Abstract
Background
Leprosy causes nerve damage which may result in nerve function impairment and disability. Decompressive surgery is used for treating nerve damage, although the effect is uncertain. This is an update of a review first published in 2009 and previously updated in 2010.
Objectives
To assess the effects of decompressive surgery on nerve damage in leprosy.
Search methods
We searched the Cochrane Neuromuscular Disease Group Specialized Register (15 October 2012), CENTRAL (2012, Issue 9 in The Cochrane Library), MEDLINE (January 1966 to October 2012), EMBASE (January 1980 to October 2012), AMED (January 1985 to October 2012), CINAHL Plus (January 1937 to October 2012) and LILACS (from January 1982 to October 2012). We checked reference lists of the studies identified, the Current Controlled Trials Register (www.controlled‐trials.com) (1 November 2012), conference proceedings and contacted trial authors.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs of decompressive surgery for nerve damage in leprosy.
Data collection and analysis
The primary outcome was improvement in sensory and motor nerve function after one year. Secondary outcomes were improvement in nerve function after two years, change in nerve pain and tenderness, and adverse events. Two authors independently extracted data and assessed trial quality. We contacted trial authors for additional information. We collected adverse effects information from the trials and non‐randomised studies.
Main results
We included two RCTs involving 88 participants. The trials were at high risk of bias. The trials examined the added benefit of surgery over prednisolone for treatment of nerve damage of less than six months duration. After two years' follow‐up there was only very low quality evidence of no significant difference in nerve function improvement between participants treated with surgery plus prednisolone or with prednisolone alone. Adverse effects of decompressive surgery were not adequately described.
Authors' conclusions
Decompressive surgery is used for treating nerve damage in leprosy but the available evidence from RCTs is of very low quality and does not show a significant added benefit of surgery over steroid treatment alone. Well‐designed RCTs are needed to establish the effectiveness of the combination of surgery and medical treatment compared to medical treatment alone.
Keywords: Humans; Administration, Oral; Combined Modality Therapy; Combined Modality Therapy/methods; Decompression, Surgical; Decompression, Surgical/methods; Glucocorticoids; Glucocorticoids/administration & dosage; Leprosy; Leprosy/complications; Peripheral Nerve Injuries; Peripheral Nerve Injuries/drug therapy; Peripheral Nerve Injuries/surgery; Prednisolone; Prednisolone/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Decompressive surgery for treating nerve damage in leprosy
Leprosy is a chronic infectious disease. Leprosy bacteria cause damage to skin and peripheral nerves which may result in nerve function impairment and disability. Decompressive surgery is used for treating nerve damage although its effect is uncertain. Two randomised controlled trials (RCTs) were included in the review and examined the added benefit of surgery over prednisolone for treatment of nerve damage of less than six months duration. Both trials were at high risk of bias. Two years from the start there was very low quality evidence of no significant difference in nerve function improvement between people treated with surgery plus prednisolone or with prednisolone alone. Adverse effects of decompressive surgery were not adequately described. No additional trials were identified when searches were updated in 2010 and 2012. Decompressive surgery is used for treating nerve damage in leprosy but the available evidence from RCTs is of very low quality and does not show a significant added benefit of surgery over steroid treatment alone. Well‐designed RCTs are needed to establish the effectiveness of the combination of surgery and medical treatment compared to medical treatment alone.
Summary of findings
Summary of findings for the main comparison. Medial epicondylectomy plus oral steroids versus oral steroids alone for treating nerve damage in leprosy.
| Medial epicondylectomy plus oral steroids versus oral steroids alone for treating nerve damage in leprosy | ||||||
| Patient or population: patients with nerve damage in leprosy Settings: hospital Intervention: medial epicondylectomy plus oral steroids versus oral steroids alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | medial epicondylectomy plus oral steroids versus oral steroids alone | |||||
| Change in sensory score after one year | The mean change in sensory score after one year ranged across control groups from 0.06‐3.94 points | The mean change in sensory score after one year in the intervention groups was 0.08 higher (2.45 lower to 2.61 higher) | 57 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| Proportion of ulnar nerves with sensory improvement after one year | 516 per 1000 | 578 per 1000 (366 to 913) | RR 1.12 (0.71 to 1.77) | 62 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |
| Change in motor score after one year | The mean change in motor score after one year ranged across control groups from 0.21‐4.31 points | The mean change in motor score after one year in the intervention groups was 0.82 higher (1.34 lower to 2.98 higher) | 57 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| Proportion of ulnar nerves with motor improvement after one year | 710 per 1000 | 646 per 1000 (454 to 909) | RR 0.91 (0.64 to 1.28) | 62 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | |
| Change in sensory score after two years | The mean change in sensory score after two years ranged across control groups from 0.73‐5.09 points | The mean change in sensory score after two years in the intervention groups was 0.02 lower (2.82 lower to 2.78 higher) | 57 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| Change in motor score after two years | The mean change in motor score after two years ranged across control groups from 0.49‐4.65 points | The mean change in motor score after two years in the intervention groups was 0.22 higher (2.39 lower to 2.83 higher) | 57 (1 study) | ⊕⊝⊝⊝ very low1,2,3 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Randomisation by alternation. Blinding of patients and clinicians not possible; blinding of outcome assessor not reported. 17% loss to follow‐up of nerves; no intention to treat analysis was performed. 2 Data from only one trial available. 3 No separate analysis was done using only one independent outcome from each patient (results from a patient contributing outcomes from more than one nerve will be treated, in the analysis, as having more weight as a patient contributing only one nerve).
Background
Description of the condition
Leprosy is a chronic infectious disease caused by the bacillus Mycobacterium leprae. Leprosy bacilli are spread in tiny droplets from the nose or mouth from infected and untreated individuals. When the immune system fails to respond effectively to the antigens of the bacilli, leprosy will develop. Leprosy bacilli may directly or indirectly cause damage. Often, the first sign of leprosy is a skin lesion. Damage to peripheral nerves may cause symptoms such as loss of sweating, sensation and muscle strength. Leprosy appears in various clinical forms, dependent on the response of the immune system. Some people have only a few skin lesions and the number of bacilli is relatively small. This is classified as paucibacillary (PB) leprosy. Other people may have many skin lesions with multiple nerves involved and a high number of bacilli in their body and are then classified as having multibacillary (MB) leprosy (ILEP 2001; WHO 2006).
Leprosy can be effectively treated with a combination of antibiotics (rifampicin, dapsone and clofazimine). Since the introduction of this multidrug therapy (MDT), the number of people with leprosy has decreased substantially. At the beginning of 2007 the reported prevalence was about 225,000 individuals worldwide. This is the registered number of people on MDT treatment. The number of newly detected people reported was approximately 259,000 in 2006 (WHO 2007).
Causes
The body's immune response to the antigens of the leprosy bacilli may cause periods of inflammation in the skin and peripheral nerves and sometimes also in other organs: so‐called 'reactions'. There are two types of potentially nerve damaging reactions: a type 1 or reversal reaction (RR) and a type 2 reaction or erythema nodosum leprosum (ENL). Reactions may occur before, during and after MDT and are the main cause of nerve damage and consequently of impairment in leprosy (ILEP 2002; Lockwood 2005; WHO 1998). Nerve damage may develop slowly and is often unnoticed until very late. It is often the symptoms of a reaction that force people to seek medical help (Job 1989; Nicholls 2003).
Impact
Leprosy is a most important disabling disease. The World Health Organization estimated the number of people living with physical disabilities due to leprosy at two to three million worldwide (WHO 2004) in spite of the low official prevalence figure. Despite a fast declining trend in the number of newly detected people with leprosy, the decline in the number of people living with physical disabilities is much slower. In the near future, we can still expect about one million people to be affected by leprosy disability (Meima 2008). People affected by leprosy, especially those with visible deformities and disabilities, fear discrimination and stigmatisation. These people may experience severe social and psychological problems (Heijnders 2004; Leekassa 2004; Rafferty 2005).
Treatment
Corticosteroids, especially prednisolone, are used as first‐line treatment of severe reversal reactions and nerve damage in leprosy. They work by controlling the acute inflammation and relieving the pain (Britton 1998; Lockwood 2000). The earlier corticosteroids are given after the onset of nerve damage, the more likely permanent nerve function impairment will be prevented (Becx‐Bleumink 1990; Naafs 1996). Prednisolone seems to be a very effective drug, but it has some shortcomings. Long‐term therapy may cause serious adverse effects, such as peptic ulcer, cataract or psychosis (Richardus 2003; Sugumaran 1998; WHO 1998). A considerable proportion of people treated for severe reversal reactions and nerve damage does not benefit from standard corticosteroid treatment (Croft 2000; Lockwood 1993; Saunderson 2000; Schreuder 1998). The long‐term benefit of corticosteroids for treating nerve damage is still uncertain and trials establishing the optimal regimen and effectiveness are needed (Van Veen 2007).
Other immunosuppressant drugs for treating severe reversal reactions and nerve damage have been tested or are under examination, such as azathioprine (Marlowe 2004) and ciclosporin (Lockwood 2000; Sena 2006). It is plausible that these drugs may be effective for treating nerve damage, but evidence from randomised controlled trials (RCTs) is very limited.
Decompressive surgery or neurolysis as treatment for nerve damage has been used for several decades. The objective of this surgery is to relieve mechanical compression, due to oedema caused by neuritis, of the affected nerve. Decompression is done by incision of the thickened nerve sheath (epineurium) where the nerve is enlarged and often tender on palpation. This incision is often of a considerable length at the place before entering the fibro‐osseous tunnel which, during surgery, also needs to be opened. Results from non‐randomised studies of surgery have been widely published (e.g. Brandsma 1983; Carayon 1993; Chaise 1985; Dandapat 1991; Droogenbroeck 1977; Ramarorazana 1995; Rao 1989), but there is little evidence from RCTs that surgery is better than medical treatment alone (Boucher 1999; Ebenezer 1996; Pannikar 1984). Decompressive surgery is not recommended without medical treatment. Indications for surgery are mainly based on common practice but are not well‐defined. These may include the presence of nerve abscess, nerve pain or nerve function impairment that does not respond to medical treatment (Chaise 2004; Kazen 1996; Malaviya 2004b; Palande 1980; Richard 2004).
