and was available for review. The table also shows the total number of patients where cardiac arrhythmia was observed in the patients. Cardiac arrhythmia was observed in 4 of 17 patients (24 %) in group-A (24-hr inpatient); 9 of 14 patients (64 %) in Group-B (12-week ambulatory). Fig. 6 shows examples of device stored true cardiac arrhythmia episodes. Highest diagnostic yield was observed in device detected AT/AF episodes followed by patient activated and device detected bradycardia episodes. All device detected pause and tachycardia were false detections due to motion artifacts and temporary device removal. An example of falsely detected pause episode is shown in Fig. 7. It was observed that most false episodes had <20 RR intervals in last 30 s prior to detection or had more than two RR intervals <220 ms (180 ms for tachycardia episodes) or had more than two RR intervals >1200 ms or had more than two beat to beat RR interval difference was >1000 ms. Simple post-hoc algorithm modifications, that employs counting short intervals, long intervals and change in intervals above the thresholds mentioned above, was able to reduce the number of false detections reported in Table 1 by 97 %.
Table 1.
Total number of investigational device recorded episodes that were reviewable. The total number of patients with true arrhythmia observed in any of those reviewable episodes.
| Group-A |
Group-B |
||
|---|---|---|---|
| Patients |
17 |
14 |
|
| Monitoring Duration | 24 h | 12 weeks | |
| Number of device recorded episodes with ECG (patients) | AT/AF | 33 (7) | 33 (7) |
| Bradycardia | 7 (3) | 37 (7) | |
| Pause | 198 (16) | 537 (13) | |
| Tachycardia | 24 (9) | 103 (13) | |
| Patient Activated | 0 (0) | 52 (11) | |
| Number of patients with true cardiac arrhythmia | AT/AF | 3 | 2 |
| Bradycardia/pause | 0 | 2 | |
| PVC | 0 | 3 | |
| Sinus Arrhythmia | 0 | 1 | |
| Sinus Tachycardia | 0 | 4 | |
| Junctional Rhythm | 1 | 0 |
| Device detected episode type | Group A – 24-h monitoring (N = 17 patients) |
Group B – 12-week monitoring (N = 14 patients) |
||||
|---|---|---|---|---|---|---|
| Number of detected episodes | Patients with detected episodes | Patients with true episodes | Number of detected episodes | Patients with detected episodes | Patients with true episodes | |
| AT/AF | 33 | 7 | 3 AF, 1 junctional rhythm |
33 | 7 | 2 AF |
| Bradycardia | 7 | 3 | 0 | 37 | 7 | 1 Bradycardia 1 pause |
| Pause | 198 | 16 | 0 | 537 | 13 | 0 |
| Tachycardia | 24 | 9 | 0 | 103 | 13 | 0 |
| Patient Activated | 0 | 0 | 0 | 52 | 12 | 3 PVC 4 sinus tachycardia 1 sinus arrhythmia |
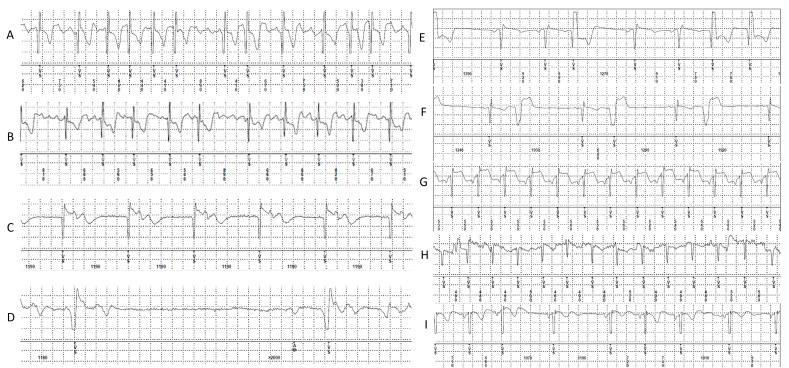
Fig. 6.
Examples of different kind of true arrhythmia detected and stored by investigational ECM device. A–B: atrial arrhythmia; C: Junctional rhythm; D: sinus pause; E–F: premature ventricular contractions; G–H: sinus tachycardia; I: sinus arrhythmia.
Fig. 7.
Examples of false arrhythmia detected and stored by investigational ECM device.
