Abstract
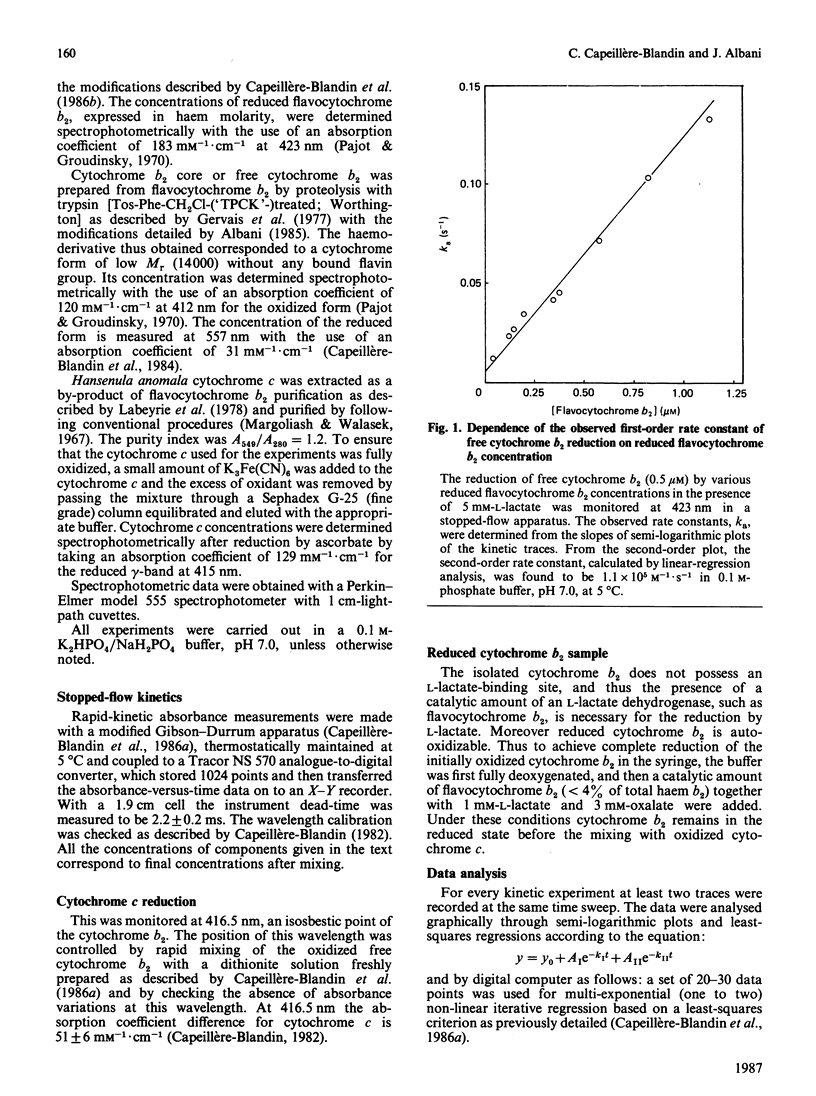
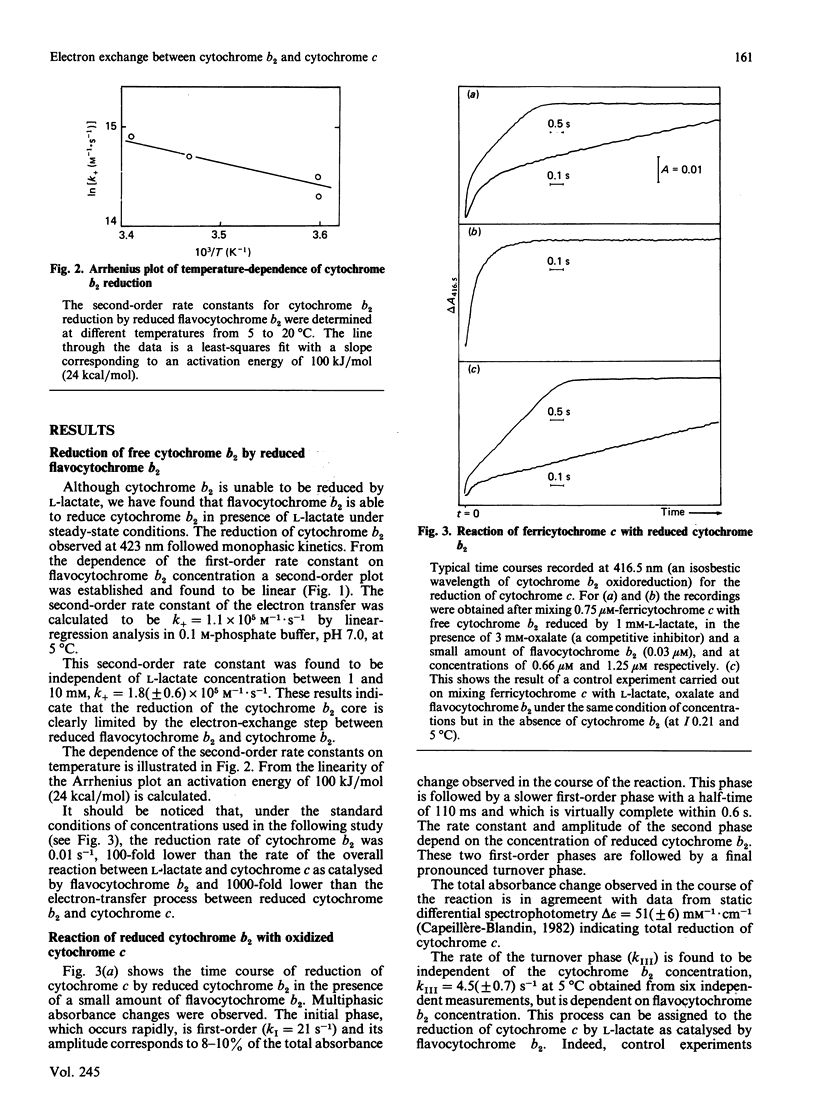
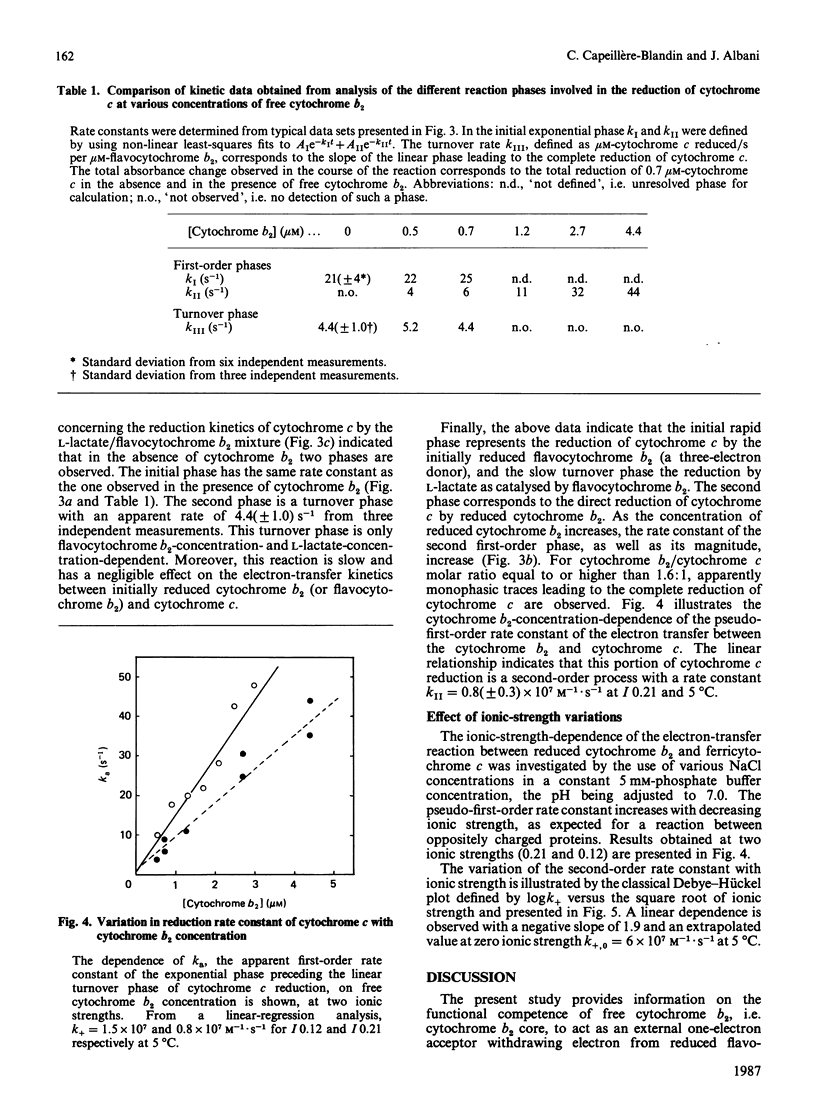
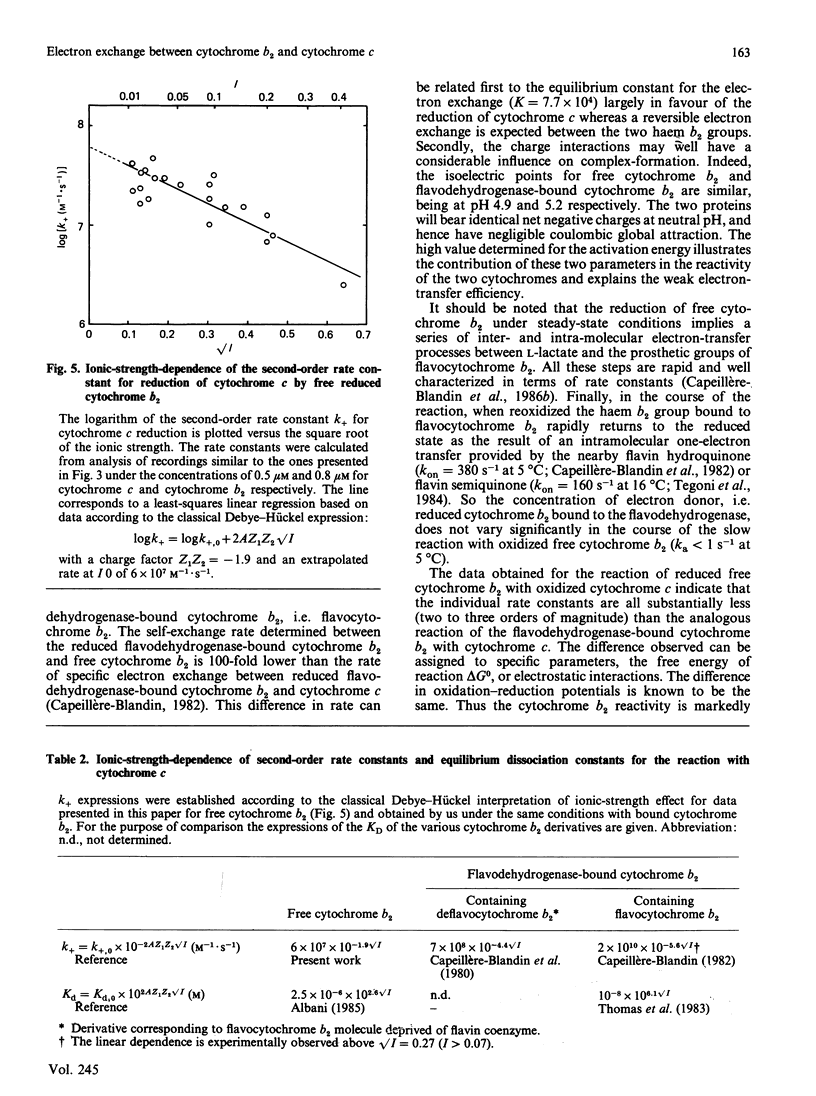
The oxidation-reduction properties of free cytochrome b2 isolated by controlled proteolysis from flavocytochrome b2, i.e. the flavodehydrogenase-bound cytochrome b2, were investigated by using stopped-flow spectrophotometry. The rapid kinetics of the reduction of cytochrome b2 by flavocytochrome b2 in the presence of L-lactate are reported. The self-exchange rate constant between reduced cytochrome b2 bound to the flavodehydrogenase and free cytochrome b2 was determined to be 10(5) M-1 X S-1 at 5 degrees C, I 0.2 and pH 7.0. The specific electron-transfer reaction between reduced cytochrome b2 and cytochrome c was also studied, giving an apparent second-order rate constant of 10(7) M-1 X S-1 at 5 degrees C, I 0.2 and pH 7.0. This electron-exchange rate is slightly modulated by ionic strength, following the Debye-Hückel relationship with a charge factor Z1Z2 = -1.9. Comparison of these data with those for the reduction of cytochrome c by flavodehydrogenase-bound cytochrome b2 [Capeillère-Blandin (1982) Eur. J. Biochem. 