Why it is important to do this review
Decompressive surgery is frequently used for treating nerve damage in leprosy. The effect of surgery, especially in the long term, is uncertain and it is unclear whether surgery is more beneficial than medical treatment alone. While this review focused on evidence from RCTs, it was expected that only a few RCTs would have been conducted in this area. Therefore, the results were also considered in the light of non‐randomised evidence in the Discussion section.
This review was first published in 2009 and updated in 2010 and 2012.
Objectives
To assess the effects of decompressive surgery for treating nerve damage in leprosy.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs of any design.
Types of participants
Anyone with leprosy confirmed by appropriate clinical signs or symptoms according to Ridley 1966 or WHO 1998 classification and leprosy‐related nerve damage or severe leprosy type 1 reaction, requiring corticosteroid treatment. Nerve damage or nerve function impairment was defined as clinically detectable impairment of motor or sensory nerve function. It did not include impairment of nerve conduction that was only detectable by electrophysiological means (Croft 1999).
Types of interventions
Decompressive surgery or neurolysis for treating nerve damage in leprosy.
The comparators were no treatment, placebo or corticosteroids.
Types of outcome measures
Primary outcomes
1. Improvement in sensory nerve function one year after registration, as determined and defined by the original authors.
Sensory nerve function was assessed with five graded nylon filaments or a ball‐point pen. We adapted the scores as defined by Van Brakel et al (Van Brakel 2005). For testing with graded nylon filaments, sensory nerve function impairment was diagnosed if the monofilament threshold was increased from normal by three or more points for any nerve. One point was given for each level that the monofilament threshold was increased from normal at each test site. The points were added for each nerve. Normal thresholds used were 200 mg for the hand and 2 g for the foot. If the score for any nerve decreased by three or more points from the baseline score, the nerve was considered as improved. When a non‐graded test was used, such as the ball‐point pen test, a nerve was diagnosed as impaired if two or more test sites did not feel the stimulus. Improvement for any nerve was defined as two or more test sites feeling the stimulus, compared to the baseline measurement (Van Brakel 2003). Improvement was a dichotomous outcome variable (improvement or not).
2. Improvement in motor nerve function one year after registration, as determined and defined by the original authors.
Improvement in motor nerve function was assessed with the modified MRC grading scale (Brandsma 1981). Motor nerve function impairment was defined as a score of less than four on the modified MRC grading scale for any nerve. Improvement was defined as at least one point improvement in score for any muscle compared to the initial score. Improvement was a dichotomous outcome variable (improvement or not).
Secondary outcomes
1. Improvement in nerve function two years after registration, as determined and defined by the original authors. Improvement was a dichotomous outcome variable (improvement or not).
2. Change in nerve pain and in nerve tenderness one year after registration, as determined and defined by the original authors or according to Pearson's Scale (Pearson 1982). Improvement was a dichotomous outcome variable (improvement or not).
3. Changes in quality of life as assessed using a recognised instrument (generic, disease specific or patient‐generated index).
4. Adverse outcomes We documented the incidence and severity of all recorded local and systemic adverse events, at any time point, in all the included studies.
Timing of outcome assessment
Data that have been recorded for less than six months were considered to reflect short‐term benefit and were analysed separately from data that were recorded after one year or more, which we considered to reflect the minimum time period to capture any long‐term benefit. The end point closest to three months (one to six months) was used for short‐term benefit and the end point closest to two years (± one year) was used for long‐term benefit. The long‐term data were considered the primary endpoint but we considered the short‐term data in order to detect rapid onset of improvement.
Economic data
We did not report data relating to costs but we addressed cost implications in the Discussion section if information was available.
Search methods for identification of studies
Electronic searches
We searched for relevant published trials in the Cochrane Neuromuscular Disease Group Specialized Register (15 October 2012), Central (2012, Issue 9 in The Cochrane Library), MEDLINE (January 1966 to October 2012), EMBASE (January 1980 to October 2012), AMED (Allied and Complementary Medicine, January 1985 to October 2012), CINAHL Plus (January 1982 to October 2012), and LILACS (Latin American and Caribbean Health Science Information database, January 1982 to October 2012). The detailed search strategies are in the appendices: MEDLINE Appendix 1, EMBASE Appendix 2, AMED Appendix 3, LILACS Appendix 4, CINAHL Plus Appendix 5 and CENTRAL Appendix 6. We searched for ongoing trials in the metaRegister of Controlled Trials (www.controlled‐trials.com) (1 November 2012) using the search term Leprosy.
Searching other resources
Reference lists
We scanned the bibliographies of the included studies and reviews for possible references to RCTs.
Unpublished literature
We attempted to find unpublished or ongoing trials via correspondence with trial authors of included and excluded trials less than 15 years old and other disease experts.
Handsearching
Conference proceedings from relevant leprosy meetings were scanned for RCTs and, where possible, the authors were contacted for further information.
Adverse effects
We did not perform a separate search for adverse events.
Language restrictions
No language restrictions were imposed when searching for publications, and translations were sought where necessary.
Data collection and analysis
Selection of studies
Two review authors (NvV and JHR) checked the titles and abstracts identified from the searches. If it was clear that the study did not refer to a RCT of surgical decompression for treating nerve damage in leprosy, we excluded it. The same two review authors independently assessed the full text version of each remaining study to determine whether it met the pre‐defined selection criteria. Any differences of opinion were resolved through discussion within the review team. We listed the excluded studies and reasons for exclusion in the 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (NvV and JHR) independently extracted data from the included studies onto a data extraction form. If there were missing data, we contacted the trial authors. We entered data into Review Manager (RevMan) (RevMan 2008). Authors were not blinded to trial author, journal or institution.
We were not able to translate reported changes in nerve function and nerve pain into the proportion of participants with improvement greater than minimal. By minimal we meant anything greater than 50% improvement from baseline on a continuous scale for sensory nerve function or a score of four or more on the MRC grading scale for motor nerve function. It was not possible to calculate the proportion of participants with full recovery of nerve function.
Assessment of risk of bias in included studies
The 'Risk of bias' assessment (Higgins 2011) included an evaluation of the following components for each included study:
the method of generation of the randomisation sequence;
the method of allocation concealment ‐ considered 'adequate' if the assignment could not be foreseen;
whether participants, personnel and outcome assessors were blinded;
selective outcome reporting;
incomplete outcome data: how many participants were lost to follow‐up in each arm, whether reasons for losses were adequately reported and whether all participants were analysed in the groups to which they were originally randomised (intention‐to‐treat principle);
other sources of bias.
In addition we reported on:
diagnostic criteria;
the baseline assessment of the participants for age, sex, duration and severity of nerve function impairment;
whether outcome measures were described.
We described our assessments, based on the 'Risk of bias' components, in the section on Risk of bias in included studies. We each graded the risk of bias in included studies as high, low or unclear. We used 'Unclear risk of bias' when the risk of bias was unknown or the entry was not relevant to the study. We included a 'Risk of bias' summary figure.
Measures of treatment effect
We used the Cochrane statistical package, RevMan (RevMan 2008) for statistical data analysis. None of the study results could be pooled, meaning that no weighted treatment effect could be calculated. Results were expressed as mean differences with 95% confidence intervals (CI) for continuous outcome measures and risk ratios (RR) with 95% CI for dichotomous outcomes. One trial reported median improvement as outcome. It was not possible to perform tests for heterogeneity or sensitivity analysis due to insufficient trials. We will perform such analyses should trials become available in the future.
Dealing with missing data
We were not able to conduct an intention‐to‐treat analysis. By contacting the authors we learned that information about the groups to which lost participants were randomised was no longer available.
Adverse outcomes
In our Discussion, we considered adverse effects by taking non‐randomised literature into account, since randomised studies rarely capture adverse events adequately.
Economic issues
We were not able to consider the costs and cost‐effectiveness of treatment due to lack of evidence.
Results
Description of studies
The number of papers found by the search strategies in the appendices (run on 15 October 2012) are Cochrane Neuromuscular Disease Group Specialized Register 3 (0 new papers); CENTRAL 5 (0 new papers); MEDLINE 14 (0 new papers); EMBASE 8 (3 new papers); AMED 0 papers; LILACS 0 papers; CINAHL Plus 1 (0 new papers).
A search of the Current Controlled Trials Register (which includes clinicaltrials.gov) retrieved one trial which was not surgical.
We identified nine potentially relevant studies and excluded seven, because they were not randomised. For a description of excluded trials see the 'Characteristics of excluded studies' table.
Two RCTs involving 88 people, were included. One trial was described in two papers: the first paper gave results after one year (Pannikar 1984) and the other paper described results after a follow‐up of two years (Ebenezer 1996). Both RCTs tested decompression surgery plus oral corticosteroids versus oral corticosteroids alone. One tested treatment of ulnar neuritis of less than six months duration (Ebenezer 1996; Pannikar 1984) and one tested treatment of neuritis of several types of less than six months duration (Boucher 1999).
For a full description of included trials, see the 'Characteristics of included studies' table.
Risk of bias in included studies
A summary of review authors' judgements about each 'Risk of bias' item for included studies is shown in Figure 1.
1.