4. Discussion
The ECM feasibility study was a pilot study designed to evaluate the feasibility of an investigational external monitoring device intended for continuous longer-term monitoring of cardiac arrhythmia in an ambulatory setting. During the 24-h inpatient monitoring, diagnostic quality ECG was observed in over 75 % of the monitoring duration during the short term study with ECG characteristics comparable to simultaneous Holter recordings using adhesive electrodes. During the 12 week ambulatory monitoring, around 5 % of patient responses indicated that the device was uncomfortable to wear with only 1.4 % responses indicating skin irritation. Cardiac arrhythmia, that might explain patient symptoms, was observed in 24 % of patients during 24-h inpatient monitoring and 64 % of patients during 12 week ambulatory monitoring.
Holter based 24-h monitoring systems with conventional adhesive electrodes has reported less than 2 % unanalyzable recording due to noise or motion artifact. [25] Adhesive patch based monitoring systems report noisy or low diagnostic quality signals anywhere between 2 % and 12 % of the monitoring time when the patch is staying on the patient depending on the setting for monitoring use [[25], [26], [27], [28], [29]]. In contrast, results from this study show that diagnostic quality ECG could not be recorded around 24 % of time using the investigational ECM prototype using dry non-adhesive electrodes in a chest strap designed for longer than 12-week monitoring. Thus, the device is functional in detecting cardia arrhythmias for around three-fourth of the time which includes time when the device is not worn or worn incorrectly. A non-adhesive dry electrode system will not have the same measurement coverage as an adhesive Holter or patch system daily, but it also enables prolonged monitoring which will increase the total duration of monitoring. The designed capability of extended monitoring duration of more than 12-weeks, enables recovery of diagnostic yield for intermittent and asymptomatic cardiac arrhythmia such as AF as has been reported elsewhere [1,18,19]. Further, it has been reported for smart watch based systems the measurement coverage is in the range from 25 % to 58 % over a 24 h monitoring period in an ambulatory setting [[30], [31], [32]]. Most of these devices uses signal quality assessment to reject segments of data due to motion artifacts to improve detection performance. Stricter the rules for signal quality, lower was the measurement coverage.
Patients mostly agreed that device in this study was comfortable to wear for prolonged duration. In around 5 % of responses over prolonged monitoring – 10 of 14 patients wore the device for more than 8 weeks - patients mentioned discomfort. Skin irritation, one of the main concerns with such a system, was negligible. Due to the non-adhesive nature of the electrodes, patients could remove the device for periods of time and wear it again without having to replace the device. In contrast, for adhesive patch based system, close to 6–7% of patients report discomfort in 10–11 days of monitoring. [1] More than 80 % of patients preferred patch monitoring compared to Holter monitoring showing that Holter monitoring systems are more uncomfortable to wear. [1] Further, adhesive based patch monitoring systems require a new patch in case the patch is removed, which is not the case for the system investigated in this study - a key advantage of non-adhesive dry electrode systems.
Holter based monitoring systems report diagnostic yield for cardiac arrhythmias in the range from 10 to 15 % and 10–14 day adhesive patch based monitoring systems report cardiac arrhythmia diagnostic yield around 40–50 % [1,[25], [26], [27], [28], [29]]. with a fair share of arrhythmia being non-clinically actionable supraventricular tachycardia. The investigational device in this study was able to diagnose cardiac arrhythmia, which may explain their symptoms, in a fair share of patients – 24 % in the 24-h monitoring and 64 % in 12-week monitoring. However, higher diagnostic yield also came at the cost of having to review large number of false detections due to motion artifacts. While remote monitoring transmissions was not utilized in this study, but with capabilities of remote monitoring that is present in these devices, further improvement in diagnostic yield is expected.
