Abstract
Sinusitis, one of the most prevalent and undertreated disorders, is a term used to describe inflammation of the paranasal sinuses caused by either infectious or non-infectious sources. Bacterial, viral, or fungal infections can all cause sinusitis. Sinusitis is classified into 3 types: acute, subacute, and chronic. Acute sinusitis lasts for less than 1 month, subacute sinusitis lasts from 1 to 3 months, and chronic sinusitis persists for over 3 months. This condition affects a significant portion of the population, imposing a substantial burden on the healthcare system. Antibiotics are the gold standard of bacterial sinusitis treatment. However, due to the rise of antimicrobial resistance, especially in immune-compromised patients, it is necessary to investigate potential adjunctive therapies. Based on the literature, vitamins (eg, vitamin D) have antioxidant, anti-inflammatory, and immune-modulatory properties and may effectively treat sinusitis and reduce mucous membrane inflammation. Besides vitamins, many other supplements like quercetin, sinupret, and echinacea have immunomodulatory effects and have shown promising results in sinusitis treatment. In this review, we look at the therapeutic role, safety, and efficacy of vitamins and nutritional supplements in sinusitis treatment.
Keywords: Sinusitis, upper respiratory tract, vitamins, nutritional supplements, zinc supplements, sinupret
Introduction
Sinusitis is the inflammation of the paranasal sinuses and nasal mucosa that leads to nasal discharge, impairment of the sense of smell, and facial pain. 1 Sinusitis accounts for many general physician visits and prescriptions of antibiotics worldwide. 2 The prevalence of sinusitis varies from 1% to 12%, 3 and despite its ubiquity and negative effect on quality of life, its etiology is not well-understood. 4 However, recent research suggests that many factors, such as infectious agents, structural abnormalities (nasal polyps), inflammatory and allergic processes, and dysfunctions in the host immune system, contribute to this condition. 4 The main treatment goals include eliminating the underlying infection and reducing inflammation. The primary treatment for sinusitis includes antibiotics, nasal corticosteroids, decongestants, and surgery for refractory cases.5,6 These current treatments have issues, such as the emergence of antibiotic-resistant bacteria due to excessive antibiotic prescription and surgery complications, especially in children. 6 Because of these problems, new treatments for sinusitis are needed. 6
Vitamin supplementation is suggested as an adjunctive therapy for inflammatory conditions, including sinusitis. Recent evidence confirms the anti-inflammatory role of certain vitamins, such as vitamin D or E.7-9 Besides its beneficial impact on bone health and its role in calcium and phosphate metabolism, vitamin D has an immunological and anti-inflammatory role and affects macrophages, T-cells, and dendritic cells. 7 Numerous studies have been conducted on ways to reduce or eliminate the symptoms of neurological and behavioral problems brought on by alterations in vitamin and other nutrient levels, including omega-3 and iron supplements. 10 Evidence has shown that patients with sinusitis have lower vitamin D levels, and vitamin D supplementation alleviates their symptoms.7,11 Vitamin E is a potent antioxidant that regulates reactive oxygen species formation and modulates cell-mediated and humoral immune responses. 12 The goal of this review is to highlight the possible therapeutic effects of vitamins and other supplements on sinusitis.
Vitamin A
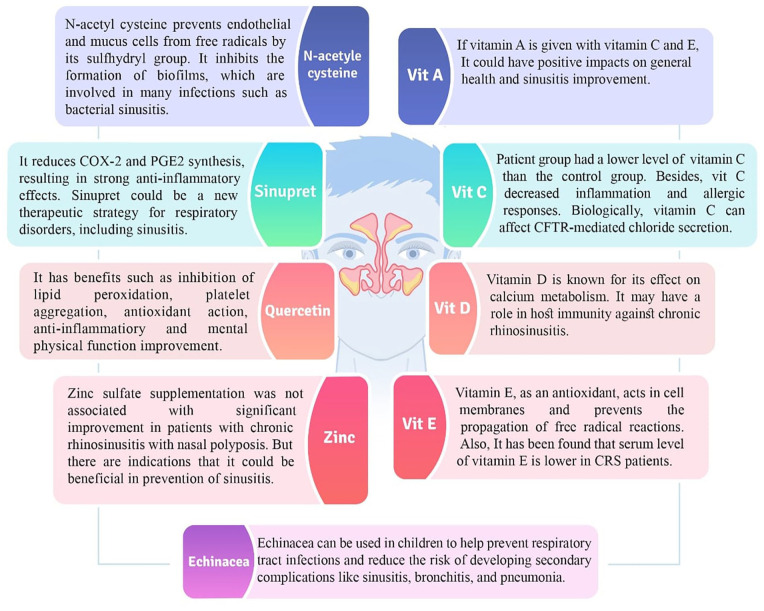
Vitamin A, also known as retinol, is a fat-soluble vitamin found in various foods such as meat, 13 fish, 14 and vegetables (provitamin A in form of certain carotenoids). 15 Its presence is crucial for the efficient functioning of organs such as the lungs, kidneys, and heart. 9
In a study by Unal et al, 16 no significant differences were found between the study group and the control group in terms of the impact of vitamin A on sinus inflammation. However, in a study conducted by Dhanawat, 17 it was reported that there was a significant effect on improving general health and reducing sinus inflammation in individuals with sinusitis when vitamin A was used in conjunction with vitamin E and vitamin C. Linday et al 6 used multivitamin-mineral and flavored cod liver oil with selenium for children with sinusitis. A suggestion was made that the use of this product can prevent sinusitis in children or at least reduce its severity. 6
Vitamin C
Vitamin C, also known as ascorbic acid, is a water-soluble vitamin that plays a crucial role in immune system regulation and the prevention of viral infections. Tomatoes, potatoes, citrus fruits such as limes, lemons, and oranges,18,19 broccoli,20,21 and kiwi22,23 are some of the known sources containing a high amount of this compound. Vitamin C content in fruit is related to some variables such as fruit color, size, and firmness19,24; cultivar24,25; technological treatment21,23; and harvest date. 26 Researchers reported that vitamin C suppresses the secretion of inflammatory mediators.27,28
The effectiveness of mucociliary clearance and hydration of the mucosal surface depends on epithelial ion transport. Cystic fibrosis transmembrane conductance regulator (CFTR) would be considered a chloride and bicarbonate channel. Vitamin C plays an important role in maintaining the normal level of airway surface liquid, thus improving the effectiveness of mucociliary clearance. Furthermore, researchers have observed that vitamin C can biologically affect CFTR-mediated chloride secretion.29-31
Considering the possible role of CFTR in chronic rhinosinusitis (CRS), Cho et al 31 experienced the effect of L-ascorbate on a distinct type of sinonasal tissues that comprised sinus mucosa from CRS, nasal mucosa from CRS, normal sinus mucosa, and normal nasal mucosa. They declared that the administration of L-ascorbate improves chloride secretion. The reduced function of CFTR-mediated chloride secretion might play a role in the progression of CRS, leading researchers to conclude that vitamin C could have a therapeutic effect by enhancing mucociliary clearance. In another ex vivo study, Zhang et al 32 assessed nasal mucus derived from 12 patients. They showed that the combination of scutellaria baicalensis, eleutherococcus senticosus, and vitamin C might suppress Th-1, Th-2, and Th-17 involved in chronic paranasal disease. Given that these cells play an essential role in the acute inflammation of CRS, suppressing them might be effective.