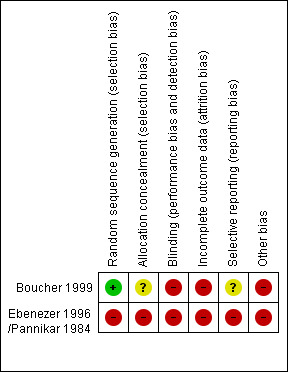
Randomisation and allocation concealment
In one trial (Boucher 1999) participants were randomly assigned to either intervention or control group and the allocation concealment was considered adequate. The other trial (Ebenezer 1996; Pannikar 1984) used alternation as the randomisation procedure which was considered inadequate.
Blinding
Participant and clinician blinding was not possible in any of the trials. Outcome assessor blinding was not reported.
Selective reporting
In neither trial was the reporting of adverse events adequate.
Incomplete outcome data
One trial (Boucher 1999) had 6% loss to follow‐up of participants, but did not report how many nerves were involved. The other trial (Ebenezer 1996; Pannikar 1984) had 17% loss to follow‐up of nerves after one year and 24% loss to follow‐up of nerves after two years. None of the trials reported how many participants or nerves were lost to follow‐up in each treatment arm. Boucher et al described the reasons for losses.
Other bias
In neither trial was there a separate analysis using only one independent outcome from each patient.
Diagnostic criteria
Both trials diagnosed and classified leprosy using the internationally accepted diagnostic criteria of Ridley and Jopling (Ridley 1966).
Baseline differences
In the trials the baseline characteristics in both arms were similar.
Explicit outcomes
The primary outcomes 'improvement in sensory nerve function one year after registration' and 'improvement in motor nerve function one year after registration' were evaluated in one trial (Pannikar 1984). The secondary outcome 'improvement in nerve function two years after registration' was evaluated in two trials (Boucher 1999; Ebenezer 1996). 'Change in nerve pain and in nerve tenderness' was assessed in one trial (Pannikar 1984) one year after registration and in two trials (Boucher 1999; Ebenezer 1996) two years after registration. None of the trials evaluated 'changes in quality of life'. Adverse events were not well‐reported in any of the trials.
Effects of interventions
See: Table 1
Medial epicondylectomy and nerve decompression plus oral corticosteroids versus oral corticosteroids alone for participants with ulnar neuritis of less than six months duration (Pannikar 1984; Ebenezer 1996)
Primary outcome measures
Improvement in sensory nerve function one year after registration
The trial compared oral prednisolone plus medial epicondylectomy and external nerve decompression (surgery group) with oral prednisolone alone (medical group) in participants with ulnar neuritis of less than six months duration (n = 57 participants with 75 nerves). One year after admission results of sensory nerve function were available for 31 nerves in the surgery group and 31 nerves in the medical group. Improvement was measured as either a mean change score between baseline and end of follow‐up or the proportion of nerves with improvement. Sensory testing was done with a No.3 and No.6 nylon thread (approximately 200 mg and 5 g, respectively). Fifteen sites on the ulnar nerve distribution area were tested. The score for each nerve depended on the number of sites felt. The score was 15 when all sites were felt with the 200 mg thread, and zero when no site was felt with either thread. Sensory improvement was defined as a positive difference between the final and initial score. Mean differences between the baseline score and the score at the end of one year were compared for the two treatment groups. Results were available for 29 nerves in the surgery group and 28 nerves in the medical group. After one year the mean difference was 2.08 (95% CI 0.28 to 3.88) in the surgery group and 2.00 (95% CI 0.06 to 3.94) in the medical group, indicating a mean improvement in both. The improvement was slightly greater in the surgery group but the mean difference 0.08 (95% CI ‐2.45 to 2.61) between the two groups was not significant (Figure 2; Analysis 1.1). In the surgery group 18 out of 31 nerves (58%) had sensory improvement compared with 16 out of 31 nerves (52%) in the medical group. The difference was not significant (RR 1.13, 95% CI 0.71 to 1.77) (Figure 3; Analysis 1.2).
2.
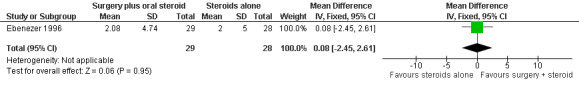
Forest plot of comparison: 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, outcome: 1.1 Change in sensory score after one year.
1.1. Analysis.
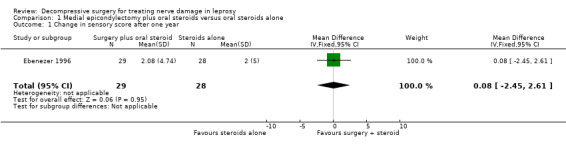
Comparison 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, Outcome 1 Change in sensory score after one year.
3.
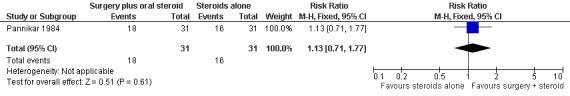
Forest plot of comparison: 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, outcome: 1.2 Proportion of ulnar nerves with sensory improvement after one year.
1.2. Analysis.
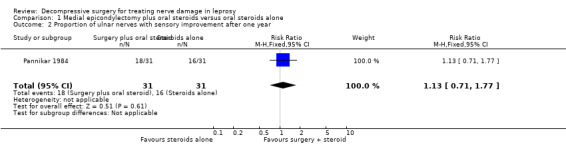
Comparison 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, Outcome 2 Proportion of ulnar nerves with sensory improvement after one year.
Improvement in motor nerve function one year after registration
The trial measured motor improvement of the ulnar nerve at one year from the time of admission (n = 57 participants with 75 nerves). Results of motor nerve function were available for 31 nerves in the surgery group and 31 nerves in the medical group. Improvement was measured as either a change score between baseline and end of follow‐up or as the proportion of nerves improved. Motor nerve function of the ulnar nerve was assessed with the MRC grading scale. The maximum score for each muscle was five and for the whole nerve 15. Motor improvement was defined as a positive difference between the final and initial score. Mean differences between the baseline score and the score at the end of one year were compared for the two treatment groups. Results were available for 29 nerves in the surgery group and 28 nerves in the medical group. After one year the mean difference was 3.08 (95% CI 2.12 to 4.04) in the surgery group and 2.26 (95% CI 0.21 to 4.31) in the medical group indicating a mean improvement in both. The improvement was greater in the surgery group but the mean difference 0.82 (95% CI ‐1.34 to 2.98) between the two groups was not significant (Figure 4; Analysis 1.3). In the surgery group 20 out of 31 nerves (65%) had motor improvement compared with 22 out of 31 nerves (71%) in the medical group. The difference was not significant (RR 0.91, 95% CI 0.64 to 1.28) (Figure 5; Analysis 1.4).
4.
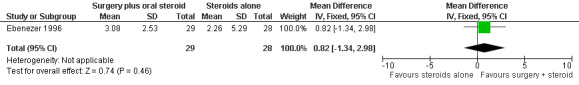
Forest plot of comparison: 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, outcome: 1.3 Change in motor score after one year.
1.3. Analysis.
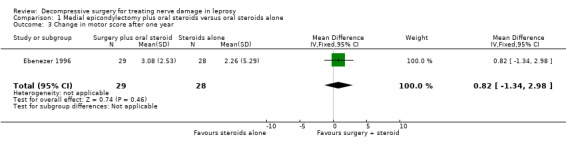
Comparison 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, Outcome 3 Change in motor score after one year.
5.
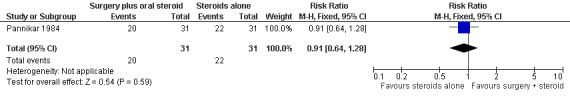
Forest plot of comparison: 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, outcome: 1.4 Proportion of ulnar nerves with motor improvement after one year.
1.4. Analysis.
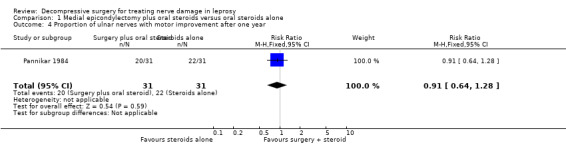
Comparison 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, Outcome 4 Proportion of ulnar nerves with motor improvement after one year.
Secondary outcome measures
Improvement in nerve function two years after registration
The trial measured nerve function improvement of the ulnar nerve two years after admission (n = 57 participants with 75 nerves). Results were available for 29 nerves in the surgery group and 28 nerves in the medical group. Improvement was measured as a change score between baseline and end of follow‐up. Mean differences between the baseline score and the score at the end of two years were compared for the two treatment groups. After two years the mean difference in sensory score was 2.89 (95% CI 0.94 to 4.84) in the surgery group and 2.91 (95% CI 0.73 to 5.09) in the medical group indicating a mean improvement in both. The improvement was slightly greater in the medical group but the mean difference ‐0.02 (95% CI ‐2.82 to 2.78) between the two groups was not significant (Figure 6; Analysis 1.5). The mean difference in motor score after two years was 2.79 (95% CI 1.03 to 4.55) in the surgery group and 2.57 (95% CI 0.49 to 4.65) in the medical group indicating a mean improvement in both. The improvement was greater in the surgery group but the mean difference 0.22 (95% CI ‐2.39 to 2.83) between the two groups was not significant (Figure 7; Analysis 1.6).
6.
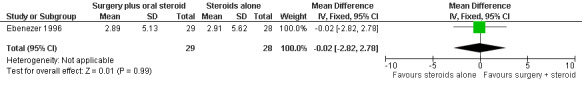
Forest plot of comparison: 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, outcome: 1.5 Change in sensory score after two years.
1.5. Analysis.
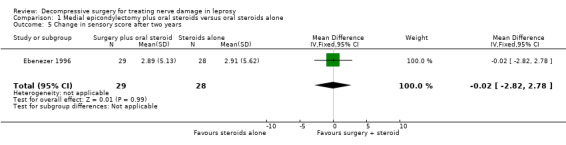
Comparison 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, Outcome 5 Change in sensory score after two years.