128, 533-542] leads to the conclusion that the intramolecular electron exchange between haem b2 and haem c within the reaction complex occurs at a rate very similar to that determined experimentally in presence of the flavodehydrogenase domain. The low reaction rate observed with free cytochrome b2 is ascribed to the low stability of the reaction complex formed between free cytochrome b2 and cytochrome c.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albani J. Fluorescence studies on the interaction between two cytochromes extracted from the yeast, Hansenula anomala. Arch Biochem Biophys. 1985 Nov 15;243(1):292–297. doi: 10.1016/0003-9861(85)90798-2. [DOI] [PubMed] [Google Scholar]
- Berg O. G., von Hippel P. H. Diffusion-controlled macromolecular interactions. Annu Rev Biophys Biophys Chem. 1985;14:131–160. doi: 10.1146/annurev.bb.14.060185.001023. [DOI] [PubMed] [Google Scholar]
- Capeillere-Blandin C., Pucheault J., Ferradini C. The reduction of flavocytochrome b2 by carboxylate radicals. A pulse radiolysis study. Biochim Biophys Acta. 1984 Apr 27;786(1-2):67–78. doi: 10.1016/0167-4838(84)90155-9. [DOI] [PubMed] [Google Scholar]
- Capeillere-Blandin C. Transient kinetics of the one-electron transfer reaction between reduced flavocytochrome b2 and oxidized cytochrome c. Evidence for the existence of a protein complex in the reaction. Eur J Biochem. 1982 Nov 15;128(2-3):533–542. doi: 10.1111/j.1432-1033.1982.tb06998.x. [DOI] [PubMed] [Google Scholar]
