Abstract
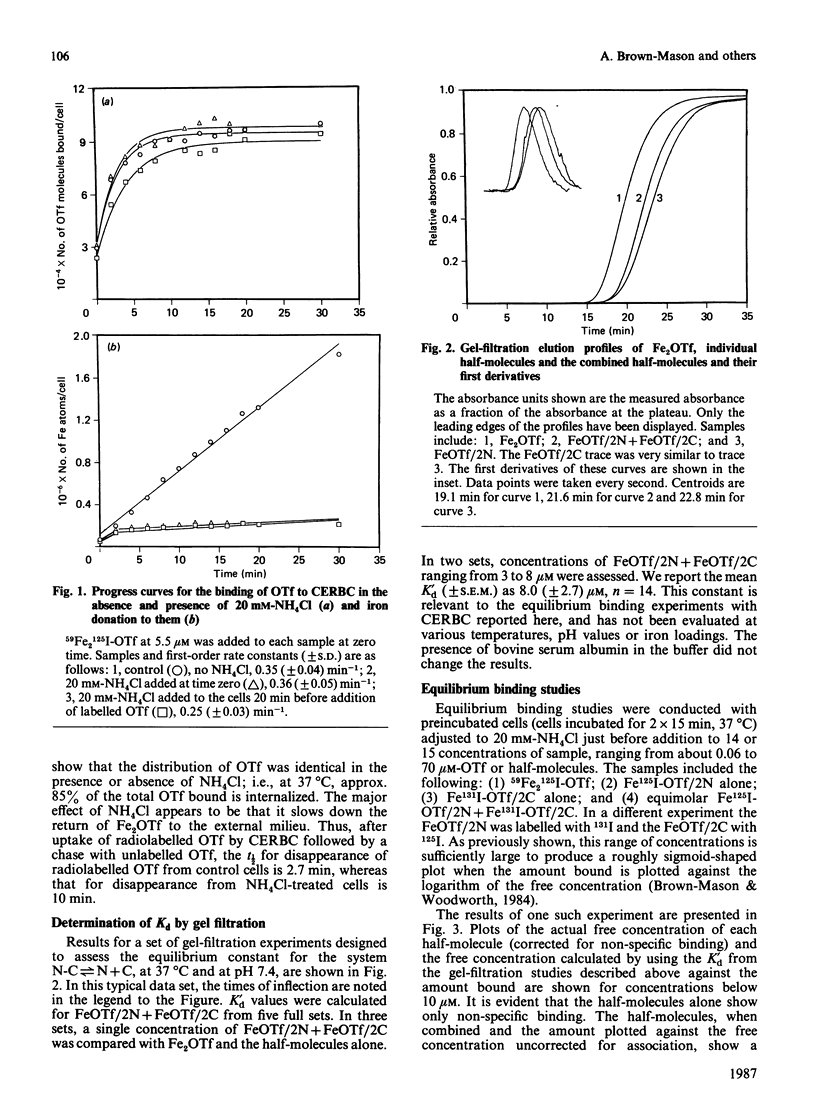
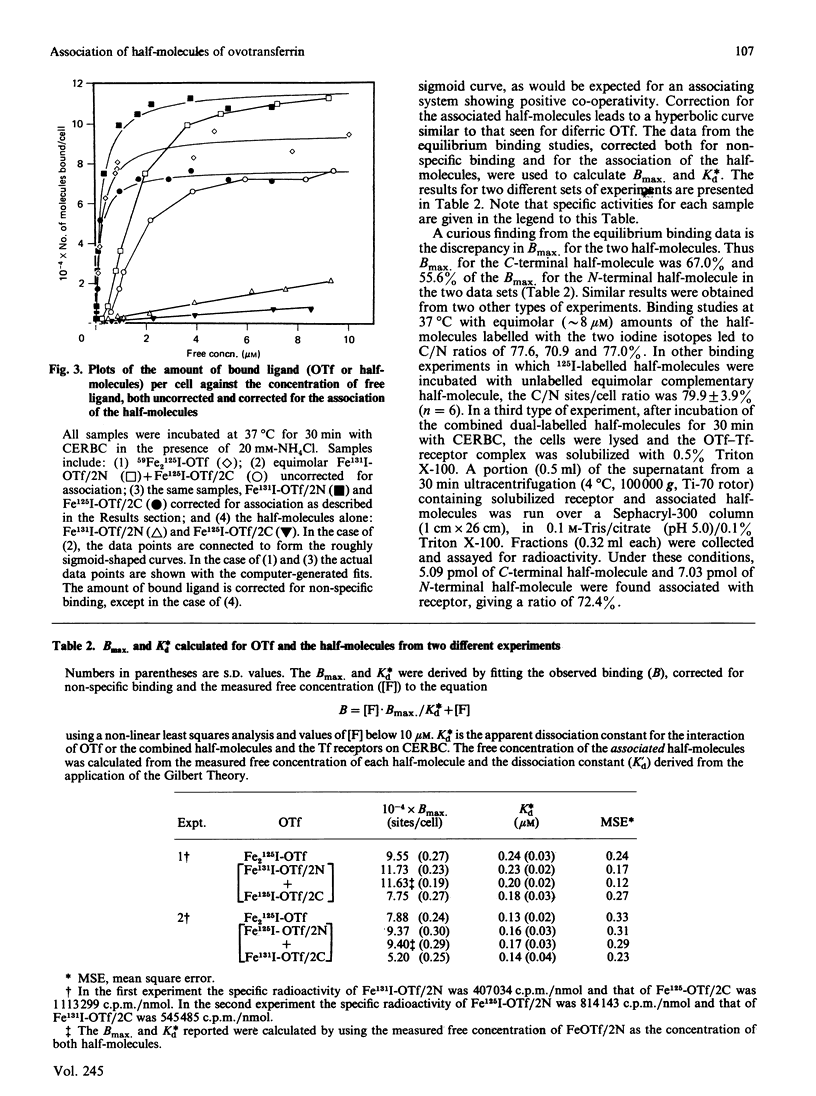
In the present paper, gel-filtration studies of diferric-ovotransferrin (Fe2OTf), the individual half-molecules of ovotransferrin (OTf) and equimolar mixtures of half-molecules have been interpreted according to the Gilbert theory as developed by Ackers & Thompson [(1965) Proc. Natl. Acad. Sci. U.S.A. 53, 342-349]. The data indicate that the half-molecules associate reversibly in solution and allow determination of a dissociation constant, Kd' = 8.0 (+/- 2.7) microM. Equilibrium binding studies have been performed using NH4Cl to block removal of iron from equimolar differentially iodine-labelled half-molecules (125I and 131I), in order to evaluate the binding of each to chick-embryo red blood cells under identical conditions. The amount of associated half-molecules over a range of concentrations has been calculated using the constant derived from the gel-filtration experiments described above. A computerized non-linear least-squares regression analysis of the data leads to determination of Kd* (the apparent dissociation constant for the interaction between OTf or half-molecules and the transferrin (Tf) receptors of chick-embryo red blood cells) and Bmax (binding at infinite free-ligand concentration) for the half-molecules similar to those found for Fe2OTf. Recent reports confirm that the two iron-binding domains of both OTf and human lactotransferrin associate non-covalently in solution. Our work shows that the isolated half-molecules of OTf are able to reassociate in solution and that this reassociation has functional significance by allowing the complex to be recognized by the Tf receptor.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K., THOMPSON T. E. DETERMINATION OF STOICHIOMETRY AND EQUILIBRIUM CONSTANTS FOR REVERSIBLY ASSOCIATING SYSTEMS BY MOLECULAR SIEVE CHROMATOGRAPHY. Proc Natl Acad Sci U S A. 1965 Feb;53:342–349. doi: 10.1073/pnas.53.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock J. H., Arzabe F. R., Richardson N. E., Deverson E. V. Characterization of monoferric fragments obtained by tryptic cleavage of bovine transferrin. Biochem J. 1978 Apr 1;171(1):73–78. doi: 10.1042/bj1710073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown-Mason A., Woodworth R. C. Physiological levels of binding and iron donation by complementary half-molecules of ovotransferrin to transferrin receptors of chick reticulocytes. J Biol Chem. 1984 Feb 10;259(3):1866–1873. [PubMed] [Google Scholar]