Automatic cardiac arrhythmia detection has improved in ICMs over the last decade [[33], [34], [35], [36]]. The current state of the art devices are able to maintain high sensitivity for detection of atrial fibrillation, pause, bradycardia, and tachycardia while also maintaining a reasonable episode review burden for clinicians using artificial intelligence algorithms to reduce inappropriate detections. [[37], [38], [39]] Artificial Intelligence algorithms (AccuRhythm™ AI) is currently being used for ICM devices, deployed in the CareLink remote monitoring cloud platform, which has reduced 88 % and 97 % of inappropriate AF and pause detections in ICM [37,38]. One of the goal of this study was to evaluate whether these algorithms when applied to non-implantable setting will also perform similarly. The main observed difference was a significant increase in false detections of pause and tachycardia due to motion artifacts. ICMs are not susceptible to motion artifacts, and thus the algorithms in those devices were not designed to reject motion artifact related false detection. Minor algorithm modifications were evaluated which would reduce false detections by over 97 %. Further artificial intelligence derived algorithms when trained to incorporate motion artifact signals can further reduce the false detections in the future. Alternatively, one can use a third-party service to curate the information presented to physicians as is done by Holter and patch based devices. Finally, the device algorithms can be programmed differently for different patient populations as has been practiced for ICM devices (e.g. only looking for pauses >4.5 s instead of 3 s for patients who do not have syncope, or requiring larger number of consecutive intervals of shorter or longer cycle lengths to detect tachycardia and bradycardia, respectively, in patient who are being monitored for AF) will also reduce false detections.
The main advantage of the investigational ECM system is the non-adhesive dry electrodes which enables multiple use in the same or additional patients without having to replace the strap or the electronics. The strap is washable and hence can be used multiple times. The electronics have batteries which will last for more than 5 years without needing to change it, thus enabling multiple use. Patients can remove the device and put it back on, which enables prolonged monitoring in patients if required. The device was not designed to replace adhesive patch based monitoring, but may be useful for patients who have intermittent and asymptomatic cardiac arrhythmia which may require longer term monitoring beyond 10–14 days. Based on the results of this feasibility study, the current investigational device is feasible for automatic detection of AT/AF and recording of preceding ECG when patients are having symptoms as not a lot of artifact induced false detections or ECG corruption was observed in these type of episodes. Pause and tachycardia detection needs algorithm modifications before it can be feasible for routine clinical use. Additionally, tighter chest straps and vests and strain gauge based automatic detection of chest strap tightness may be useful in the future. Additional data is going to be collected in an amended study with the second generation of the investigational prototype. Future enhancements being considered include multi-lead systems with additional sensors which can potentially measure other parameters to monitor for additional disease states.
The primary limitation of the study is the limited number of patients included for analysis. Additional data will be collected in the future in an expanded study with a second generation of the investigational prototype. Further, the study did not utilize wireless transmission and remote monitoring capability due to some logistical limitation during the study. Thus, device recorded ECG were only reviewed at the monthly follow-ups hence limiting the number of episodes reviewed. Despite this limitation the diagnostic yield for cardiac arrhythmia was above 50 % for longer term monitoring. Finally, the quality of the signal as well as the patient comfort level is very dependent on the size of the strap utilized and the tightness of the fit of the device for various patients. Only two sizes were available and hence the variance in diagnostic quality across different patients. Data on the tightness of the strap worn was not collected and hence could not be analyzed.
5. Conclusion
A chest strap based investigational external cardiac monitoring system using non-adhesive dry electrodes for prolonged cardiac monitoring was feasible. Recorded ECG was of similar diagnostic quality as Holter systems with adhesive electrodes with recordings devoid of noise or motion artifacts more than 75 % of time. It was mostly comfortable for patients to wear the system for a long periods daily and continuously for 4–12 weeks. Cardiac arrhythmia was diagnosed in more than 60 % of the patients during longer term monitoring along with large number of inappropriate detections.
Funding sources
Funding for this project was received from Medtronic Inc.
Patient consent
All patients provided consent at the time of enrollment to the ECM feasibility study.
Ethics statement
The clinical study was approved by Institutional ethics committees where the study was conducted.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors wish to thank Dr. Wali Mohammed and Asumitha A. for all study coordination activities at their respective investigational sites.
Footnotes
Peer review under responsibility of Indian Heart Rhythm Society.