7.
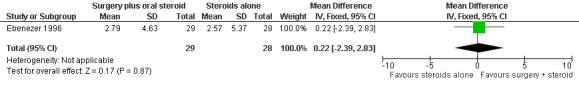
Forest plot of comparison: 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, outcome: 1.6 Change in motor score after two years.
1.6. Analysis.
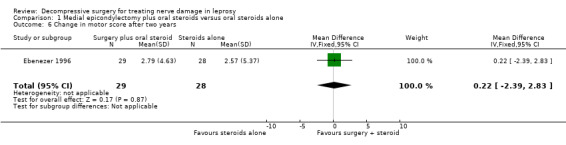
Comparison 1 Medial epicondylectomy plus oral steroids versus oral steroids alone, Outcome 6 Change in motor score after two years.
Change in nerve pain and in nerve tenderness one year after registration
Pannikar et al evaluated nerve pain and tenderness one year after registration using the scale as defined by Pearson (Pearson 1982). At the end of one year nerve pain and tenderness had disappeared in both groups. Ebenezer et al reported no new nerve pain or tenderness between the first and second year.
Changes in quality of life
The trial did not evaluate changes in quality of life.
Occurrence of adverse events
The trial did not report any adverse events or reasons of loss to follow‐up. Contacting the authors did not yield additional information.
Longitudinal epineurotomy and nerve decompression plus oral corticosteroids versus oral corticosteroids alone for participants with neuritis of less than six months duration (Boucher 1999)
Primary outcome measures
Improvement in sensory nerve function one year after registration
The trial did not report results one year after treatment.
Improvement in motor nerve function one year after registration
The trial did not report results one year after treatment.
Secondary outcome measures
Improvement in nerve function two years after registration
The trial compared oral prednisolone plus epineurotomy and external nerve decompression (surgery group) with oral prednisolone alone (medical group) in participants with neuritis of less than six months duration (n = 31 participants). The trial measured sensory nerve function improvement of ulnar, median and posterior tibial nerves and motor nerve function improvement of ulnar, median and common peroneal nerves two years after treatment. Results were available for 46 nerves in the surgery group and 47 nerves in the medical group. Improvement was measured as a change score between baseline and end of follow‐up and converted into median improvement. For example, a median improvement of 25% means that 50% of the data had greater than 25% improvement and 50% of the data had less than 25% improvement. Sensory testing was done with five graded Semmes‐Weinstein monofilaments (50 mg, 200 mg, 2 g, 4 g, 10 g) and according to a protocol described by Pearson (Pearson 1982). Sensory improvement was defined as a positive difference between the final and initial score. Outcomes were expressed as median improvement, meaning that 50% of the data had greater improvement than this value and 50% of the data had less improvement than this median. In the surgery group median sensory improvement was 25% compared to 20% median improvement in the medical group. The difference was not significant at a 5% level (Tukey box plot test) (Analysis 2.1). Motor nerve function was assessed with the MRC grading scale. The maximum score for one nerve was 10 points. Motor improvement was defined as a positive difference between the final and initial score. Median motor improvement was 30% in the surgery group and 20% in the medical group. The difference was not significant at a 5% level (Tukey box plot test) (Analysis 2.2). No numbers, test values or 95% CI values were given. Contacting the author revealed that original data were no longer available.
2.1. Analysis.
Comparison 2 Longitudinal epineurotomy plus oral steroids versus oral steroids alone, Outcome 1 Median sensory improvement after two years.
| Median sensory improvement after two years | ||||
|---|---|---|---|---|
| Study | Surgery plus oral steroid group | Steroid alone group | Test | Outcome |
| Boucher 1999 | 25% median improvement | 20% median improvement | Tukey box plot test | No significant difference at 5% level |
2.2. Analysis.
Comparison 2 Longitudinal epineurotomy plus oral steroids versus oral steroids alone, Outcome 2 Median motor improvement after two years.
| Median motor improvement after two years | ||||
|---|---|---|---|---|
| Study | Surgery plus oral steroid group | Steroid alone group | Test | Outcome |
| Boucher 1999 | 30% median improvement | 20% median improvement | Tukey box plot test | No significant difference at 5% level |
Change in nerve pain and in nerve tenderness one year after registration
Boucher et al evaluated nerve pain and tenderness two years after registration using the scale as defined by Pearson (Pearson 1982). In the surgery group median nerve pain relief was 11% compared to 0% in the medical group. The difference was significant at a 5% level (Tukey box plot test) (Analysis 2.3). No numbers, test values or 95% CI values were given. Contacting the author revealed that original data were no longer available.
2.3. Analysis.
Comparison 2 Longitudinal epineurotomy plus oral steroids versus oral steroids alone, Outcome 3 Median improvement in nerve pain and tenderness after two years.
| Median improvement in nerve pain and tenderness after two years | ||||
|---|---|---|---|---|
| Study | Surgery plus oral steroid group | Steroid alone group | Test | Outcome |
| Boucher 1999 | 11% median improvement | 0% median improvement | Tukey box plot test | Significant difference at 5% level |
Changes in quality of life
The trial did not evaluate changes in quality of life.
Occurrence of adverse events
One participant was excluded from the study due to haemorrhage, but it was unclear if it was caused by the intervention.
Discussion
Summary of main results
Decompressive surgery is frequently used in the management of nerve damage in leprosy, but evidence from RCTs for the effect of decompressive surgery is scarce.
Overall completeness and applicability and quality of the evidence
Two RCTs were available for this review. One trial compared the added benefit of medial epicondylectomy over corticosteroids for participants with ulnar neuritis of less than six months duration (Ebenezer 1996; Pannikar 1984). The other trial compared the added benefit of longitudinal epineurotomy over corticosteroids for participants with ulnar, median, common peroneal or posterior tibial nerve involvement of less than six months duration (Boucher 1999). The interventions and outcomes were too heterogeneous to be combined in a meta‐analysis. The numbers of participants included in the trials were small and did not allow for subgroup analysis. The variability between studies and the limitations in study design and sample size made it difficult to draw any robust conclusions.
None of the trials found a significant difference in improved nerve function between surgery and medical groups after a follow‐up of one or two years. This result may have been biased by the selection criteria used for inclusion of patients and nerves. Only a small proportion (about 10%) may benefit from decompressive surgery and show improvement after surgery (Naafs 2008). The other nerves need no decompression. By taking all nerves together, results may be diluted and the conclusion clouded.
The two trials had some drawbacks. One major drawback of both trials was that they sometimes used more than one nerve from individual patients in the analyses thereby considering the outcomes from each nerve independently. The trial of Pannikar and Ebenezer included 18 patients with ulnar nerve damage on both sides (bilateral involvement). The right side was allocated to the group drawn by random selection and the left side was allocated to the other group. The final results reflect the outcomes of all nerves. No separate analysis was done using only one independent outcome from each patient. Original data were not available. Boucher 1999 included 31 patients with 93 nerves in total. It is unclear how many nerves each patient contributed. The final results reflect the outcomes of all nerves. No separate analysis was done using only one independent outcome from each patient. Original data were not available. The results from these studies should be treated with considerable caution, because results from a patient contributing outcomes from more than one nerve will be treated, in the analysis, as having more weight as a patient contributing only one nerve.
Other limitations of the Pannikar study were that randomisation was done by alternation, which is considered an inadequate randomisation procedure. With regard to loss to follow‐up, 23% of the participants were lost to follow‐up after one year and 32% after two years. No reasons for these losses were reported and no intention‐to‐treat analysis was performed.
The randomisation procedure and loss to follow‐up (6%) were considered adequate in Boucher 1999. Outcomes were expressed as median improvement. No numbers or original data were available to calculate mean differences or RRs making comparison and interpretation of the results difficult. Subgroup analyses showed no difference in median improvement between operated or non‐operated nerves with respect to type of leprosy (lepromatous or non‐lepromatous), type of antibacillary drug therapy (mono or multi), type of nerve function impairment (motor or sensory), and duration of neuritis (zero to three months or three to six months). There were significant differences for pain relief and severity of the neuritis before surgery. Operated nerves had higher median pain relief compared to non‐operated nerves. In the group with considerable loss of nerve function the operated nerves had higher median improvement compared to non‐operated nerves.
The occurrence of adverse effects was not adequately reported in the trials. One study (Boucher 1999) excluded a participant with haemorrhage during the course of the trial, but it was unclear whether this was due to the intervention. The literature reviewing decompressive surgery in leprosy often does not take adverse effects into account, but stresses the importance of having adequate techniques and instruments and competent surgeons to prevent unfavourable outcomes (Bernardin 1997; Bourrel 1992; Richard 2004). Complications of decompressive surgery in general may be painful scars, wound problems, haematoma, infection and damage to nerves, arteries or tendons (Malaviya 2004a; Scholten 2007; Thoma 2004).
None of the trials included quality of life measures or cost‐effectiveness calculations which could be useful indicators of the effectiveness of interventions.
Potential biases in the review process
The search process was elaborate and to our knowledge no other RCTs were available for this review.
Agreements and disagreements with other studies or reviews
Many published and unpublished non‐randomised studies have examined the effect of decompressive surgery for treating nerve damage in leprosy. While the two RCTs give insufficient evidence in favour of decompressive surgery in addition to steroid treatment, most non‐randomised studies report beneficial effects of decompressive surgery. Relief of nerve pain and tenderness is the most frequently and consistently reported benefit. Nerve function improvement is frequently reported, but the response to surgery seems to depend on several factors, such as severity and duration of neuritis before surgery, the type of leprosy, the nerve involved and the surgical technique used. Nerves which are partially damaged, have neuritis of less than six months duration and are associated with multibacillary (MB) leprosy show better results (Chaise 2004; Kazen 1996; Malaviya 2004b; Palande 1980; Pandya 1983). Studies examining the effects of surgery reported sensory improvement varying from about 38% to 97% and motor improvement varying from about 26% to 63% (Antia 1976; Bernardin 1997; Brandsma 1983; Chaise 1982; Chaise 1985; Chaise 1987; Husain 1997; Husain 1998; Kumar 1982; Malaviya 1982; Palande 1973; Pandya 1978; Ramarorazana 1995). Comparison of these studies is difficult due to differences in surgical techniques used, duration and severity of neuritis, type of leprosy, follow‐up time, and outcome measures.