Unal et al 16 studied serum levels of antioxidant vitamins in 24 children who suffered from CRS. This case-control study showed that the group of patients had lower levels of vitamin C and vitamin Prabhakar Reddy et al 33 assessed serum levels of vitamin C in CRS. The study results showed that the patient group had a lower level of vitamin C than the control group. Besides, the study reported that vitamin C decreased inflammation and allergic responses.
Vitamin D
Vitamin D is known for its effects on calcium metabolism. However, recent studies suggest that this molecule plays an important role in the immune system. So it may have a role in host immunity against CRS. 34 Konstantinidis et al, 35 in an experiment on the human sinonasal epithelium of patients with eosinophilic CRS, suggest that vitamin D supplementation in eosinophilic CRS patients is effective for innate immunity regulation. Mulligan et al 36 examined the impact of vitamin D deficiencies on inflammation in a mouse model of CRS involving aspergillus fumigatus. They found out that a low level of vitamin D in the diet selectively intensifies immunological alterations related to CRS with aspergillus fumigatus. Mostafa Bel et al, 7 in 74 participants, determined the level of vitamin D in patients with allergic fungal sinusitis (AFRS) and CRS and found that the level of vitamin D in patients with CRS with nasal polyps and AFRS is incredibly lower than that in those with CRS without nasal polyps and healthy controls. Many other studies have confirmed that the levels of vitamin D are lower in patients with CRS with nasal polyps.37-42 Sansoni et al 43 conducted a clinical trial of 14 CRS patients with polyps and 31 CRS patients without polyps, as well as 12 control patients with noninflammatory pathology. Their blood was assayed for vitamin D and their sinonasal mucus for RANTES and basic fibroblast growth factor (bFGF). Only in CRS patients with polyps a negative correlation was observed between vitamin D status and RANTES and bFGF. They found that vitamin D might be crucial in regulating RANTES and basic fibroblast growth factor expression in CRS. The research by Wang et al 44 used cultivated fibroblasts from 3 patients’ nasal polyps. RANTES and eotaxin were produced in vitro by the cells after being activated with IL-1. Vitamin D derivatives calcitriol and tacalcitrol, which were also given to cultures, blocked this effect.
Tomaszewska et al 45 measured levels of vitamin D in serum and the expression of vitamin D receptors in 52 patients with CRS without nasal polyps and 55 patients with CRS with nasal polyps and then showed that vitamin D and the expression of its receptors may be associated with CRS. Bavi et al, 46 in another study with 166 patients with CRS with nasal polyps and 172 healthy subjects, noticed a considerable decrease in vitamin D levels in Iranian CRS patients with nasal polyps, which shows a positive relevance to disease intensity. Boeva et al, 47 in a study with 50 patients and 14 subjects comprising the control group, revealed higher levels of vitamin D in the blood serum of the control subjects compared to the patients with chronic polypous rhinosinusitis. Moreover, the patients of the latter group more frequently exhibited the variant of the LCT CT-13910 gene polymorphism, suggesting latent hypolactasia. In contrast, the subjects comprising the control group more frequently had the variant of the LCT CT-13910 gene polymorphism indicative of the normal tolerance of lactose. Duţu et al, 48 in a study with 6 CRS patients and 21 non-smoking controls, showed that both chitotriosidase and vitamin D could be considered non-invasive biomarkers whose utility in the follow-up of CRS-associated inflammation requires further investigation. Patients with polyps exhibited lower vitamin D values than those without polyps, while patients with microbial biofilms had higher mean values of chitotriosidase and a lower mean value of vitamin D than their counterparts. This finding may reveal the importance of microbial biofilms as an aggravating factor in disease pathogenesis. Hashemian et al, 49 in a study with 40 CRS patients with nasal polyps, demonstrated vitamin D supplementation’s efficacy in reducing the recurrence of polyposis after functional endoscopic sinus surgery in patients with CRS with nasal polyps. Kalińczak-Górna et al, 11 in a study of 40 patients suffering from chronic sinusitis, discovered a relationship between a decrease in inflammation and increased vitamin D levels and reduced mental and physical symptoms of chronic inflammation. Konstantinidis et al 35 showed that both CRS and CF patients have vitamin D deficiency and nasal polyps. The lower the serum vitamin D level, the more severe the mucosal disease was observed. Lee et al 50 indicated that low serum vitamin D levels may not significantly correlate with an elevated CRS frequency in Korean adults. However, CRS patients had higher serum vitamin D levels than the control group. In a meta-analysis by Li et al, 51 low vitamin D levels in patients with CRS were observed, suggesting that vitamin D supplements can benefit individuals. Therefore, because of the heterogeneity of the studies, better-designed futuristic RCTs should be performed to further evaluate these findings for the common population in the future. Moreover, Baruah et al, 52 in a study with 200 CRS patients with vitamin D deficiency and 100 patients that were given oral vitamin D supplements, found that 100 patients on placebo did not have vitamin D deficiency, and there were practically no differences in outcomes for both groups of patients.
Vitamin E
Vitamin E is one of the lipid-soluble vitamins, which comprises 4 isoforms of tocopherol and 4 isoforms of tocotrienol. 53 This vitamin can be found in many sources, some of which are: fruits, peanut oil, corn oil, 54 extra virgin olive oil, pomace olive oil, soybean oil, palm oil, 55 apricot seed oil, hemp seed oil, flaxseed oil, and sunflower oil. 56 Vitamin E, as an antioxidant, plays a role in cell membranes and blocks the propagation of free radical reactions. 57 Vitamin E isomers are well-known for their gene-regulation effects and anti-inflammatory properties. 58 Unal et al, 16 in a study on 24 children aged 7 to 12 years with CRS and 20 healthy controls, measured serum levels of copper, magnesium, antioxidant vitamins, and zinc. They found that the serum level of vitamin E is lower in CRS patients. However, Westerveld et al 59 measured antioxidant levels in a mucosal biopsy of the uncinate process of the ethmoid bone in patients with CRS and healthy controls. There was no difference in levels of vitamin E between the 2 groups.
