Abstract
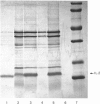
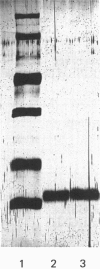
Recombinant human interleukin-2 (IL-2) expressed as Escherichia coli was isolated as insoluble aggregates of protein (inclusion bodies) after cell breakage. IL-2 and contaminants were dissolved in 6 M-guanidinium chloride/10 mM-dithiothreitol, pH 8.5, and further purified in reduced and denatured form by gel-permeation chromatography in the same solvent. Renaturation was effected by dilution and autoxidation; IL-2 of native specific activity was isolated at over 95% purity by reversed-phase h.p.l.c.; an additional peak of reduced protein was also observed. Most losses of native IL-2 occurred on refolding, probably because of an aggregation process; concentrations around 1 microgram/ml were necessary to achieve 30% recovery. It was essential to maintain the denatured protein in reduced form before renaturation and autoxidation, which was most efficient at pH 8.5 with 1.5 microM-CuSO4. A procedure based on these observations has been used to prepare IL-2 on the 50 micrograms scale.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. K., Schaffer S. W., Wetlaufer D. B. Nonenzymic reactivation of reduced bovine pancreatic ribonuclease by air oxidation and by glutathione oxidoreduction buffers. J Biol Chem. 1975 Nov 10;250(21):8477–8482. [PubMed] [Google Scholar]
- Anderson W. L., Wetlaufer D. B. A new method for disulfide analysis of peptides. Anal Biochem. 1975 Aug;67(2):493–502. doi: 10.1016/0003-2697(75)90323-1. [DOI] [PubMed] [Google Scholar]
- Browning J. L., Mattaliano R. J., Chow E. P., Liang S. M., Allet B., Rosa J., Smart J. E. Disulfide scrambling of interleukin-2: HPLC resolution of the three possible isomers. Anal Biochem. 1986 May 15;155(1):123–128. doi: 10.1016/0003-2697(86)90236-8. [DOI] [PubMed] [Google Scholar]
- Christie G. E., Farnham P. J., Platt T. Synthetic sites for transcription termination and a functional comparison with tryptophan operon termination sites in vitro. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4180–4184. doi: 10.1073/pnas.78.7.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Devos R., Plaetinck G., Cheroutre H., Simons G., Degrave W., Tavernier J., Remaut E., Fiers W. Molecular cloning of human interleukin 2 cDNA and its expression in E. coli. Nucleic Acids Res. 1983 Jul 11;11(13):4307–4323. doi: 10.1093/nar/11.13.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erard F., Corthesy P., Nabholz M., Lowenthal J. W., Zaech P., Plaetinck G., MacDonald H. R. Interleukin 2 is both necessary and sufficient for the growth and differentiation of lectin-stimulated cytolytic T lymphocyte precursors. J Immunol. 1985 Mar;134(3):1644–1652. [PubMed] [Google Scholar]
- Farrar W. L., Johnson H. M., Farrar J. J. Regulation of the production of immune interferon and cytotoxic T lymphocytes by interleukin 2. J Immunol. 1981 Mar;126(3):1120–1125. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L. E., Hewetson J. F., Hopkins R. F., 3rd, Sowder R. C., Neubauer R. H., Rabin H. A rapid, large scale purification procedure for gibbon interleukin 2. J Immunol. 1983 Aug;131(2):810–815. [PubMed] [Google Scholar]
- Henney C. S., Kuribayashi K., Kern D. E., Gillis S. Interleukin-2 augments natural killer cell activity. Nature. 1981 May 28;291(5813):335–338. doi: 10.1038/291335a0. [DOI] [PubMed] [Google Scholar]
- Kato K., Yamada T., Kawahara K., Onda H., Asano T., Sugino H., Kakinuma A. Purification and characterization of recombinant human interleukin-2 produced in Escherichia coli. Biochem Biophys Res Commun. 1985 Jul 31;130(2):692–699. doi: 10.1016/0006-291x(85)90472-3. [DOI] [PubMed] [Google Scholar]