Several non‐randomised studies compared operated versus non‐operated nerves. One study evaluated nerve function in nine individuals with neuritis of less than six months duration.Three patients underwent ulnar nerve decompression, three patients received corticosteroid therapy for ulnar neuritis and three patients underwent median nerve decompression. The study found an average nerve function improvement of 35% for ulnar nerve decompression (n = 3), 32% for steroid treatment of eight weeks (n = 3) and 18%, for median nerve decompression (n = 3) six months after surgery or start of treatment (Shah 1986).
Three studies examined surgery alone versus surgery plus steroids. One study compared medial epicondylectomy alone (n = 7) with medial epicondylectomy plus steroids (n = 7) given two weeks postoperatively for ulnar neuritis of less than one month's duration. After a five‐month follow‐up motor improvement was not better in the group receiving additional steroids (Oommen 1979). Another study compared neurolysis (n = 21) with neurolysis in combination with perineural corticosteroid injections (n = 18) for ulnar neuritis of less than six months duration. The injections were administered around the thickened nerve after surgery and two and three weeks later. One year after surgery the mean difference between final and initial nerve function score was 14 for the surgery only group and 21 for the surgery plus steroids group (Dandapat 1991). The third study compared decompressive surgery alone (n = 59) with surgery plus steroids (n = 25) given for three to four months for sensory impairment of the posterior tibial nerve of varying duration. Satisfactory recovery of nerves with duration of anaesthesia of less than six months was 61% in the surgery group and 83% in the surgery plus steroids group four weeks after surgery (Rao 1989).
One study compared operated nerves with contralateral non‐operated nerves. Prior to surgery all participants had received three months of steroid treatment. The most affected nerves underwent surgical decompression and were compared with the contralateral non‐operated nerves one year or more after surgery. Of the more than 100 nerve decompressions four operated nerves had decreased nerve function after one year of follow‐up. The other operated nerves had unchanged or improved nerve function one year after surgery. It is unclear how many of the contralateral non‐operated nerves improved or deteriorated (Droogenbroeck 1977).
After losses to follow‐up, another study compared operated nerves (n = 195) of 95 patients with non‐operated nerves of 96 patients, matched for type of leprosy, age and duration of sensory loss but not randomised, on changes in sensation. Participants in whom no improvement of sensory nerve function was found after a standard steroid treatment (40 mg prednisolone daily for three weeks after which the dosage was reduced by 5 mg per week) were included in the study. Between 27% and 66% of the nerves had definite improvement two years after surgery compared to 7% of the non‐operated nerves (Theuvenet 2006). Improvement was more likely if the sensory loss had been present for a shorter time.
Studies from Carayon et al favour surgery plus medical treatment above medical treatment alone (Carayon 1985a; Carayon 1985b; Carayon 1993).
Corticosteroids are the cornerstone of management in acute nerve damage in leprosy, are recommended by the WHO and are widely available. But corticosteroids have some shortcomings. The effects of corticosteroids in the long term remain uncertain and a considerable proportion of people treated for nerve damage do not benefit from corticosteroid treatment. Long‐term therapy may cause serious adverse effects, such as peptic ulcer, cataract or psychosis. Spontaneous improvement or recovery of nerve function in untreated or placebo treated individuals has been reported and needs more investigation. The limitations of corticosteroids urge the need to find alternative therapeutic approaches (Van Veen 2007). Surgery alone as therapy for treating neuritis is not recommended, but there is discussion about whether the combination of surgery and medical treatment (e.g. steroids) will give better results than medical treatment alone and there is a call for appropriate trials examining this question (Bourrel 1992; Malaviya 2004b; Richard 2004 ).
Authors' conclusions
Implications for practice.
Very low quality evidence from the two RCTs is insufficient to draw robust conclusions about the effect of decompressive surgery for treating nerve damage in leprosy. Two trials examining the added benefit of surgery over steroids for neuritis of less than six months duration did not show significantly better outcomes with steroids plus surgery than steroids alone in the long term. Adverse effects of decompressive surgery for treating nerve damage in leprosy are not well documented.
Implications for research.
There is a need to identify factors which will predict a favourable response to decompressive surgery or groups of patients or nerves that will be likely to benefit from surgery. Future RCTs should be well‐designed to establish the usefulness and effectiveness of the combination of decompressive surgery and medical treatment compared to medical treatment alone. New trials should pay more attention to non‐clinical aspects, such as costs and impact on quality of life, because these are highly relevant indicators for both policy makers and participants.
What's new
| Date | Event | Description |
|---|---|---|
| 31 October 2012 | New citation required but conclusions have not changed | No new studies for inclusion. Content of plain language summary and abstract revised. Risk of bias text edited with no change to assessments. Published notes added. |
| 15 October 2012 | New search has been performed | Searches updated (October 2012). |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 15 February 2010 | New search has been performed | This review has been updated with a new search (August 2010) but no new relevant studies were found. 'Risk of bias' and 'Summary of findings' tables have been included. |
| 27 May 2008 | Amended | Converted to new review format. |
| 14 November 2007 | New citation required and conclusions have changed | Substantive amendment |
Notes
As trials are rarely conducted in this field, this review will be updated every four years instead of the usual two years. However, if new data emerge an earlier update will be planned.
Acknowledgements
We would like to thank Dr P Bourrel, Dr M Ebenezer, Dr J Millan and Dr B Naafs for providing additional information and the Cochrane Neuromuscular Disease Group for advice and help.
The editorial base of the Cochrane Neuromuscular Disease Group is supported by the MRC Centre for Neuromuscular Diseases.
Appendices
Appendix 1. MEDLINE (Ovid)search strategy
Database: Ovid MEDLINE(R) <1946 to October Week 1 2012> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (338952) 2 controlled clinical trial.pt. (85368) 3 randomized.ab. (241738) 4 placebo.ab. (135435) 5 drug therapy.fs. (1576027) 6 randomly.ab. (173480) 7 trial.ab. (250444) 8 groups.ab. (1134703) 9 or/1‐8 (2934469) 10 exp animals/ not humans.sh. (3793849) 11 9 not 10 (2492833) 12 leprosy.mp. or exp Leprosy/ (21113) 13 hansen disease.mp. (66) 14 12 or 13 (21121) 15 exp Decompression/ or decompression$.mp. (28810) 16 neurolysis.mp. (1408) 17 epicondylectomy.mp. (92) 18 epineurotomy.mp. (35) 19 or/15‐18 (30081) 20 neuritis.mp. or Neuritis/ (12398) 21 nerve damage.mp. (3396) 22 peripheral nervous system diseases.mp. or exp Peripheral Nervous System Diseases/ (116793) 23 nerve loss.mp. (75) 24 Peripheral Nerves/ (19886) 25 neuropath$.mp. (87793) 26 nerve function impairment.mp. (68) 27 nerve problem.mp. (17) 28 nerve involvement.mp. (1815) 29 (nerve pain or neuralgia).mp. or Neuralgia/ (15249) 30 or/20‐29 (197412) 31 11 and 14 and 19 and 30 (14) 32 31 and 20100701:20121015.(ed). (0)
Appendix 2. EMBASE (Ovid) search strategy
Database: Embase <1980 to 2012 Week 41> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (35263) 2 double‐blind procedure.sh. (111398) 3 single‐blind procedure.sh. (16509) 4 randomized controlled trial.sh. (330814) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (905431) 6 trial.ti. (136362) 7 clinical trial/ (872711) 8 or/1‐7 (1505885) 9 (animal/ or nonhuman/ or animal experiment/) and human/ (1214260) 10 animal/ or nonanimal/ or animal experiment/ (3322220) 11 10 not 9 (2750537) 12 8 not 11 (1417057) 13 limit 12 to embase (1098843) 14 leprosy.mp. or exp Leprosy/ (24619) 15 hansen disease.mp. (94) 16 14 or 15 (24626) 17 exp Decompression/ or decompression$.mp. (38755) 18 neurolysis.mp. (2722) 19 epicondylectomy.mp. (121) 20 epineurotomy.mp. (43) 21 or/17‐20 (41200) 22 neuritis.mp. or Neuritis/ (14248) 23 nerve damage.mp. (4468) 24 peripheral nervous system diseases.mp. or exp Peripheral Nervous System Diseases/ (45650) 25 nerve loss.mp. (105) 26 Peripheral Nerves/ (17757) 27 neuropath$.mp. (184945) 28 nerve function impairment.mp. (97) 29 nerve problem.mp. (19) 30 nerve involvement.mp. (2297) 31 (nerve pain or neuralgia).mp. or Neuralgia/ (20283) 32 or/22‐31 (229118) 33 13 and 16 and 21 and 32 (8)
Appendix 3. AMED (Ovid) search strategy
Database: AMED (Allied and Complementary Medicine) <1985 to October 2012> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 leprosy.mp. or exp Leprosy/ (67) 2 hansen disease.mp. (0) 3 1 or 2 (67) 4 exp Decompression/ or decompression$.mp. (282) 5 neurolysis.mp. (44) 6 epicondylectomy.mp. (1) 7 epineurotomy.mp. (0) 8 or/4‐7 (323) 9 neuritis.mp. or Neuritis/ (68) 10 nerve damage.mp. (46) 11 peripheral nervous system diseases.mp. or exp Peripheral Nervous System Diseases/ (1) 12 nerve loss.mp. (0) 13 Peripheral Nerves/ (330) 14 neuropath$.mp. (1446) 15 nerve function impairment.mp. (2) 16 nerve problem.mp. (3) 17 nerve involvement.mp. (22) 18 (nerve pain or neuralgia).mp. or Neuralgia/ (252) 19 or/9‐18 (2084) 20 Randomized controlled trials/ (1553) 21 Random allocation/ (304) 22 Double blind method/ (450) 23 Single‐Blind Method/ (32) 24 exp Clinical Trial/ (3219) 25 (clin$ adj25 trial$).tw. (5501) 26 ((singl$ or doubl$ or treb$ or trip$) adj25 (blind$ or mask$ or dummy)).tw. (2265) 27 placebos/ (522) 28 placebo$.tw. (2521) 29 random$.tw. (12977) 30 research design/ (1681) 31 Prospective Studies/ (509) 32 cross over studies.mp,et. (6) 33 meta analysis/ (111) 34 (meta?analys$ or systematic review$).tw. (1913) 35 control$.tw. (27934) 36 (multicenter or multicentre).tw. (739) 37 ((study or studies or design$) adj25 (factorial or prospective or intervention or crossover or cross‐over or quasi‐experiment$)).tw. (9872) 38 or/20‐37 (43054) 39 3 and 8 and 19 and 38 (0)