Other Nutritional Supplements
Echinacea
Echinacea is a dietary supplement for the common cold and other infections; the idea is that it could stimulate the immune system to act more effectively against infections. 60 Echinacea, prepared from the root of echinacea purpurea, is sometimes also derived from echinacea pallida and echinacea angustifolia. 61
Ogal et al 60 and Vimalanathan et al 61 showed that using echinacea in children from 3 to 12 years old can be helpful for the prevention of RTI and secondary complications such as sinusitis, bronchitis, and pneumonia. The outcome indicated significant differences between the study and control groups in the intensity of sinusitis in the studied patients.
N-acetylcysteine
N-acetyl cysteine is a tolerable and safe dietary supplement. 62 It is the precursor to L-cysteine, an amino acid that is used to form glutathione molecules. 63 N-acetyl cysteine is the antidote to acetaminophen toxicity and a mucolytic agent. 64 Furthermore, it protects endothelial and mucus cells from free radicals by its sulfhydryl group and increases glutathione levels. 63 Evidence has shown that N-acetyl cysteine inhibits bacterial aggregate formation (biofilms), which are involved in many infections, such as bacterial sinusitis. 65
Several studies have shown the positive impacts of N-acetyl cysteine on sinusitis treatment due to its antioxidant and antimicrobial effects.63,65,66 Bezshapochniy et al 63 examined the positive effect of N-acetyl cysteine on treating viral sinusitis. They combined 6% N-acetyl cysteine with 3% normal saline and added it to the usual sinusitis treatment (topical corticosteroids). In this study, 56 participants (29 patients in the experimental group and 27 patients in the control group) received classical sinusitis treatment with either 3% normal saline or 3% normal saline plus 6% N-acetyl cysteine. In this research, the combination of N-acetyl cysteine and normal saline improved patients’ symptoms with viral sinusitis. Another study investigated the role of N-acetyl cysteine in bacterial biofilms and its possible benefits in treating bacterial sinusitis. Blasi et al 65 reviewed both in vitro and clinical studies. They showed that N-acetyl cysteine inhibits the formation of biofilms and may have some therapeutic effect on sinusitis.
Sinupret
Sinupret is a well-known herbal medicine with evidence-based efficacy for several respiratory disorders, such as sinusitis or acute and chronic bronchitis.67,68 Primula veris, verbena officinalis, sambucus nigra, gentiana lutea, and rumex acetosa are among the plants used to make Sinupret. 69 Since 1934, this herbal preparation has been used to repair and maintain the physiological activity of the mucosa in the paranasal sinuses in both liquid and sugar-coated tablet form.69,70 Antibacterial, antiviral, secretolytic, mucolytic, and immunological activities are all found in sinupret extract.67,69,71,72 It reduces cyclo-oxygenase-rhino2 expression and prostaglandin E-2 synthesis, resulting in strong anti-inflammatory effects when taken orally. 73 Sinupret’s general safety has been thoroughly documented. 69 In some related studies, Zhang et al 68 observed that sinupret raises airway surface liquid depth, enhances ciliary beat frequency, and promotes transepithelial chloride secretion, which is expected to improve mucociliary clearance. Sinupret, according to their findings, could be employed as a new therapeutic strategy for common respiratory disorders, including sinusitis.
Quercetin
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a kind of flavonoid compound. It is derived from phenylalanine and produced through the phenylpropanoid pathway. Quercetin can be found in a variety of sources, including different kinds of berries, 74 tomatoes, 75 vegetables, fruits, nuts, and beverages, 76 onions, 77 apples, capers, grapes, and tea, 78 shallots, 79 and honey.80,81 Researchers studied whether it might have a role in improving mental and physical function and reducing infection risk. 82
Since medical plants have lower prices and fewer side effects, they are more popular. 83 Some studies have mentioned that some plants decrease inflammatory factors and have an inhibitory effect on a transcription factor that enhances the secretion of mediators. 82 Studies have found that quercetin inhibits lipid peroxidation, platelet aggregation, and capillary permeability. 84 Additionally, a new review published in 2020 by Jafarinia et al 85 showed some anti-inflammatory effects of quercetin. Zhang et al 86 investigated the effect of quercetin on chloride secretion and ciliary bite frequency. Their results indicated that quercetin can increase chloride secretion and ciliary beat frequency.
Zinc supplements
Zinc is necessary for cells to develop and function properly by mediating nonspecific immunity, including neutrophils and natural killer cells. 87 The availability and importance of zinc in diseases and its essential presence in biological fluids should not be ignored. 88
A study by Mohammadhossein et al, 89 which used 50 patients with nasal polyposis, suggested that there is no significant difference in sinusitis, but there are signs showing zinc may be useful in the prevention of sinusitis. However, with that being said, in the study by Akbari Dilmaghani et al 90 it is reported that the treatment group had mild superiority in general health improvement but not in symptoms. Add-on therapy with zinc sulfate supplementation showed no remarkable improvement in patients suffering from CRS and nasal polyposis.
Conclusion
Multiple therapies are required to treat sinusitis patients, including nutritional interventions. In this review, we discussed the possible therapeutic effects of vitamins and a wide range of nutrition on sinusitis. We indicated their pathogenetic role in sinusitis. As shown in Table 1 and Figure 1, vitamin C, vitamin D, echinacea, N-acetyl cysteine, sinupret, and quercetin have shown promise in the treatment of sinusitis based on growing positive evidence. The effects of vitamins and nutritional supplements on sinusitis need further evaluation due to a lack of sufficient evidence. Clinicians are encouraged to consider these useful supplements in sinusitis management whenever possible. By complementing these add-on therapies, additional antimicrobial resistance is avoided, and the final treatment outcome might be improved. However, studies on the efficacy of many of these supplements are insufficient, and more research, especially clinical trials with large study samples, is required to assess their effectiveness and potential side effects.
Table 1.
Therapeutic effects of vitamins and other nutritional supplements on sinusitis.