- Capeillère-Blandin C., Barber M. J., Bray R. C. Comparison of the processes involved in reduction by the substrate for two homologous flavocytochromes b2 from different species of yeast. Biochem J. 1986 Sep 15;238(3):745–756. doi: 10.1042/bj2380745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais M., Groudinsky O., Risler Y., Labeyrie F. Dissection of flavocytochrome b2-a bifunctional enzyme-into a cytochrome core and a flavoprotein molecule. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1543–1551. doi: 10.1016/s0006-291x(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Iwatsubo M., Mével-Ninio M., Labeyrie F. Rapid kinetic studies of partial reactions in the heme free derivative of L-lactate cytochrome c oxidoreductase (flavocytochrome b2); the flavodehydrogenase function. Biochemistry. 1977 Aug 9;16(16):3558–3566. doi: 10.1021/bi00635a009. [DOI] [PubMed] [Google Scholar]
- Koppenol W. H. Effect of a molecular dipole on the ionic strength dependence of a biomolecular rate constant. Identification of the site of reaction. Biophys J. 1980 Mar;29(3):493–507. doi: 10.1016/S0006-3495(80)85148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeyrie F., Baudras A., Lederer F. Flavocytochrome b 2 or L-lactate cytochrome c reductase from yeast. Methods Enzymol. 1978;53:238–256. doi: 10.1016/s0076-6879(78)53030-9. [DOI] [PubMed] [Google Scholar]