- Bürgisser E. Model testing in radioligand/receptor interaction by Monte Carlo simulation. J Recept Res. 1983;3(1-2):261–281. doi: 10.3109/10799898309041940. [DOI] [PubMed] [Google Scholar]
- Dautry-Varsat A., Ciechanover A., Lodish H. F. pH and the recycling of transferrin during receptor-mediated endocytosis. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2258–2262. doi: 10.1073/pnas.80.8.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney T. A., Morgan W. H., Morgan E. H. Chemical, but not functional, differences between the iron-binding sites of rabbit transferrin. Biochim Biophys Acta. 1982 Mar 4;701(3):295–304. doi: 10.1016/0167-4838(82)90232-1. [DOI] [PubMed] [Google Scholar]
- Egyed A. Availability, distribution and kinetics of the transferrin receptors of rabbit reticulocytes. Br J Haematol. 1984 Apr;56(4):563–570. doi: 10.1111/j.1365-2141.1984.tb02181.x. [DOI] [PubMed] [Google Scholar]
- GLAZER A. N., McKENZIE H. A. The denaturation of proteins. IV. Conalbumin and iron(III)-conalbumin in urea solution. Biochim Biophys Acta. 1963 Apr 2;71:109–123. doi: 10.1016/0006-3002(63)90990-9. [DOI] [PubMed] [Google Scholar]
- Harding C., Stahl P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun. 1983 Jun 15;113(2):650–658. doi: 10.1016/0006-291x(83)91776-x. [DOI] [PubMed] [Google Scholar]
- Harris D. C., Aisen P. Functional equivalence of the two iron-binding sites of human transferrin. Nature. 1975 Oct 30;257(5529):821–823. doi: 10.1038/257821a0. [DOI] [PubMed] [Google Scholar]
- Huebers H., Csiba E., Josephson B., Huebers E., Finch C. Interaction of human diferric transferrin with reticulocytes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):621–625. doi: 10.1073/pnas.78.1.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch J. M., Chambon P. The complete nucleotide sequence of the chicken ovotransferrin mRNA. Eur J Biochem. 1982 Feb;122(2):291–295. doi: 10.1111/j.1432-1033.1982.tb05879.x. [DOI] [PubMed] [Google Scholar]
- Keung W. M., Azari P. Structure and function of ovotransferrin. II. Iron-transferring activity of iron-binding fragments of ovotransferrin with chicken embryo red cells. J Biol Chem. 1982 Feb 10;257(3):1184–1188. [PubMed] [Google Scholar]
- Klausner R. D., Ashwell G., van Renswoude J., Harford J. B., Bridges K. R. Binding of apotransferrin to K562 cells: explanation of the transferrin cycle. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2263–2266. doi: 10.1073/pnas.80.8.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Van Renswoude J., Ashwell G., Kempf C., Schechter A. N., Dean A., Bridges K. R. Receptor-mediated endocytosis of transferrin in K562 cells. J Biol Chem. 1983 Apr 25;258(8):4715–4724. [PubMed] [Google Scholar]