| Author | Type of study | Intervention or measured compound | Participants | Outcome | |
|---|---|---|---|---|---|
| Ünal et al 91 | Animal study | Intervention | Vitamin A | 20 animals | There was no difference between the study and control groups’ sinus inflammation changes. |
| Fang et al 92 | Within-subject control study | Intervention | Vitamin A | 30 patients | Antrostomy stenosis prevention, mucociliary promotion, and adhesion reduction are achieved by topical vitamin A. |
| Guven et al 93 | Prospective controlled animal trial | Intervention | Vitamin A | 20 New Zealand hybrid rabbits | There is no significant statistical difference between the control and study groups. |
| Unal et al 16 | Case-control | Measured | Vitamin A, vitamin E, vitamin C, zinc, and copper | 24 patients between 7 and 12 y | In the patients’ group, the levels of vitamin E, vitamin C, copper (Cu), and zinc (Zn) were found to be significantly lower compared to the control group. However, no significant difference was observed in the levels of vitamin A and magnesium (Mg) between the 2 groups. |
| Tomiki 94 | Case-control | Intervention | Vitamin A and vitamin D | 265 school children | Vitamin A was ineffective in the treatment but useful in preventing CRS in children. |
| Dhanawat 17 | Clinical trial | Intervention | Prabhakar Reddy et al | 53 patients with allergic rhinitis | Vitamins A, E, and C’s role (antioxidant, anti-inflammatory) in the treatment of allergic rhinitis was significant. |
| Prabhakar Reddy et al 33 | Case-control | Measured | Vitamin C | 40 cases and 40 controls | A lower vitamin C level in the cases was observed. |
| Ogal et al 60 | Randomized clinical trial | Intervention | Vitamin C and Echinacea | 94 Children (intervention group) and 93 children (control group) |
Echinacea prevents RTI better than vitamin C. |
| Zhang et al 32 | In vitro | Intervention | Combination of Vitamin C with Scutellaria baicalensis And Eleutherococcus senticosus | Nasal mucosal tissue of 12 patients | Suppression of Th-1, Th-2, and Th-17. |
| Baruah et al 52 | Cross-sectional | Intervention | Vitamin D | 100 CRS patients were given oral Vitamin D, and 100 CRS patients were treated with placebo | At baseline, the pretreatment TNSS exhibited an average score of 11.92. Following a duration of 3 mo, the scores experienced a notable reduction with an average decrease of 10.65 points, demonstrating a statistically significant difference. |
| Bavi et al 46 | Cross-sectional | Measured | Vitamin D | 166 CRS patients with nasal polyps and 172 healthy subjects | Decreased vitamin D levels in Iranian patients with CRS with nasal polyps, with an association to disease intensity |
| Boeva et al 47 | Cross-sectional | Measured | Vitamin D | 50 patients with sinusitis and 14 healthy subjects | Higher levels of vitamin D in the control subjects compared to patients with chronic polypous rhinosinusitis. |
| Duţu et al 48 | Cross-sectional | Measured | Vitamin D | 6 CRS patients and 21 non-smoking controls | Reducing inflammation increases vitamin D levels. |
| Elbistanlı et al 95 | Prospective case-control | Measured | Vitamin D | Group 1 consisted of fifteen pediatric patients with ARS-induced preseptal cellulitis complication, Group 2 consisted of fifteen ARS patients without complication, and Group 3 consisted of fifteen healthy volunteers. At the first admission of research participants, serum vitamin D levels (nmol/l) were assessed in addition to regular blood testing. | There was a statistically significant difference according to the existence of ARS (Group-1 and Group 2) and absence of ARS (Group-3) based on categorization in which vitamin D levels were compared with normal values (p 0.05). Between Group 1 and Group 3, a statistically significant difference was also discovered. |
| Faghih Habibi et al 96 | Cross-sectional controlled study | Measured | Vitamin D | 32 CRS patients with nasal polyps, 35 CRS patients without nasal polyps, and 50 healthy controls | The serum level of Vitamin D was considerably lower in CRS patients compared with the other group. |
| Hashemian et al 49 | Cross-sectional | Intervention | Vitamin D | 40 patients with CRS with nasal polyposis | Vitamin D efficiently reduced the recurrence of polyposis after FESS in CRS patients with nasal polyps. |
| Kalińczak-Górna et al 11 | Cross-sectional | Measured | Vitamin D | 40 CRS patients | Reducing inflammation increases vitamin D levels. |
| Konstantinidis et al 35 | Cross-sectional | Measured | Vitamin D | 27 CF patients with nasal polyps, 31 CF patients without nasal polyps, 32 CRS patients with nasal polyps, 30 patients without nasal polyps, and 32 healthy controls | Vitamin D deficiency with nasal polyps appears similar in those with CRS and CF. The lower the serum vitamin D level, the more severe the mucosal disease. |
| Mostafa Bel et al 7 | Case-control | Measured | Vitamin D | 25 patients with allergic fungal rhinosinusitis, 15 CRS patients with nasal polyps, 15 CRS patients without nasal polyps, and 19 healthy controls | Vitamin D levels in CRS patients with nasal polyps and AFRS were meaningfully lower than those in CRS patients without nasal polyps. |
| Schlosser et al 37 | Case-control | Measured | Vitamin D | Blood and sinus tissue | CRS Patients with nasal polyps and AFRS patients both have decreased sinonasal vitamin D. |
| Shanaki et al 40 | Case-control | Measured | Vitamin D | 45 CRS patients with nasal polyps and 45 controls | CRS patients with nasal polyps have lower levels of vitamin D in their serum compared to controls. |
| Zand et al 41 | Case-control | Measured | Vitamin D | 93 CRS patients with nasal polyps | An association between vitamin D levels and disease intensities in CRS patients with nasal polyps. |
| Tomaszewska et al 45 | Case-control | Measured | Vitamin D | 52 CRS patients without nasal polyps, 55 CRS patients with nasal polyps, and 59 controls | Vitamin D and its receptor expression may be associated with CRS. |
| Thakur and Potluri 42 | Case-control | Measured | Vitamin D | 30 CRS patients without nasal polyps, 30 CRS patients with nasal polyps, and 31 controls | Vitamin D was a predictive factor for the Occurrence of CRS. |
| Ma et al 97 | Clinical trial | Measured | Vitamin D | Human sinonasal epithelium of fifteen patients with eosinophilic CRS and healthy controls | Topical vitamin D in CRS patients was beneficial for the innate immune system. |
| Mulligan et al 36 | Clinical trial | Intervention | Vitamin D | Female mice (8 wk old) | Loss of vitamin D in the diet intensified immunological changes related to Aspergillus fumigatus-CRS. |