- Matthew J. B., Weber P. C., Salemme F. R., Richards F. M. Electrostatic orientation during electron transfer between flavodoxin and cytochrome c. Nature. 1983 Jan 13;301(5896):169–171. doi: 10.1038/301169a0. [DOI] [PubMed] [Google Scholar]
- Pajot P., Groudinsky O. Molecular weight and quaternary structure of yeast L-lactate dehydrogenase (cytochrome b2). 2. Revised heme extinction coefficients and minimal molecular weight. Eur J Biochem. 1970 Jan;12(1):158–164. doi: 10.1111/j.1432-1033.1970.tb00833.x. [DOI] [PubMed] [Google Scholar]
- Prats M. Complexes entre les L (+) lactate: cytochrome c oxydoréductase extraite des levures Saccharomyces cerevisiae ou Hansenula anomala et le cytochrome c de coeur de cheval. Biochimie. 1977;59(7):621–626. doi: 10.1016/s0300-9084(77)80171-5. [DOI] [PubMed] [Google Scholar]
- Silvestrini M. C., Brunori M., Tegoni M., Gervais M., Labeyrie F. Kinetics of electron transfer between two Hansenula anomala flavocytochrome b2 derivatives and two simple copper proteins (azurin and stellacyanin). Eur J Biochem. 1986 Dec 1;161(2):465–472. doi: 10.1111/j.1432-1033.1986.tb10467.x. [DOI] [PubMed] [Google Scholar]