Appendix 4. LILACS search strategy
(leprosy or lepra or hanseniase or hansen's disease or MH:C01.252.410.040.552.386$)
AND (MH:E.04.188$ or decompression or decompresion or descompressao or neurolysis or epicondylectomy) and (neuritis or neurite or nerve damage or MH:C10.668.829$ or Enfermedades del Sistema Nervioso Periferico or Doencas do Sistema Nervoso Periferico or nerve loss or peripheral nerves or Nervios Perifericos or Nervos Perifericos or neuropath$ or nerve or neuralgia)
and ((PT:"Randomized Controlled Trial" or "Randomized Controlled trial" or "Ensayo Clínico Controlado Aleatorio" or "Ensaio Clínico Controlado Aleatório" or PT:"Controlled Clinical Trial" or "Ensayo Clínico Controlado" or "Ensaio Clínico Controlado" or "Random allocation" or "Distribución Aleatoria" or "Distribuição Aleatória" or randon$ or Randomized or randomly or "double blind" or "duplo‐cego" or "duplo‐cego" or "single blind" or "simples‐cego" or "simples cego" or placebo$ or trial or groups) AND NOT (B01.050$ AND NOT (humans or humanos or humanos)))
Appendix 5. CINAHL (EBSCOhost)search strategy
Monday, October 15, 2012 11:56:23 AM S41 S39 and S18 1 S40 S39 and S21 1 S39 S38 and S26 and S21 1 S38 S37 or S36 or S35 or S34 or S33 or S32 or S31 or S30 or S29 or S28 or S27 30193 S37 nerve pain 995 S36 (neuralgia) or (MH "Neuralgia") 2988 S35 nerve involvement 248 S34 nerve problem 24 S33 nerve function impairment 20 S32 neuropath* 12174 S31 (Peripheral Nerves) or (MH "Peripheral Nerves") 2082 S30 nerve loss 224 S29 (peripheral nervous system diseases) or (MH "Peripheral Nervous System Diseases+") 21885 S28 nerve damage 549 S27 (neuritis) or (MH "Neuritis") 657 S26 S25 or S24 or S23 or S22 3792 S25 epineurotomy 2 S24 epicondylectomy 10 S23 neurolysis 170 S22 (Decompression) or (MH "Decompression, Surgical") 3641 S21 S20 or S19 976 S20 hansen disease 11 S19 (leprosy) or (MH "Leprosy") 975 S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 565233 S17 ABAB design* 78 S16 TI random* or AB random* 114866 S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 236487 S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 80165 S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 23816 S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 18634 S11 PT ("clinical trial" or "systematic review") 105563 S10 (MH "Factorial Design") 845 S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 188555 S8 (MH "Meta Analysis") 14850 S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 30 S6 (MH "Quasi‐Experimental Studies") 5602 S5 (MH "Placebos") 7787 S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 25165 S3 (MH "Clinical Trials+") 149100 S2 (MH "Crossover Design") 9734 S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 58490
Appendix 6. CENTRAL search strategy
#1 leprosy #2 MeSH descriptor Leprosy explode all trees #3 "hansen disease" #4 (#1 OR #2 OR #3) #5 Decompression #6 MeSH descriptor Decompression explode all trees #7 neurolysis #8 epicondylectomy #9 epineurotomy #10 (#5 OR #6 OR #7 OR #8 OR #9) #11 neuritis #12 MeSH descriptor Neuritis explode all trees #13 "nerve damage" #14 "peripheral nervous system diseases" #15 MeSH descriptor Peripheral Nervous System Diseases explode all trees #16 "nerve loss" #17 MeSH descriptor Peripheral Nerves, this term only #18 neuropath* #19 "nerve function impairment" #20 "nerve problem" #21 "nerve involvement" #22 ("nerve pain" or neuralgia) #23 MeSH descriptor Neuralgia, this term only #24 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23) #25 (#4 AND #10 AND #24) #26 (#25)
Data and analyses
Comparison 1. Medial epicondylectomy plus oral steroids versus oral steroids alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in sensory score after one year | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐2.45, 2.61] |
| 2 Proportion of ulnar nerves with sensory improvement after one year | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.71, 1.77] |
| 3 Change in motor score after one year | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 0.82 [‐1.34, 2.98] |
| 4 Proportion of ulnar nerves with motor improvement after one year | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.64, 1.28] |
| 5 Change in sensory score after two years | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐2.82, 2.78] |
| 6 Change in motor score after two years | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 0.22 [‐2.39, 2.83] |
Comparison 2. Longitudinal epineurotomy plus oral steroids versus oral steroids alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Median sensory improvement after two years | Other data | No numeric data | ||
| 2 Median motor improvement after two years | Other data | No numeric data | ||
| 3 Median improvement in nerve pain and tenderness after two years | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boucher 1999.
| Methods | Randomised, parallel group trial Randomisation by a computer random number table Blinding not possible | |
| Participants | 31 leprosy patients with nerve deficit < 6 months duration Unit of randomisation: ulnar, median, common peroneal or posterior tibial nerve. Unit of analysis: nerve Nerves randomised: unclear Nerves analysed: 93 (a: 47 , b: 46) | |
| Interventions | (a) Prednisone start at 40 mg/day for 15 days and thereafter gradually tapered with 5 mg/15 or 30 days until 6 months completed (total 3450 mg) (b) Same intervention plus external nerve decompression and a simple, longitudinal epineurotomy | |
| Outcomes | Change in: (1) Sensory score after 2 years (2) Voluntary muscle testing (VMT) score after 2 years (3) Nerve pain after 2 years | |
| Notes | Single centre Conducted in Senegal | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by a computer random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of patients and clinicians not possible; blinding of outcome assessor not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/31 (6%) of participants lost to follow‐up, but unclear how many nerves were involved; no intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | The occurrence of adverse effects was not adequately reported |
| Other bias | High risk | No separate analysis was done using only one independent outcome from each patient |
Ebenezer 1996.
| Methods | Randomised, parallel group trial Randomisation by alternation Blinding not possible | |
| Participants | 57 leprosy patients with ulnar neuritis < 6 months duration Unit of randomisation: person Unit of analysis: ulnar nerve Persons randomised: 57 with 75 ulnar nerves (18 bilateral cases) Nerves analysed: 57 of 39 persons (a: 28, b: 29) | |
| Interventions | (a) Prednisolone 30 mg/day for 1 week, reducing the daily dose by 5 mg every week for 6 weeks (total 735 mg) (b) Same intervention plus external nerve decompression and a simple, subperiosteal medial epicondylectomy | |
| Outcomes | Change in: (1) Sensory score after 2 years (2) VMT score after 2 years | |
| Notes | Single centre Conducted in India Follow‐up study of Pannikar et al | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternation |
| Allocation concealment (selection bias) | High risk | Alternation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of patients and clinicians not possible; blinding of outcome assessor not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18/75 (24%) loss to follow‐up of nerves, 18/57 participants (32%); no intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | High risk | The occurrence of adverse effects was not reported |
| Other bias | High risk | No separate analysis was done using only one independent outcome from each patient |
Pannikar 1984.
| Methods | Randomised, parallel group trial Randomisation by alternation Blinding not possible | |
| Participants | 57 leprosy patients with complaints suggestive of ulnar nerve dysfunction < 24 weeks duration Unit of randomisation: person Unit of analysis: ulnar nerve Persons randomised: 57 with 75 ulnar nerves (18 bilateral cases) Nerves analysed: 62 of 44 persons (a: 31, b: 31) | |
| Interventions | a) Prednisolone 30 mg/day for 1 week, reducing the daily dose by 5 mg every week for 6 weeks (total 735 mg) (b) Same intervention plus external nerve decompression and a simple, subperiosteal medial epicondylectomy | |
| Outcomes | Change in: (1) Sensory score after 1 year (2) VMT score after 1 year (3) Nerve pain and tenderness after 1 year (4) Stretch test | |
| Notes | Single centre Conducted in India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternation |
| Allocation concealment (selection bias) | High risk | Alternation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of patients and clinicians not possible; blinding of outcome assessor not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13/75 (17%) loss to follow‐up of nerves, 13/57 participants (23%); no intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | High risk | The occurrence of adverse effects was not reported |
| Other bias | High risk | No separate analysis was done using only one independent outcome from each patient |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carayon 1985a | Unclear randomisation procedure |
| Carayon 1985b | Unclear randomisation procedure |
| Carayon 1993 | Unclear randomisation procedure |
| Dandapat 1991 | Unclear randomisation procedure |
| Droogenbroeck 1977 | No randomisation procedure |
| Oommen 1979 | No randomisation procedure |
| Rao 1989 | No randomisation procedure |
Differences between protocol and review
We updated the risk of bias methodology, added a 'Risk of bias' figure and included a 'Summary of findings' table. The 'Risk of bias' section was further revised in 2012 (Higgins 2011).