| Sansoni et al 43 | Clinical trial | Measured | Vitamin D | 57 patients in groups of CRS with and without nasal polyposis | Vitamin D may have a role in the regulation of the immune system. |
| Westerveld 59 | Cross-sectional | Measured | Vitamin E | Mucosa samples of 9 patients with CRS and 10 healthy persons | There was no difference in levels of vitamin E between patients and the healthy group. |
| Ishrefova et al 98 | Cohort | Intervention | Echinacea | A number of children in the time 2008 to 2014 | Using Echinacea lowers the rate of complications of RTI such as sinusitis, bronchitis, and adenoiditis. |
| Popov et al 66 | Clinical trial | Intervention | N-acetyl cysteine | 48 patients in the intervention group 50 patients in the control group | N-acetyl cysteine alleviated the symptoms, decreased relapses, and increased the efficacy of surgery among the study group. |
| Bezshapochniy et al 63 | Clinical trial | Intervention | N-acetyl cysteine | 29 patients in the intervention group 27 patients in the control group | Saline and N-acetyl cysteine solutions alleviated the symptoms. |
| Perić et al 99 | Randomized comparative study | Intervention | Sinupret | 20 CRS patients without nasal polyps and 20 patients who used fluticasone spray | Sinupret may be a safe and effective alternative to intranasal corticosteroids for the treatment of CRS patients without nasal polyps. |
| Sen’kevich et al 100 | Randomized prospective comparative clinical study | Intervention | Sinupret | 107 children aged 4 to 5 y with ARS | The use of Sinupret in the treatment of children with acute rhinosinusitis (ARS) caused by a viral etiology is associated with a more pronounced favorable outcome. |
| Vishnyakov and Sinkov 101 | Cohort | Intervention | Sinupret | 25 patients and 25 control group | Overall satisfaction with the treatment was higher in the herbal medication group compared to the antibiotic drug control group. |
| Popovych et al 102 | Randomized comparative study | Intervention | Sinupret | 292 children aged 6 to 11 y with ARS | Sinupret was beneficial for ARS in both adults and children. |
| Passali et al 69 | Prospective open-label Study | Intervention | Sinupret | 30 patients with ARS were treated with sinupret, and 30 patients used fluticasone | Symptoms improved significantly in all of the patients using sinupret. |
| Neubauer and März 70 | Clinical trial | Intervention | Sinupret | 160 patients with acute bacterial sinusitis | Sinupret improved sinusitis symptoms. |
| Palm et al 67 | Clinical trial | Intervention | Sinupret | 929 patients with CRS | Sinupret extract was found to be effective in treating CRS patients. |
| Jund et al 103 | Clinical trial | Intervention | Sinupret | 589 patients with acute viral rhinosinusitis | Sinupret is an effective and safe herbal drug for viral sinusitis therapy. |
| Mohammadhossein et al 89 | Case-control | Intervention | Zinc supplements | 50 participants with nasal polyposis and 50 participants as the control group | The serum level of zinc was low in patients suffering from nasal polyposis. |
| Akbari Dilmaghani et al 90 | Randomized clinical trial | Intervention | Zinc supplements | 44 participants | Using zinc supplements offers several advantages for improving general health. |
Abbreviation: AFRS, allergic fungal rhinosinusitis; ARS, acute rhinosinusitis; CF, cystic fibrosis; CI, chloride; CRS, chronic rhinosinusitis; FESS, functional endoscopic sinus surgery; RTI, respiratory tract infection; TNSS, total nasal symptom score.
Figure 1.
A review of therapeutic effects of vitamins and various nutrition on sinusitis. Vitamins and nutritional supplements can reduce sinusitis symptoms.
Footnotes
Author Contributions: Conceived and designed the analysis: Mohammad Ghaheri, Narjes Sadat Farizani Gohari and Dorsa Abolhasani. Collected data: Sadaf Parvin, Mohammad Mahdi Ghodrati and Shekoufeh Mohammadpour. Contributed data or analysis tools: Erfan Kazemi, Fatemeh Mohammadyari and Armin Alinezhad. Performed the analysis: Farhad Nikzad, Shahin Sabbaghi and Sara Saeedpour. Designed the study: Mohadeseh Poudineh, Sepehr Olangian-Tehrani and Farbod Heydarasadi. Wrote the paper: Mohadeseh Poudineh, Farhad Nikzad, Sadaf Parvin, Mohammad Ghaheri, Shahin Sabbaghi, Erfan Kazemi, Mohammad Mahdi Ghodrati, Fatemeh Mohammadyari, Sara Saeedpour, Shekoufeh Mohammadpour, Narjes Sadat Farizani Gohari, Farbod Heydarasadi, Dorsa Abolhasani, Sepehr Olangian-Tehrani and Armin Alinezhad. All authors reviewed the results and approved the final version of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Stokes PJ, Rimmer J. The relationship between serum vitamin D and chronic rhinosinusitis: a systematic review. Am J Rhinol Allergy. 2016;30:23-28. [DOI] [PubMed] [Google Scholar]
- 2. Battisti AS, Modi P, Pangia J. Sinusitis. StatPearls Publishing LLC; 2021. [PubMed] [Google Scholar]
- 3. Xu Y, Quan H, Faris P, et al. Prevalence and incidence of diagnosed chronic rhinosinusitis in Alberta, Canada. Otolaryngol Head Neck Surg. 2016;142:1063-1069. [DOI] [PubMed] [Google Scholar]
- 4. Philpott CM, Erskine S, Hopkins C, et al. Prevalence of asthma, aspirin sensitivity and allergy in chronic rhinosinusitis: data from the UK National Chronic Rhinosinusitis Epidemiology Study. Respir Res. 2018;19:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schilling AL, Carcella AR, Moore J, et al. Compatibility of a thermoresponsive and controlled release system for promoting sinonasal cilia regeneration. Macromol Biosci. 2021;21:2100277. [DOI] [PubMed] [Google Scholar]
- 6. Linday LA, Dolitsky JN, Shindledecker RD. Nutritional supplements as adjunctive therapy for children with chronic/recurrent sinusitis: pilot research. Int J Pediatr Otorhinolaryngol. 2004;68:785-793. [DOI] [PubMed] [Google Scholar]
- 7. Mostafa Bel D, Taha MS, Abdel Hamid T, Omran A, Lotfi N. Evaluation of vitamin D levels in allergic fungal sinusitis, chronic rhinosinusitis, and chronic rhinosinusitis with polyposis. Int Forum Allergy Rhinol. 2016;6:185-190. [DOI] [PubMed] [Google Scholar]
- 8. Bishop LE, Ismailova A, Dimeloe S, Hewison M, White JH. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. JBMR Plus. 2021;5:e10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reifen R. Vitamin A as an anti-inflammatory agent. Proc Nutr Soc. 2002;61:397-400. [DOI] [PubMed] [Google Scholar]
- 10. Olangian-Tehrani S, Poudineh M, Parvin S, et al. The effects of vitamin therapy on ASD and ADHD: a narrative review. CNS Neurol Disord Drug Targets. 2023;22:711-735. [DOI] [PubMed] [Google Scholar]