- Kunitani M., Hirtzer P., Johnson D., Halenbeck R., Boosman A., Koths K. Reversed-phase chromatography of interleukin-2 muteins. J Chromatogr. 1986 May 30;359:391–402. doi: 10.1016/0021-9673(86)80093-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang S. M., Allet B., Rose K., Hirschi M., Liang C. M., Thatcher D. R. Characterization of human interleukin 2 derived from Escherichia coli. Biochem J. 1985 Jul 15;229(2):429–439. doi: 10.1042/bj2290429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S. M., Thatcher D. R., Liang C. M., Allet B. Studies of structure-activity relationships of human interleukin-2. J Biol Chem. 1986 Jan 5;261(1):334–337. [PubMed] [Google Scholar]
- Mulé J. J., Shu S., Rosenberg S. A. The anti-tumor efficacy of lymphokine-activated killer cells and recombinant interleukin 2 in vivo. J Immunol. 1985 Jul;135(1):646–652. [PubMed] [Google Scholar]
- Orsini G., Goldberg M. E. The renaturation of reduced chymotrypsinogen A in guanidine HCl. Refolding versus aggregation. J Biol Chem. 1978 May 25;253(10):3453–3458. [PubMed] [Google Scholar]
- Robb R. J. Interleukin 2 and its cell-surface receptor. Behring Inst Mitt. 1985 Aug;(77):56–67. [PubMed] [Google Scholar]
- Robb R. J., Kutny R. M., Panico M., Morris H. R., Chowdhry V. Amino acid sequence and post-translational modification of human interleukin 2. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6486–6490. doi: 10.1073/pnas.81.20.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Construction and analysis of in vivo activity of E. coli promoter hybrids and promoter mutants that alter the -35 to -10 spacing. Gene. 1982 Dec;20(2):231–243. doi: 10.1016/0378-1119(82)90042-7. [DOI] [PubMed] [Google Scholar]
- Saxena V. P., Wetlaufer D. B. Formation of three-dimensional structure in proteins. I. Rapid nonenzymic reactivation of reduced lysozyme. Biochemistry. 1970 Dec 8;9(25):5015–5023. doi: 10.1021/bi00827a028. [DOI] [PubMed] [Google Scholar]
- Sgaramella V., Khorana H. G. CXII. Total synthesis of the structural gene for an alanine transfer RNA from yeast. Enzymic joining of the chemically synthesized polydeoxynucleotides to form the DNA duplex representing nucleotide sequence 1 to 20. J Mol Biol. 1972 Dec 28;72(2):427–444. doi: 10.1016/0022-2836(72)90155-6. [DOI] [PubMed] [Google Scholar]
- Stern A. S., Pan Y. C., Urdal D. L., Mochizuki D. Y., DeChiara S., Blacher R., Wideman J., Gillis S. Purification to homogeneity and partial characterization of interleukin 2 from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1984 Feb;81(3):871–875. doi: 10.1073/pnas.81.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi T., Matsui H., Fujita T., Takaoka C., Kashima N., Yoshimoto R., Hamuro J. Structure and expression of a cloned cDNA for human interleukin-2. Nature. 1983 Mar 24;302(5906):305–310. doi: 10.1038/302305a0. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- Yamada T., Kato K., Kawahara K., Nishimura O. Separation of recombinant human interleukin-2 and methionyl interleukin-2 produced in Escherichia coli. Biochem Biophys Res Commun. 1986 Mar 28;135(3):837–843. doi: 10.1016/0006-291x(86)91004-1. [DOI] [PubMed] [Google Scholar]
- Yutani K., Yutani A., Imanishi A., Isemura T. The mechanism of refording of the reduced random coil form of lysozyme. J Biochem. 1968 Oct;64(4):449–455. doi: 10.1093/oxfordjournals.jbchem.a128916. [DOI] [PubMed] [Google Scholar]