Contributions of authors
Link with editorial base and co‐ordinate contributions from co‐authors (NvV). Draft protocol (NvV with input from all). Run search (NvV). Identify relevant titles and abstracts from searches (NvV, JHR). Obtain copies of trials (NvV). Selection of trials (NvV, JHR). Extract data from trials (NvV, JHR). Enter data into RevMan (NvV). Carry out analysis (NvV, JHR). Interpret data (NvV, TS, WT, JHR). Draft final review (NvV with input from all). Update review (NvV, JHR).
Sources of support
Internal sources
The Netherlands Leprosy Relief, Netherlands.
External sources
No sources of support supplied
Declarations of interest
NVV and JHR: work on this review was supported by an institutional grant from the Netherlands Leprosy Relief.
Other authors: none declared.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Boucher 1999 {published data only}
- Boucher P, Millan J, Parent M, Moulia‐Pela JP. Randomized controlled trial of medical and medico‐surgical treatment of Hansen's neuritis [Essai compare randomise du traitement medical et medico‐chirurgical des nevrites hanseniennes]. Acta Leprologica 1999;11(4):171‐7. [PUBMED: 10987048] [PubMed] [Google Scholar]
Ebenezer 1996 {published data only}
- Ebenezer M, Andrews P, Solomon S. Comparative trial of steroids and surgical intervention in the management of ulnar neuritis. International Journal of Leprosy and Other Mycobacterial Diseases 1996;64(3):282‐6. [PUBMED: 8862262 ] [PubMed] [Google Scholar]
Pannikar 1984 {published data only}
- Pannikar VK, Ramprasad S, Reddy NR, Andrews P, Ravi K, Fritschi EP. Effect of epicondylectomy in early ulnar neuritis treated with steroids. International Journal of Leprosy and Other Mycobacterial Diseases 1984;52(4):501‐5. [PUBMED: 6535823 ] [PubMed] [Google Scholar]
References to studies excluded from this review
Carayon 1985a {published data only}
- Carayon A, Droogenbroeck J, Languillon J. Surgical decompression of neuritis of Hansen's disease [Decompression chirurgicale des nevrites de la maladie de Hansen]. Acta Leprologica 1985;3(1):37‐66. [PubMed] [Google Scholar]
Carayon 1985b {published data only}
- Carayon A, Droogenbroeck JB, Boucher P, Hirzel C. Results of treatment of 206 patients with recent neuritis (C.M.S.‐P.E.R.) [Resultats du traitement de 206 porteurs de nevrites recentes (C.M.S.‐P.E.R.)]. Acta Leprologica 1985;3(2):155‐62. [PubMed] [Google Scholar]
Carayon 1993 {published data only}
- Carayon A, Droogenbroeck J, Courbil J, Boucher P, Naafs N. Treatment of leprotic neuritis. Exclusive medical treatment or combined with decompression [Evolution du traitement des nevrites hanseniennes. Traitement medical exclusif ou associe a la decompression]. Medecine Tropicale (Marseille) 1993;53(4):493‐504. [PubMed] [Google Scholar]
Dandapat 1991 {published data only}
- Dandapat MC, Sahu DM, Mukherjee LM, Panda C, Baliarsing AS. Treatment of leprous neuritis by neurolysis combined with perineural corticosteroid injection. Leprosy Review 1991;62(1):27‐34. [DOI] [PubMed] [Google Scholar]
Droogenbroeck 1977 {published data only}
- Droogenbroeck JBA, Naafs B. Surgical nerve release in leprosy: a study with comparison with non operated opposite nerves [Etude comparative d'une serie de nerfs lepreux decomprimes chirurgicalement par rapport aux nerfs controlateraux non operes]. Medecine Tropicale (Marseille) 1977;37(6):771‐6. [Google Scholar]
Oommen 1979 {published data only}
- Oommen PK. Ulnar nerve decompression by medial epicondylectomy of the humerus and a method of assessing muscle power status by totalling the muscle grading. Leprosy in India 1979;51(3):336‐40. [PubMed] [Google Scholar]
Rao 1989 {published data only}
- Rao KS, Siddalinga Swamy MK. Sensory recovery in the plantar aspect of the foot after surgical decompression of posterior tibial nerve. Possible role of steroids along with decompression. Leprosy Review 1989;60(4):283‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Antia 1976
- Antia NH, Vankani B, Pandya NJ. Surgical decompression of the ulnar nerve in leprous neuritis. Leprosy in India 1976;48(4):362‐70. [PubMed] [Google Scholar]
Becx‐Bleumink 1990
- Becx‐Bleumink M, Berhe D, Mannetje W. The management of nerve damage in the leprosy control services. Leprosy Review 1990;61(1):1‐11. [DOI] [PubMed] [Google Scholar]
Bernardin 1997
- Bernardin R, Thomas B. Surgery for neuritis in leprosy: indications for and results of different types of procedures. Leprosy Review 1997;68(2):147‐54. [DOI] [PubMed] [Google Scholar]
Bourrel 1992
- Bourrel P. Preliminary recommendations on the use of surgery for the treatment of leprosy neuritis: caution concerning the use of surgery in prevention of deformities. ILEP Technical Bulletin 1992, issue 4:1‐2.
Brandsma 1981
- Brandsma W. Basic nerve function assessment in leprosy patients. Leprosy Review 1981;52(2):161‐70. [DOI] [PubMed] [Google Scholar]
Brandsma 1983
- Brandsma JW, Nugteren WA, Andersen JB, Naafs B. Functional changes of the ulnar nerve in leprosy patients following neurolysis. Leprosy Review 1983;54(1):31‐8. [DOI] [PubMed] [Google Scholar]
Britton 1998
- Britton WJ. The management of leprosy reversal reactions. Leprosy Review 1998;69(3):225‐34. [DOI] [PubMed] [Google Scholar]
Chaise 1982
- Chaise F, Sedel L, Medevielle D, Witvoet J. Ulnar neuritis in Hansen's disease results of fifty neurolyses in the arm and elbow. Annales de Chirurgie de la Main 1982;1(4):326‐35. [DOI] [PubMed] [Google Scholar]
Chaise 1985
- Chaise F, Roger B. Neurolysis of the common peroneal nerve in leprosy. A report on 22 patients. Journal of Bone and Joint Surgery ‐ British Volume 1985;67(3):426‐9. [DOI] [PubMed] [Google Scholar]
Chaise 1987
- Chaise F, Boucher P. Remote results of the surgical decompression of the posterior tibial nerve in the neuropathies of Hansen's disease [Les resultats eloignes de la decompression chirurgicale du nerf tibial posterieur dans les neuropathies de la maladie de Hansen]. Journal de Chirurgie (Paris) 1987;124(5):315‐8. [PubMed] [Google Scholar]
Chaise 2004
- Chaise F. Current management of hand leprosy [La prise en charge actuelle des mains lepreuses]. Chirurgie de la Main 2004;23(1):1‐16. [DOI] [PubMed] [Google Scholar]
Croft 1999
- Croft RP, Richardus JH, Nicholls PG, Smith WC. Nerve function impairment in leprosy: design, methodology, and intake status of a prospective cohort study of 2664 new leprosy cases in Bangladesh (The Bangladesh Acute Nerve Damage Study). Leprosy Review 1999;70(2):140‐59. [DOI] [PubMed] [Google Scholar]
Croft 2000
- Croft RP, Nicholls PG, Richardus JH, Smith WC. The treatment of acute nerve function impairment in leprosy: results from a prospective cohort study in Bangladesh. Leprosy Review 2000;71(2):154‐68. [DOI] [PubMed] [Google Scholar]
Heijnders 2004
- Heijnders ML. The dynamics of stigma in leprosy. International Journal of Leprosy and Other Mycobacterial Diseases 2004;72(4):437‐47. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Husain 1997
- Husain S, Mishra B, Prakash V, Malaviya GN. Evaluation of results of surgical decompression of median nerve in leprosy in relation to sensory‐motor functions. Acta Leprologica 1997;10(4):199‐201. [PubMed] [Google Scholar]
Husain 1998
- Husain S, Mishra B, Prakash V, Malaviya GN. Results of surgical decompression of ulnar nerve in leprosy. Acta Leprologica 1998;11(2):17‐20. [PubMed] [Google Scholar]
ILEP 2001
- The International Federation of Anti‐Leprosy Associations (ILEP). How to diagnose and treat leprosy. London: ILEP, 2001. [Google Scholar]
ILEP 2002
- The International Federation of Anti‐Leprosy Associations (ILEP). How to recognise and manage reactions. London: ILEP, 2002. [Google Scholar]
Job 1989
- Job CK. Nerve damage in leprosy. International Journal of Leprosy and Other Mycobacterial Diseases 1989;57(2):532‐9. [PubMed] [Google Scholar]
Kazen 1996
- Kazen R. Role of surgery of nerves in leprosy in the restoration of sensibility in hands and feet of leprosy patients. Indian Journal of Leprosy 1996;68(1):55‐65. [PubMed] [Google Scholar]
Kumar 1982
- Kumar K. Surgical management of leprous ulnar neuritis. Clinical Orthopaedics and Related Research 1982;163:235‐42. [PubMed] [Google Scholar]
Leekassa 2004
- Leekassa R, Bizuneh E, Alem A. Prevalence of mental distress in the outpatient clinic of a specialized leprosy hospital. Addis Ababa, Ethiopia, 2002. Leprosy Review 2004;75(4):367‐75. [PubMed] [Google Scholar]
Lockwood 1993
- Lockwood DN, Vinayakumar S, Stanley JN, McAdam KP, Colston MJ. Clinical features and outcome of reversal (type 1) reactions in Hyderabad, India. International Journal of Leprosy and Other Mycobacterial Diseases 1993;61(1):8‐15. [PubMed] [Google Scholar]
Lockwood 2000
- Lockwood DN. Steroids in leprosy type 1 (reversal) reactions: mechanisms of action and effectiveness. Leprosy Review 2000;71(Supplement):S111‐4. [DOI] [PubMed] [Google Scholar]
Lockwood 2005
- Lockwood DN, Suneetha S. Leprosy: too complex a disease for a simple elimination paradigm. Bulletin of the World Health Organization 2005;83(3):230‐5. [PMC free article] [PubMed] [Google Scholar]
Malaviya 1982
- Malaviya GN, Ramu G. Role of surgical decompression in ulnar neuritis of leprosy. Leprosy in India 1982;54(2):287‐302. [PubMed] [Google Scholar]
Malaviya 2004a
- Malaviya GN. Unfavourable outcomes after reconstructive surgery in leprosy. In: Schwarz R, Brandsma W editor(s). Surgical reconstruction & rehabilitation in leprosy and other neuropathies. Kathmandu, Nepal: Ekta Books, 2004:47‐64. [Google Scholar]
Malaviya 2004b
- Malaviya GN. Shall we continue with nerve trunk decompression in leprosy?. Indian Journal of Leprosy 2004;76(4):331‐42. [PubMed] [Google Scholar]
Marlowe 2004
- Marlowe SN, Hawksworth RA, Butlin CR, Nicholls PG, Lockwood DN. Clinical outcomes in a randomized controlled study comparing azathioprine and prednisolone versus prednisolone alone in the treatment of severe leprosy type 1 reactions in Nepal. Transactions of the Royal Society of Tropical Medicine and Hygiene 2004;98(10):602‐9. [DOI] [PubMed] [Google Scholar]
Meima 2008
- Meima A, Veen NHJ, Richardus JH. Future prevalence of WHO grade 2 impairment in relation to incidence trends in leprosy: an exploration. Tropical Medicine and International Health 2008;13(2):241‐6. [DOI] [PubMed] [Google Scholar]
Naafs 1996
- Naafs B. Treatment of reactions and nerve damage. International Journal of Leprosy and Other Mycobacterial Diseases 1996;64(4 Supplement):S21‐8. [PubMed] [Google Scholar]
Naafs 2008
- Letter to authors 2008 August. Personal correspondence.