- 11. Kalińczak-Górna P, Radajewski K, Burduk P. Relationship between the severity of inflammatory changes in chronic sinusitis and the level of vitamin D before and after the FESS procedure. J Clin Med. 2021;10:2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee GY, Han SN. The role of vitamin E in immunity. Nutrients. 2018;10:1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carazo A, Macáková K, Matoušová K, et al. Vitamin A update: forms, sources, kinetics, detection, function, deficiency, therapeutic use and toxicity. Nutrients. 2021;13:1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schwartz SG, Wang X, Chavis P, Kuriyan AE, Abariga SA. Vitamin A and fish oils for preventing the progression of retinitis pigmentosa. Cochrane Database Syst Rev. 2020;6:Cd008428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Debelo H, Novotny JA, Ferruzzi MG. Vitamin A. Adv Nutr. 2017;8:992-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Unal M, Tamer L, Pata YS, et al. Serum levels of antioxidant vitamins, copper, zinc and magnesium in children with chronic rhinosinusitis. J Trace Elem Med Biol. 2004;18:189-192. [DOI] [PubMed] [Google Scholar]
- 17. Dhanawat GS. Rhinitis, sinusitis and ocular disease – 2100. New approach to treat allergic rhinitis with vitamin E, cod liver oil and vitamin C with use of nasal steroidal spray. World Allergy Organ J. 2013;6:175-175. [Google Scholar]
- 18. Valdés F. Vitamina C. Actas Dermosifiliogr. 2006;97:557-568. [DOI] [PubMed] [Google Scholar]
- 19. Giuffrè A, Nobile R. Citrus bergamia, Risso: the peel, the juice and the seed oil of the bergamot fruit of Reggio Calabria (South Italy). Emirates J Food Agric. 2020;32:522-532. [Google Scholar]
- 20. Kathi S, Laza H, Singh S, et al. Vitamin C biofortification of broccoli microgreens and resulting effects on nutrient composition. Front Plant Sci. 2023;14:1145992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Meng Z, Malik AU, Zhang S, Wang Q. Maintaining the quality of postharvest broccoli by inhibiting ethylene accumulation using diacetyl. Front Nutr. 2022;9:1055651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kumar J, Sen A. The role of vitamin C: from prevention of pneumonia to treatment of covid-19. Mater Today Proc. Published online November 18, 2022. doi: 10.1016/j.matpr.2022.11.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bhat TA, Hussain SZ, Wani SM, et al. The impact of different drying methods on antioxidant activity, polyphenols, vitamin C and rehydration characteristics of Kiwifruit. Food Biosci. 2022;48:101821. [Google Scholar]
- 24. Ali MN, Serçe S. Vitamin C and fruit quality consensus in breeding elite European strawberry under multiple interactions of environment. Mol Biol Rep. 2022;49:11573-11586. [DOI] [PubMed] [Google Scholar]
- 25. Giuffrè AM. Bergamot (Citrus bergamia, Risso): the effects of cultivar and harvest date on functional properties of juice and Cloudy Juice. Antioxidants. 2019;8:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dobón-Suárez A, Giménez MJ, Castillo S, García-Pastor ME, Zapata PJ. Influence of the phenological stage and harvest date on the bioactive compounds content of green pepper fruit. Molecules. 2021;26:3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruiz-Valdepeñas Montiel V, Sempionatto JR, Vargas E, et al. Decentralized vitamin C & D dual biosensor chip: toward personalized immune system support. Biosens Bioelectron. 2021;194:113590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khan H, Hussain F, Samad A. Cure and prevention of diseases with vitamin C into perspective: an overview. J Crit Rev. 2020;7:289-293. [Google Scholar]
- 29. Fischer H, Schwarzer C, Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci. 2004;101:3691-3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fischer H, Illek B. Vitamin C and flavonoids potentiate CFTR CL transport in human airway epithelia. In: Schultz C, ed. Defects of Secretion in Cystic Fibrosis. Springer; 2005;129-144. [Google Scholar]
- 31. Cho DY, Hwang PH, Illek B. Effect of L-ascorbate on chloride transport in freshly excised sinonasal epithelia. Am J Rhinol Allergy. 2009;23:294-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang N, Van Crombruggen K, Holtappels G, Bachert C. A herbal composition of Scutellaria baicalensis and Eleutherococcus senticosus shows potent anti-inflammatory effects in an ex vivo human mucosal tissue model. Evid Based Complement Alternat Med. 2012;2012:673145-673149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Prabhakar Reddy E, Muthukumaraswamy B, Venkataramanan R, Sai Ravi Kiran B, Mohanalakshmi T. Evaluation of thyroid profile and oxidative stress and antioxidants parameters in chronic sinusitis. Int J Pharm Sci Res. 2017;8:455-458. [Google Scholar]
- 34. Shahangian A, Schlosser RJ. Role of vitamin D in pathogenesis of chronic sinusitis with nasal polyposis. Adv Otorhinolaryngol. 2016;79:86-90. [DOI] [PubMed] [Google Scholar]
- 35. Konstantinidis I, Fotoulaki M, Iakovou I, et al. Vitamin D₃ deficiency and its association with nasal polyposis in patients with cystic fibrosis and patients with chronic rhinosinusitis. Am J Rhinol Allergy. 2017;31:395-400. [DOI] [PubMed] [Google Scholar]
- 36. Mulligan JK, Pasquini WN, Carroll WW, et al. Dietary vitamin D3 deficiency exacerbates sinonasal inflammation and alters local 25(OH)D3 metabolism. PLoS One. 2017;12:e0186374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schlosser RJ, Carroll WW, Soler ZM, Pasquini WN, Mulligan JK. Reduced sinonasal levels of 1α-hydroxylase are associated with worse quality of life in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2016;6:58-65. [DOI] [PubMed] [Google Scholar]
- 38. Wang LF, Lee CH, Chien CY, et al. Serum 25-hydroxyvitamin D levels are lower in chronic rhinosinusitis with nasal polyposis and are correlated with disease severity in Taiwanese patients. Am J Rhinol Allergy. 2013;27:e162-e165. [DOI] [PubMed] [Google Scholar]
- 39. Schlosser RJ, Soler ZM, Schmedes GW, Storck K, Mulligan JK. Impact of vitamin D deficiency upon clinical presentation in nasal polyposis. Int Forum Allergy Rhinol. 2014;4:196-199. [DOI] [PubMed] [Google Scholar]
- 40. Shanaki M, Doulabi SRM, Dilmaghani N. Circulatory levels of 25-OHD and vitamin D binding protein in patients with chronic rhinosinusitis with polyposis: A case-control study. Biomed Res. 2017;28:4625-4629. [Google Scholar]