- Strickland S., Palmer G., Massey V. Determination of dissociation constants and specific rate constants of enzyme-substrate (or protein-ligand) interactions from rapid reaction kinetic data. J Biol Chem. 1975 Jun 10;250(11):4048–4052. [PubMed] [Google Scholar]
- Tegoni M., Silvestrini M. C., Labeyrie F., Brunori M. A temperature-jump study of the electron transfer reactions in Hansenula anomala flavocytochrome b2. Eur J Biochem. 1984 Apr 2;140(1):39–45. doi: 10.1111/j.1432-1033.1984.tb08064.x. [DOI] [PubMed] [Google Scholar]
- Thomas M. A., Capellere-Blandin C., Pucheault J., Ferradini C. Pulse radiolysis study of a yeast cytochrome c from Hansenula anomala. Biochimie. 1986 May;68(5):745–755. doi: 10.1016/s0300-9084(86)80169-9. [DOI] [PubMed] [Google Scholar]
- Thomas M. A., Gervais M., Favaudon V., Valat P. Study of the Hansenula anomala yeast flavocytochrome-b2-cytochrome-c complex 2. Localization of the main association area. Eur J Biochem. 1983 Oct 3;135(3):577–581. doi: 10.1111/j.1432-1033.1983.tb07691.x. [DOI] [PubMed] [Google Scholar]
- Wherland S., Gray H. B. Metalloprotein electron transfer reactions: analysis of reactivity of horse heart cytochrome c with inorganic complexes. Proc Natl Acad Sci U S A. 1976 Sep;73(9):2950–2954. doi: 10.1073/pnas.73.9.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]