- Lineback-Zins J., Brew K. Preparation and characterization of an NH2-terminal fragment of human serum transferrin containing a single iron-binding site. J Biol Chem. 1980 Jan 25;255(2):708–713. [PubMed] [Google Scholar]
- Martin A. W., Huebers E., Huebers H., Webb J., Finch C. A. A mono-sited transferrin from a representative deuterostome: the ascidian Pyura stolonifera (subphylum Urochordata). Blood. 1984 Nov;64(5):1047–1052. [PubMed] [Google Scholar]
- Morgan E. H. Effect of pH and iron content of transferrin on its binding to reticulocyte receptors. Biochim Biophys Acta. 1983 Jul 14;762(4):498–502. doi: 10.1016/0167-4889(83)90052-6. [DOI] [PubMed] [Google Scholar]
- Morgan E. H. Inhibition of reticulocyte iron uptake by NH4Cl and CH3NH2. Biochim Biophys Acta. 1981 Mar 20;642(1):119–134. doi: 10.1016/0005-2736(81)90143-7. [DOI] [PubMed] [Google Scholar]
- Rao K., van Renswoude J., Kempf C., Klausner R. D. Separation of Fe+3 from transferrin in endocytosis. Role of the acidic endosome. FEBS Lett. 1983 Aug 22;160(1-2):213–216. doi: 10.1016/0014-5793(83)80969-7. [DOI] [PubMed] [Google Scholar]
- Williams J., Elleman T. C., Kingston I. B., Wilkins A. G., Kuhn K. A. The primary structure of hen ovotransferrin. Eur J Biochem. 1982 Feb;122(2):297–303. doi: 10.1111/j.1432-1033.1982.tb05880.x. [DOI] [PubMed] [Google Scholar]
- Williams S. C., Woodworth R. C. The interaction of iron-conalbumin (anion) complexes with chick embryo red blood ccells. J Biol Chem. 1973 Aug 25;248(16):5848–5853. [PubMed] [Google Scholar]
- Woodworth R. C., Brown-Mason A., Christensen T. G., Witt D. P., Comeau R. D. An alternative model for the binding and release of diferric transferrin by reticulocytes. Biochemistry. 1982 Aug 31;21(18):4220–4225. doi: 10.1021/bi00261a005. [DOI] [PubMed] [Google Scholar]
- Young S. P. Evidence for the functional equivalence of the iron-binding sites of rat transferrin. Biochim Biophys Acta. 1982 Sep 17;718(1):35–41. doi: 10.1016/0304-4165(82)90006-x. [DOI] [PubMed] [Google Scholar]
- van Renswoude J., Bridges K. R., Harford J. B., Klausner R. D. Receptor-mediated endocytosis of transferrin and the uptake of fe in K562 cells: identification of a nonlysosomal acidic compartment. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6186–6190. doi: 10.1073/pnas.79.20.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]