- 41. Zand V, Baradaranfar M, Vaziribozorg S, et al. Correlation of serum vitamin D levels with chronic rhinosinusitis disease severity. Iran J Otorhinolaryngol. 2020;32:35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thakur P, Potluri P. Association of serum vitamin D with chronic rhinosinusitis in adults residing at high altitudes. Eur Arch Otorhinolaryngol. 2021;278:1067-1074. [DOI] [PubMed] [Google Scholar]
- 43. Sansoni ER, Sautter NB, Mace JC, et al. Vitamin D3 as a novel regulator of basic fibroblast growth factor in chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol. 2015;5:191-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang LF, Chien CY, Tai CF, Chiang FY, Chen JY. Vitamin D decreases the secretion of eotaxin and RANTES in nasal polyp fibroblasts derived from Taiwanese patients with chronic rhinosinusitis with nasal polyps. Kaohsiung J Med Sci. 2015;31:63-69. [DOI] [PubMed] [Google Scholar]
- 45. Tomaszewska M, Sarnowska E, Rusetska N, et al. Role of vitamin D and its receptors in the pathophysiology of chronic rhinosinusitis. J Am Coll Nutr. 2019;38:108-118. [DOI] [PubMed] [Google Scholar]
- 46. Bavi F, Movahed R, Salehi M, Hossaini S, Bakhshaee M. Chronic rhinosinusitis with polyposis and serum vitamin D levels. Acta Otorhinolaryngol Ital. 2019;39:336-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boeva VI, Kokorina OV, Archba RR, Dvoryanchikov VV, Kolhyubaeva SN. [The influence of the vitamin D3 level in the blood serum of lactase gene polymorphism on the development of chronic polypous rhinosinusitis]. Vestn Otorinolaringol. 2018;83:49-54. [DOI] [PubMed] [Google Scholar]
- 48. Duţu AG, Vlad D, Drugan C, et al. Biochemical markers of inflammatory syndrome in chronic rhinosinusitis. Rom Biotechnol Lett. 2020;25:1456-1464. [Google Scholar]
- 49. Hashemian F, Sadegh S, Jahanshahi J, Seif Rabiei MA, Hashemian F. Effects of vitamin D supplementation on recurrence of nasal polyposis after endoscopic sinus Surgery. Iran J Otorhinolaryngol. 2020;32:21-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee EJ, Hwang CS, Kim KS. Lack of correlation between serum 25(OH)D level and endoscopy-based chronic rhinosinusitis in Korean adults. Rhinology. 2019;57:139-146. [DOI] [PubMed] [Google Scholar]
- 51. Li B, Wang M, Zhou L, Wen Q, Zou J. Association between serum vitamin D and chronic rhinosinusitis: a meta-analysis. Braz J Otorhinolaryngol. 2021;87:178-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Baruah B, Gupta A, Kumar A, Kumar A. The role of oral vitamin D3 supplementation in the treatment of chronic rhinosinusitis in adults with vitamin D deficiency. J Fam Med Prim Care. 2020;9:2877-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abraham A, Kattoor AJ, Saldeen T, Mehta JL. Vitamin E and its anticancer effects. Crit Rev Food Sci Nutr. 2019;59:2831-2838. [DOI] [PubMed] [Google Scholar]
- 54. Shahidi F, Pinaffi-Langley ACC, Fuentes J, Speisky H, de Camargo AC. Vitamin E as an essential micronutrient for human health: common, novel, and unexplored dietary sources. Free Radic Biol Med. 2021;176:312-321. [DOI] [PubMed] [Google Scholar]
- 55. Giuffrè A, Caracciolo M, Zappia C, Capocasale M, Poiana M. Effect of heating on chemical parameters of extra virgin olive oil, pomace olive oil, soybean oil and palm oil. Ital J Food Sci. 2018;30. [Google Scholar]
- 56. Kostadinović Veličkovska S, Brühl L, Mitrev S, Mirhosseini H, Matthäus B. Quality evaluation of cold-pressed edible oils from Macedonia. Eur J Lipid Sci Technol. 2015;117:2023-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Herrera E, Barbas C. Vitamin E: action, metabolism and perspectives. J Physiol Biochem. 2001;57:43-56. [PubMed] [Google Scholar]
- 58. Zaaboul F, Liu Y. Vitamin E in foodstuff: nutritional, analytical, and food technology aspects. Compr Rev Food Sci Food Saf. 2022;21:964-998. [DOI] [PubMed] [Google Scholar]
- 59. Westerveld GJ, Dekker I, Voss HP, Bast A, Scheeren RA. Antioxidant levels in the nasal mucosa of patients with chronic sinusitis and healthy controls. Arch Otolaryngol Head Neck Surg. 1997;123:201-204. [DOI] [PubMed] [Google Scholar]
- 60. Ogal M, Johnston SL, Klein P, Schoop R. Echinacea reduces antibiotic usage in children through respiratory tract infection prevention: a randomized, blinded, controlled clinical trial. Eur J Med Res. 2021;26:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vimalanathan S, Schoop R, Suter A, Hudson J. Prevention of influenza virus induced bacterial superinfection by standardized Echinacea purpurea, via regulation of surface receptor expression in human bronchial epithelial cells. Virus Res. 2017;233:51-59. [DOI] [PubMed] [Google Scholar]
- 62. Mokhtari V, Afsharian P, Shahhoseini M, Kalantar S, Moini A. A review on various uses of N-acetyl cysteine. Cell J. 2017;19:11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bezshapochniy SB, Loburets VV, Loburets AV, Dzhirov OR, Podovzhniy OG. Peculiarities of nasal irrigation in acute viral rhinosinusitis. World -med Biol. 2020;16:007. [Google Scholar]
- 64. Šalamon Š, Kramar B, Marolt TP, Poljšak B, Milisav I. Medical and dietary uses of N-Acetylcysteine. Antioxidants. 2019;8:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Blasi F, Page C, Rossolini GM, et al. The effect of N-acetylcysteine on biofilms: Implications for the treatment of respiratory tract infections. Respir Med. 2016;117:190-197. [DOI] [PubMed] [Google Scholar]
- 66. Popov IB, Shcherbakov DA, Tyryk OB, Aleksanyan TA. [New approach to treatment of polypous rhinosinusitis]. Vestn Otorinolaringol. 2020;85:48-51. [DOI] [PubMed] [Google Scholar]
- 67. Palm J, Steiner I, Abramov-Sommariva D, et al. Assessment of efficacy and safety of the herbal medicinal product BNO 1016 in chronic rhinosinusitis. Rhinology. 2017;55:142-151. [DOI] [PubMed] [Google Scholar]
- 68. Zhang S, Skinner D, Hicks SB, et al. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS One. 2014;9:e104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Passali D, Loglisci M, Passali GC, et al. A prospective open-label study to assess the efficacy and safety of a herbal medicinal product (sinupret) in patients with acute rhinosinusitis. ORL. 2015;77:27-32. [DOI] [PubMed] [Google Scholar]
- 70. Neubauer N, März RW. Placebo-controlled, randomized double-blind clinical trial with sinupret® sugar coated tablets on the basis of a therapy with antibiotics and decongestant nasal drops in acute sinusitis. Phytomedicine. 1994;1:177-181. [DOI] [PubMed] [Google Scholar]
- 71. Ross SM. An integrative approach to rhinosinusitis in children, Part II. Holist Nurs Pract. 2010;24:49-51. [DOI] [PubMed] [Google Scholar]