References
- 1.Barrett P.M., Komatireddy R., Haaser S., Topol S., Sheard J., Encinas J., Fought A.J., Topol E.J. Comparison of 24-hour Holter monitoring with 14-day novel adhesive patch electrocardiographic monitoring. Am J Med. 2014;127(1):95.e11–95.e17. doi: 10.1016/j.amjmed.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pothineni N.V.K., Soliman E.Z., Cushman M., Howard G., Howard V.J., Kasner S.E., Judd S., Rhodes J.D., Marchlinski F.E., Deo R. Continuous cardiac rhythm monitoring post-stroke: a feasibility study in REGARDS. J Stroke Cerebrovasc Dis. 2022;31(11) doi: 10.1016/j.jstrokecerebrovasdis.2022.106662. [DOI] [PubMed] [Google Scholar]
- 3.Lau J.K., Lowres N., Neubeck L., Brieger D.B., Sy R.W., Galloway C.D., Albert D.E., Freedman S.B. iPhone ECG application for community screening to detect silent atrial fibrillation: a novel technology to prevent stroke. Int J Cardiol. 2013;165(1):193–194. doi: 10.1016/j.ijcard.2013.01.220. [DOI] [PubMed] [Google Scholar]
- 4.Halcox J.P.J., Wareham K., Cardew A., Gilmore M., Barry J.P., Phillips C., Gravenor M.B. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation. 2017;136(19):1784–1794. doi: 10.1161/CIRCULATIONAHA.117.030583. [DOI] [PubMed] [Google Scholar]
- 5.Perez M.V., Mahaffey K.W., Hedlin H., Rumsfeld J.S., Garcia A., Ferris T., Balasubramanian V., Russo A.M., Rajmane A., Cheung L., Hung G., Lee J., Kowey P., Talati N., Nag D., Gummidipundi S.E., Beatty A., Hills M.T., Desai S., Granger C.B., Desai M., Turakhia M.P. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019;381(20):1909–1917. doi: 10.1056/NEJMoa1901183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lubitz S.A., Faranesh A.Z., Selvaggi C., Atlas S.J., McManus D.D., Singer D.E., Pagoto S., McConnell M.V., Pantelopoulos A., Foulkes A.S. Detection of atrial fibrillation in a large population using wearable devices: the fitbit heart study. Circulation. 2022;146(19):1415–1424. doi: 10.1161/CIRCULATIONAHA.122.060291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sana F., Isselbacher E.M., Singh J.P., Heist E.K., Pathik B., Armoundas A.A. Wearable devices for ambulatory cardiac monitoring: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(13):1582–1592. doi: 10.1016/j.jacc.2020.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duarte R., Stainthorpe A., Greenhalgh J., Richardson M., Nevitt S., Mahon J., Kotas E., Boland A., Thom H., Marshall T., Hall M., Takwoingi Y. Lead-I ECG for detecting atrial fibrillation in patients with an irregular pulse using single time point testing: a systematic review and economic evaluation. Health Technol Assess. 2020;24(3):1–164. doi: 10.3310/hta24030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soni A., Karna S., Fahey N., Sanghai S., Patel H., Raithatha S., Thanvi S., Nimbalkar S., Freedman B., Allison J., McManus D.D. Age-and-sex stratified prevalence of atrial fibrillation in rural Western India: results of SMART-India, a population-based screening study. Int J Cardiol. 2019;280:84–88. doi: 10.1016/j.ijcard.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saggu D.K., Rangaswamy V.V., Yalagudri S., Sundar G., Reddy N.K., Shah V., K K., Shankar M., Chennapragada S., Narasimhan C. Prevalence, clinical profile, and stroke risk of atrial fibrillation in rural Andhra Pradesh, India (the AP-AF study) Indian Heart J. 2022;74(2):86–90. doi: 10.1016/j.ihj.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krahn A.D., Klein G.J., Yee R., Takle-Newhouse T., Norris C. Use of an extended monitoring strategy in patients with problematic syncope. Circulation. 1999;99(3):406–410. doi: 10.1161/01.cir.99.3.406. [DOI] [PubMed] [Google Scholar]
- 12.Pürerfellner H., Sanders P., Pokushalov E., Di Bacco M., Bergemann T., Dekker L.R. Miniaturized Reveal LINQ insertable cardiac monitoring system: first-in-human experience. Heart Rhythm. 2015 Jun;12(6):1113–1119. doi: 10.1016/j.hrthm.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Krahn A.D., Klein G.J., Yee R., Skanes A.C. Detection of asymptomatic arrhythmias in unexplained syncope. Am Heart J. 2004;148:326–332. doi: 10.1016/j.ahj.2004.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Farwell D.J., Freemantle N., Sulke A.N. Use of implantable loop recorders in the diagnosis and management of syncope. Eur Heart J. 2004;25(14):1257–1263. doi: 10.1016/j.ehj.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 15.Verma A., Champagne J., Sapp J., Essebag V., Novak P., Skanes A., Morillo C.A., Khaykin Y., Birnie D. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med. 2013;173:149–156. doi: 10.1001/jamainternmed.2013.1561. [DOI] [PubMed] [Google Scholar]