Nicholls 2003
- Nicholls PG, Croft RP, Richardus, JH, Withington SG, Smith WC. Delay in presentation, an indicator for nerve function status at registration and for treatment outcome ‐ the experience of the Bangladesh Acute Nerve Damage Study cohort. Leprosy Review 2003;74(4):349‐56. [PubMed] [Google Scholar]
Palande 1973
- Palande DD. A review of twenty‐three operations on the ulnar nerve in leprous neuritis. Journal of Bone and Joint Surgery ‐ American Volume 1973;55(7):1457‐64. [PubMed] [Google Scholar]
Palande 1980
- Palande DD. Preventive nerve surgery in leprosy. Leprosy in India 1980;52(2):276‐98. [PubMed] [Google Scholar]
Pandya 1978
- Pandya NJ. Surgical decompression of nerves in leprosy. An attempt at prevention of deformities. A clinical, electrophysiologic, histopathologic and surgical study. International Journal of Leprosy 1978;46(1):47‐55. [PubMed] [Google Scholar]
Pandya 1983
- Pandya SS. Surgery on the peripheral nerves in leprosy. Neurosurgical Review 1983;6(3):153‐4. [DOI] [PubMed] [Google Scholar]
Pearson 1982
- Pearson JM. The evaluation of nerve damage in leprosy. Leprosy Review 1982;53(2):119‐30. [DOI] [PubMed] [Google Scholar]
Rafferty 2005
- Rafferty J. Curing the stigma of leprosy. Leprosy Review 2005;76(2):119‐26. [PubMed] [Google Scholar]
Ramarorazana 1995
- Ramarorazana S, Rene JP, Schwartzl E, Randrianomenjanahary J, Razafindramboa H, Schino M. One‐year follow‐up of 466 nerve decompressions in 123 lepers during multidrug therapy in Madagascar [Resultats a un an de 466 decompressions nerveuses realisees chez 123 lepreux en cours de polychimiotherapie a Madagascar]. Medecine Tropicale (Marseille) ‐ Revue du Corps de Sante Colonial 1995;55(2):146‐50. [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Richard 2004
- Richard B. Surgical management of neuritis. In: Schwarz R, Brandsma W editor(s). Surgical reconstruction & rehabilitation in leprosy and other neuropathies. Kathmandu, Nepal: Ekta Books, 2004. [Google Scholar]
Richardus 2003
- Richardus JH, Withington SG, Anderson AM, Croft RP, Nicholls PG, Brakel WH, et al. Adverse events of standardized regimens of corticosteroids for prophylaxis and treatment of nerve function impairment in leprosy: results from the 'TRIPOD' trials. Leprosy Review 2003;74(4):319‐27. [PubMed] [Google Scholar]
Ridley 1966
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five‐group system. International Journal of Leprosy and Other Mycobacterial Diseases 1966;34(3):255‐73. [PubMed] [Google Scholar]
Saunderson 2000
- Saunderson P, Gebre S, Desta K, Byass P, Lockwood DN. The pattern of leprosy‐related neuropathy in the AMFES patients in Ethiopia: definitions, incidence, risk factors and outcome. Leprosy Review 2000;71(3):285‐308. [DOI] [PubMed] [Google Scholar]
Scholten 2007
- Scholten RJ, Mink van der Molen A, Uitdehaag BM, Bouter LM, Vet HC. Surgical treatment options for carpal tunnel syndrome. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003905.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schreuder 1998
- Schreuder PA. The occurrence of reactions and impairments in leprosy: experience in the leprosy control program of three provinces in northeastern Thailand, 1987‐1995 [correction of 1978‐1995]. III. Neural and other impairments. International Journal of Leprosy and Other Mycobacterial Diseases 1998;66(2):170‐81. [PubMed] [Google Scholar]
Sena 2006
- Sena CB, Salgado CG, Tavares CM, Cruz CA, Xavier MB, Do Nascimento JL. Cyclosporine A treatment of leprosy patients with chronic neuritis is associated with pain control and reduction in antibodies against nerve growth factor. Leprosy Review 2006;77(2):121‐9. [PubMed] [Google Scholar]
Shah 1986
- Shah A. Evaluation of nerve function deficit. Its improvement by nerve decompression or corticosteroid therapy. Indian Journal of Leprosy 1986;58(2):216‐24. [PubMed] [Google Scholar]
Sugumaran 1998
- Sugumaran DS. Leprosy reactions ‐ complications of steroid therapy. International Journal of Leprosy and Other Mycobacterial Diseases 1998;66(1):10‐5. [PubMed] [Google Scholar]
Theuvenet 2006
- Theuvenet WJ, Gavin‐Finlay K, Roche PW. Change of sensation in leprosy by selective meshing of the epineurium. European Journal of Plastic Surgery 2006;28(6):393‐9. [Google Scholar]
Thoma 2004
- Thoma A, Veltri K, Haines T, Duku E. A systematic review of reviews comparing the effectiveness of endoscopic and open carpal tunnel decompression. Plastic and Reconstructive Surgery 2004;113(4):1184‐91. [DOI] [PubMed] [Google Scholar]
Van Brakel 2003
- Brakel WH, Anderson AM, Withington SG, Croft RP, Nicholls PG, Richardus JH, et al. The prognostic importance of detecting mild sensory impairment in leprosy: a randomized controlled trial (TRIPOD 2). Leprosy Review 2003;74(4):300‐10. [PubMed] [Google Scholar]
Van Brakel 2005
- Brakel WH, Nicholls PG, Das L, Barkataki P, Suneetha SK, Jadhav RS, et al. The INFIR Cohort Study: investigating prediction, detection and pathogenesis of neuropathy and reactions in leprosy. Methods and baseline results of a cohort of multibacillary leprosy patients in north India. Leprosy Review 2005;76(3):14‐34. [PubMed] [Google Scholar]
Van Veen 2007
- Veen NH, Nicholls PG, Smith WC, Richardus JH. Corticosteroids for treating nerve damage in leprosy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005491] [DOI] [PubMed] [Google Scholar]
WHO 1998
- World Health Organization. WHO Expert Committee on Leprosy [editorial]. World Health Organization Technical Report Series 1998; Vol. 874:1‐43. [PubMed]
WHO 2004
- World Health Organization. WHO Leprosy Elimination Project: Status Report 2003, draft. http://www.searo.who.int/LinkFiles/Leprosy_s20042.pdf (accessed 19 March 2007).
WHO 2006
- World Health Organization. Leprosy. http://www.who.int/mediacentre/factsheets/fs101/en/index.html (accessed 26 June 2007).
WHO 2007
- World Health Organization. Global leprosy situation, 2007. Weekly Epidemiological Record 2007;82(25):225‐32. [Google Scholar]
References to other published versions of this review
van Veen 2008
- Veen NHJ, Schreuders TAR, Theuvenet WJ, Agrawal A, Richardus JH. Decompression surgery for treating nerve damage in leprosy. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006983] [DOI] [Google Scholar]
Van Veen 2009
- Veen NHJ, Schreuders TAR, Theuvenet WJ, Agrawal A, Richardus JH. Decompressive surgery for treating nerve damage in leprosy. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006983.pub2] [DOI] [PubMed] [Google Scholar]