- 72. Glatthaar-Saalmüller B, Rauchhaus U, Rode S, Haunschild J, Saalmüller A. Antiviral activity in vitro of two preparations of the herbal medicinal product sinupret® against viruses causing respiratory infections. Phytomedicine. 2011;19:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rossi A, Dehm F, Kiesselbach C, et al. The novel sinupret® dry extract exhibits anti-inflammatory effectiveness in vivo. Fitoterapia. 2012;83:715-720. [DOI] [PubMed] [Google Scholar]
- 74. Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999;47:2274-2279. [DOI] [PubMed] [Google Scholar]
- 75. Mitchell AE, Hong YJ, Koh E, et al. Ten-year comparison of the influence of organic and conventional crop management practices on the content of flavonoids in tomatoes. J Agric Food Chem. 2007;55:6154-6159. [DOI] [PubMed] [Google Scholar]
- 76. Tutel’ian VA, Lashneva NV. [Biologically active substances of plant origin. flavanols and flavones: prevalence, dietary sources and consumption]. Vopr Pitan. 2013;82:4-22. [PubMed] [Google Scholar]
- 77. Smith C, Lombard K, Peffley E, Liu W. Genetic analysis of quercetin in onion (Allium cepa L.)’Lady Raider. Tex J Agric Nat Resour. 2003;16:24-28. [Google Scholar]
- 78. Williamson G, Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr. 2005;81:243s-255s. [DOI] [PubMed] [Google Scholar]
- 79. Wiczkowski W, Romaszko J, Bucinski A, et al. Quercetin from shallots (Allium cepa L. Var. Aggregatum) is more bioavailable than its glucosides. J Nutr. 2008;138:885-888. [DOI] [PubMed] [Google Scholar]
- 80. Petrus K, Schwartz H, Sontag G. Analysis of flavonoids in honey by HPLC coupled with coulometric electrode array detection and electrospray ionization mass spectrometry. Anal Bioanal Chem. 2011;400:2555-2563. [DOI] [PubMed] [Google Scholar]
- 81. Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19). Front Immunol. 2020;11:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li Y, Yao J, Han C, et al. Quercetin, inflammation and immunity. Nutrients. 2016;8:167-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Jafarinia M, Jafarinia M. A review of medicinal properties of some Asteraceae family plants on immune system. Rep Heal Care. 2019;5:1-7. [Google Scholar]
- 84. Mlcek J, Jurikova T, Skrovankova S, Sochor J. Quercetin and its anti-allergic immune response. Molecules. 2016;21:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jafarinia M, Sadat Hosseini M, kasiri N, et al. Quercetin with the potential effect on allergic diseases. Allergy Asthma Clin Immunol. 2020;16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Zhang S, Smith N, Schuster D, et al. Quercetin increases cystic fibrosis transmembrane conductance regulator–mediated chloride transport and ciliary beat frequency: therapeutic implications for chronic rhinosinusitis. Am J Rhinol Allergy. 2011;25:307-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Prasad A, Fitzgerald J, Hess J, et al. Zinc deficiency in elderly patients. Nutrition. 1993;9:218-224. [PubMed] [Google Scholar]
- 88. Prasad AS. Discovery and importance of zinc in human nutrition. Fed Proc. 1984;43:2829-2834. [PubMed] [Google Scholar]
- 89. Mohammadhossein D, Mohammad E, Mohammadhossein B, Mojtaba M, Vahid Z, et al. Evaluation of the zinc, copper and iron serum levels in patients with nasal polyposis. Exp Rhinol Otolaryngol. 2018;2. [Google Scholar]
- 90. Akbari Dilmaghani N, Alani N, Fazeli S. A randomized clinical trial of elemental zinc add-on therapy on clinical outcomes of patients with chronic rhinosinusitis with nasal polyposis (CRSwNP). Iran J Pharm Res Summer. 2019;18:1595-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Ünal M, Öztürk C, Aslan G, Aydin Görür K. The effect of high single dose parenteral vitamin A in addition to antibiotic therapy on healing of maxillary sinusitis in experimental acute sinusitis. Int J Pediatr Otorhinolaryngol. 2002;65:219-223. [DOI] [PubMed] [Google Scholar]
- 92. Fang KM, Wang CT, Chen YW, Huang TW. Reduction of adhesions and antrostomy stenosis with topical vitamin A after endoscopic sinus surgery. Am J Rhinol Allergy. 2015;29:430-434. [DOI] [PubMed] [Google Scholar]
- 93. Guven M, Aladag I, Eyibilen A, et al. Experimentally induced acute sinusitis and efficacy of vitamin A. Acta Otolaryngol. 2007;127:855-860. [DOI] [PubMed] [Google Scholar]
- 94. Tomiki K. Conservative treatment of chronic sinusitis in children. Nippon Jibiinkoka Gakkai Kaiho. 1958;61:685-693. [Google Scholar]
- 95. Elbistanlı MS, Koçak HE, Güneş S, et al. Vit D deficiency is a possible risk factor in ARS. Eur Arch Otorhinolaryngol. 2017;274:3391-3395. [DOI] [PubMed] [Google Scholar]
- 96. Faghih Habibi A, Gerami H, Banan R, et al. Serum 25-hydroxy vitamin D in chronic rhinosinusitis with and without nasal polyposis: a case-control study. Iran J Otorhinolaryngol. 2019;31:19-24. [PMC free article] [PubMed] [Google Scholar]
- 97. Ma SW, Ende JA, Alvarado R, et al. Topical vitamin D may modulate human sinonasal mucosal responses to house dust mite antigen. Am J Rhinol Allergy. 2020;34:471-481. [DOI] [PubMed] [Google Scholar]
- 98. Ishrefova LR, Lyalina LV, Lioznov DA, et al. Argumentation of acute respiratory viral infections nonspecific prevention in groups of children. Russ J Infect Immun. 2016;6:184-188. [Google Scholar]
- 99. Perić A, Gaćeša D, Aleksić A, Kopacheva-Barsova G, Perić AV. Herbal drug BNO 1016 versus fluticasone propionate nasal spray in the treatment of chronic rhinosinusitis without nasal polyps: a preliminary report. Eur J Ther. 2020;26:192-201. [Google Scholar]
- 100. Sen’kevich OA, Sidorenko SV, Ditrikh OA. [Comparative efficacy of various treatment regimens for children 2-5 years old with symptoms of acute viral rhinosinusitis]. Vestn Otorinolaringol. 2021;86:46-50. [DOI] [PubMed] [Google Scholar]
- 101. Vishnyakov VV, Sinkov DE. Herbal medicine as add-on therapy in acute rhinosinusitis. Z Civilistische Phytother. 2014;34:262-265. [Google Scholar]
- 102. Popovych VI, Beketova HV, Koshel IV, et al. An open-label, multicentre, randomized comparative study of efficacy, safety and tolerability of the 5 plant - extract BNO 1012 in the delayed antibiotic prescription method in children, aged 6 to 11 years with acute viral and post-viral rhinosinusitis. Am J Otolaryngol. 2020;41:102564. [DOI] [PubMed] [Google Scholar]
- 103. Jund R, Mondigler M, Stammer H, Stierna P, Bachert C. Herbal drug BNO 1016 is safe and effective in the treatment of acute viral rhinosinusitis. Acta Otolaryngol. 2015;135:42-50. [DOI] [PMC free article] [PubMed] [Google Scholar]