- 16.Sanna T., Diener H.C., Passman R.S., DiLazzaro V., Bernstein R.A., Morillo C.A., Rymer M.M., Thijs V., Rogers T., Beckers F., Lindborg K., Brachmann J. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 17.Mittal S., Rogers J., Sarkar S., Koehler J., Passman R.S. Real-world incidence of pacemaker and defibrillator implantation following diagnostic monitoring with an insertable cardiac monitor. Am J Cardiol. 2019 Jun 15;123(12):1967–1971. doi: 10.1016/j.amjcard.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Ziegler P.D., Koehler J.L., Mehra R. Comparison of continuous versus intermittent monitoring of atrial arrhythmias. Heart Rhythm. 2006;3:1445–1452. doi: 10.1016/j.hrthm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Hanke T., Charitos E.I., Stierle U., Karluss A., Kraatz E., Graf B., Hagemann A., Misfeld M., Sievers H.H. Twenty four-hour Holter monitor follow-up does not provide accurate heart rhythm status after surgical atrial fibrillation ablation therapy: upto12 months experience with a novel permanently implantable heart rhythm monitor device. Circulation. 2009;120:S177–S184. doi: 10.1161/CIRCULATIONAHA.108.838474. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao P.J., Chiu C.C., Lin K.H., Hu F.K., Tsai P.J., Wu C.T., Pang Y.K., Lin Y., Kuo M.H., Chen K.H., Wu Y.S., Wu H.Y., Chang Y.T., Chang Y.T., Cheng C.S., Chuu C.P., Lin F.H., Chang C.W., Li Y.K., Chan J.S., Chu C.M. Usability of wearable devices with a novel cardiac force index for estimating the dynamic cardiac function: observational study. JMIR Mhealth Uhealth. 2020 Jul 21;8(7) doi: 10.2196/15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin I.M., Wang S.Y., Fan S.Y., Peper E., Chen S.P., Huang C.Y. A single session of heart rate variability biofeedback produced greater increases in heart rate variability than autogenic training. Appl Psychophysiol Biofeedback. 2020 Dec;45(4):343–350. doi: 10.1007/s10484-020-09483-y. [DOI] [PubMed] [Google Scholar]
- 22.Parak J., Salonen M., Myllymäki T., Korhonen I. Comparison of heart rate monitoring accuracy between chest strap and vest during physical training and implications on training decisions. Sensors. 2021 Dec 16;21(24):8411. doi: 10.3390/s21248411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chatterjee T., Bhattacharyya D., Yadav A., Pal M. Quantification of physiological and mental workloads of faster and slower finishers of a long-distance military training activity. BMJ Mil Health. 2022 Oct 25 doi: 10.1136/military-2022-002154. [DOI] [PubMed] [Google Scholar]
- 24.Miyazaki Y., Toyoda K., Iguchi Y., Hirano T., Metoki N., Tomoda M., Shiozawa M., Koge J., Okada Y., Terasawa Y., Kikuno M., Okano H., Hagii J., Nakajima M., Komatsu T., Yasaka M. Atrial fibrillation after ischemic stroke detected by chest strap-style 7-day holter monitoring and the risk predictors: educate-esus. J Atheroscler Thromb. 2021 May 1;28(5):544–554. doi: 10.5551/jat.58420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karaoğuz M.R., Yurtseven E., Aslan G., Deliormanlı B.G., Adıgüzel Ö., Gönen M., Li K.M., Yılmaz E.N. The quality of ECG data acquisition, and diagnostic performance of a novel adhesive patch for ambulatory cardiac rhythm monitoring in arrhythmia detection. J Electrocardiol. 2019;54:28–35. doi: 10.1016/j.jelectrocard.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Liu C.M., Chang S.L., Yeh Y.H., Chung F.P., Hu Y.F., Chou C.C., Hung K.C., Chang P.C., Liao J.N., Chan Y.H., Lo L.W., Wu L.S., Lin Y.J., Wen M.S., Chen S.A. Enhanced detection of cardiac arrhythmias utilizing 14-day continuous ECG patch monitoring. Int J Cardiol. 2021 Jun 1;(332):78–84. doi: 10.1016/j.ijcard.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Kwon S., Lee S.R., Choi E.K., Ahn H.J., Song H.S., Lee Y.S., Oh S., Lip G.Y.H. Comparison between the 24-hour holter test and 72-hour single-lead electrocardiogram monitoring with an adhesive patch-type device for atrial fibrillation detection: prospective cohort study. J Med Internet Res. 2022 May 9;24(5) doi: 10.2196/37970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fruytier L.A., Janssen D.M., Campero Jurado I., van de Sande D.A., Lorato I., Stuart S., Panditha P., de Kok M., Kemps H.M. The utility of a novel electrocardiogram patch using dry electrodes technology for arrhythmia detection during exercise and prolonged monitoring: proof-of-concept study. JMIR Form Res. 2023 Nov 30;7 doi: 10.2196/49346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen W.C., Wu Y.L., Hsu Y.C., Hsu J.T., Tseng H.P., Chen C.C., Chiang M.H., Hsiao J.F., Chin S.K., Huang Y.L., Lei M.H. Comparison of continuous 24-hour and 14-day ECG monitoring for the detection of cardiac arrhythmias in patients with ischemic stroke or syncope. Clin Cardiol. 2024 Feb;47(3) doi: 10.1002/clc.24247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eerikäinen L.M., Bonomi A.G., Dekker L.R.C., Vullings R., Aarts R.M. Atrial fibrillation monitoring with wrist-worn photoplethysmography-based wearables: state-of-the-art review. Cardiovasc Digit Health J. 2020 Aug 26;1(1):45–51. doi: 10.1016/j.cvdhj.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira T., Tran N., Gadhoumi K., Pelter M.M., Do D.H., Lee R.J., Colorado R., Meisel K., Hu X. Photoplethysmography based atrial fibrillation detection: a review. NPJ Digit Med. 2020;3:3. doi: 10.1038/s41746-019-0207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saarinen H.J., Joutsen A., Korpi K., Halkola T., Nurmi M., Hernesniemi J., Vehkaoja A. Wrist-worn device combining PPG and ECG can be reliably used for atrial fibrillation detection in an outpatient setting. Front Cardiovasc Med. 2023 Feb 9;10 doi: 10.3389/fcvm.2023.1100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarkar S., Ritscher D., Mehra R. A detector for a chronic implantable atrial tachyarrhythmia monitor. IEEE Trans Biomed Eng. 2008 Mar;55(3):1219–1224. doi: 10.1109/TBME.2007.903707. [DOI] [PubMed] [Google Scholar]
- 34.Pürerfellner H., Pokushalov E., Sarkar S., Koehler J., Zhou R., Urban L., Hindricks G. P-wave evidence as a method for improving algorithm to detect atrial fibrillation in insertable cardiac monitors. Heart Rhythm. 2014;11(9):1575–1583. doi: 10.1016/j.hrthm.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Passman R.S., Rogers J.D., Sarkar S., Reiland J., Reisfeld E., Koehler J., Mittal S. Development and validation of a dual sensing scheme to improve accuracy of bradycardia and pause detection in an insertable cardiac monitor. Heart Rhythm. 2017;14(7):1016–1023. doi: 10.1016/j.hrthm.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 36.Pürerfellner H., Sanders P., Sarkar S., Reisfeld E., Reiland J., Koehler J., Pokushalov E., Urban L., Dekker L.R.C. Adapting detection sensitivity based on evidence of irregular sinus arrhythmia to improve atrial fibrillation detection in insertable cardiac monitors. Europace. 2018 Nov 1;20(FI_3):f321–f328. doi: 10.1093/europace/eux272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng YJ, Ousdigian KT, Sarkar S, Koehler J, Cho YK, Kloosterman EM. Innovative artificial intelligence application reduces false pause alerts while maintaining perfect true pause alert sensitivity for insertable cardiac monitors. Heart Rhythm 18 (8), S293-S294.
- 38.Radtke A.P., Ousdigian K.T., Haddad T.D., Koehler J.L., Colombowala I.K. Artificial intelligence enables dramatic reduction of false atrial fibrillation alerts from insertable cardiac monitors. Heart Rhythm. 2021 Aug;18(8 Supplement) [Google Scholar]
- 39.Sarkar S., Majumder S., Koehler J.L., Landman S.R. An ensemble of features based deep learning neural network for reduction of inappropriate atrial fibrillation detection in implantable cardiac monitors. Heart Rhythm O2. 2022 Nov 1;4(1):51–58. doi: 10.1016/j.hroo.2022.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]