Abstract
The glycosphingolipid binding specificities of neuraminidase-sensitive (simian SA11 and bovine NCDV) and neuraminidase-insensitive (bovine UK) rotavirus strains were investigated using the thin-layer chromatogram binding assay. Both triple-layered and double-layered viral particles of SA11, NCDV, and UK bound to nonacid glycosphingolipids, including gangliotetraosylceramide (GA1; also called asialo-GM1) and gangliotriaosylceramide (GA2; also called asialo-GM2). Binding to gangliosides was observed with triple-layered particles but not with double-layered particles. The neuraminidase-sensitive and neuraminidase-insensitive rotavirus strains showed distinct ganglioside binding specificities. All three strains bound to sialylneolactotetraosylceramide and GM2 and GD1a gangliosides. However, NeuAc-GM3 and the GM1 ganglioside were recognized by rotavirus strain UK but not by strains SA11 and NCDV. Conversely, NeuGc-GM3 was bound by rotaviruses SA11 and NCDV but not by rotavirus UK. Thus, neuraminidase-sensitive strains bind to external sialic acid residues in gangliosides, while neuraminidase-insensitive strains recognize gangliosides with internal sialic acids, which are resistant to neuraminidase treatment. By testing a panel of gangliosides with triple-layered particles of SA11 and NCDV, the terminal sequence sialyl-galactose (NeuGc/NeuAcα3-Galβ) was identified as the minimal structural element required for the binding of these strains. The binding of triple-layered particles of SA11 and NCDV to NeuGc-GM3, but not to NeuAc-GM3, suggested that the sequence NeuGcα3Galβ is preferred to NeuAcα3Galβ. Further dissection of this binding epitope showed that the carboxyl group and glycerol side chain of sialic acid played an important role in the binding of such triple-layered particles.
Rotaviruses, members of the family Reoviridae, are a major cause of diarrhea in young mammals. Rotavirus infections cause 600,000 childhood deaths in developing countries and are an important cause of childhood morbidity in industrialized countries (36). Rotavirus infections also result in important economical losses in agriculture due to diarrhea in calf, pig, sheep, and poultry rearing. The first step of a productive rotavirus infection is the viral recognition of and virus binding to the villus tip cells of the small intestine. Suppression of virus binding to host cells by competition with receptor analogs offers the possibility to interrupt the infectious cycle. The therapy of rotavirus infection by receptor competition is attractive, because the small intestine, the target organ for rotavirus replication, can easily be reached by oral feeding.
The design of a successful receptor analog hinges on a detailed knowledge of the first steps of rotavirus-enterocyte interaction. This knowledge could also contribute to an understanding of the epidemiology and pathogenesis of rotavirus infections. It has been suggested that the recognition of a specific receptor on the host cell surface determines, at least in part, the tissue, host, and age specificities of rotavirus infections (2, 54, 60, 66). Rotavirus replication is restricted to the small intestine, and susceptibility to this disease is limited to a narrow age range (36). Under field conditions rotavirus infections show remarkable host specificity. According to serological and epidemiological data, rotavirus cross-species infections are rare events (10, 20, 56). However, the species barrier is not absolute. An avian-like rotavirus has been isolated from a symptomatic calf (11), and bovine-like rotavirus strains have been isolated from symptomatic infants in Italy (21) and neonates in India (16). Genetic analysis identified the latter strains as likely natural reassortants between human and bovine rotaviruses (16, 21). Furthermore, infants can be infected with a derivative of bovine rotavirus NCDV under experimental conditions, as demonstrated in early vaccination trials (35), and calves can be infected with human rotavirus isolates (46).
The chemical nature of the rotavirus receptors is a topical area of rotavirus research, but no consensus has yet emerged (2, 24, 42, 53, 54). For example, infection of cell culture by the simian rotavirus strain SA11 is neuraminidase sensitive, suggesting the involvement of sialic acid on the cell surface in the infection process (14, 19). Infection with rotavirus SA11 can also be inhibited by sialylated glycoproteins of various origins (40, 67, 69, 70) and by the GM1 ganglioside (62). Paradoxically, it was demonstrated in a study using the thin-layer chromatogram (TLC) binding assay that rotavirus SA11 binds to the nonacid glycosphingolipid gangliotetraosylceramide (GA1; also called asialo-GM1), but not to gangliosides (60, 66). Still other investigations implicated integrins α2β1 and α4β1 for rotavirus SA11 attachment and entry into cells (28).
Biochemical studies demonstrated that GM3 gangliosides, consisting of either N-acetylneuraminic acid (NeuAc) or N-glycolylneuraminic acid (NeuGc), isolated from piglet intestine are relevant cell surface receptors for the porcine rotavirus strain OSU (53, 54). Other studies with rhesus rotavirus showed binding to a glycoprotein on murine enterocytes, with O-linked sialic acid residues being required for virus binding (2). It has recently been suggested that gangliosides are also involved in infection by the so-called neuraminidase-insensitive human rotaviruses (24, 42).
To clarify these conflicting results, the glycosphingolipid binding specificities of two neuraminidase-sensitive (simian SA11, bovine NCDV) and one neuraminidase-insensitive (bovine UK) rotavirus strain were investigated using the TLC binding assay (26, 38, 39). Notably, triple-layered particles (TLP) but not double-layered particles (DLP) from both types of rotaviruses bound to gangliosides. However, the ganglioside binding specificities differed for the neuraminidase-sensitive and neuraminidase-insensitive strains. A sialic acid-binding epitope required for the binding of SA11 and NCDV to gangliosides was deduced from the binding experiments.
MATERIALS AND METHODS
Chemical nomenclature.
The glycosphingolipid nomenclature follows the recommendations by the IUPAC-IUB Commission on Biochemical Nomenclature (30, 31, 32, 33). It is assumed that Gal, Glc, GlcNAc, GalNAc, NeuAc, and NeuGc are of the d configuration, Fuc is of the l configuration, and all sugars are present in the pyranose form.
In the shorthand nomenclature for fatty acids and bases, the number before the colon refers to the carbon chain length and the number after the colon gives the total number of double bonds in the molecule. Fatty acids with a 2-hydroxy group are denoted by the prefix h before the abbreviation, e.g., h16:0. For long-chain bases, d denotes dihydroxy and t denotes trihydroxy. Thus, d18:1 represents sphingosine (1,3-dihydroxy-2-aminooctadecene) and t18:0 represents phytosphingosine (1,3,4-trihydroxy-2-aminooctadecene).
Materials.
α2,3-Sialyllactose and α2,6-sialyllactose were purchased from Dextra Laboratories (Reading, Berkshire, United Kingdom). Vibrio cholerae and Arthrobacter ureafaciens neuraminidases were obtained from Behringwerke AG (Marburg, Germany) and from Roche Products (Basel, Switzerland), respectively. Glass- or aluminum-backed silica gel high-performance TLC plates (60 HPTLC) were obtained from Merck (Darmstadt, Germany). Methylamine, ethylamine, propylamine, benzylamine, and methyl iodide were purchased from Aldrich (Steinheim, Germany).
Viruses.
The bovine rotavirus strains NCDV (P1, G6) and UK (P5, G6) and the simian strain SA11 (P2, G3) were obtained from P. A. Offit, Children's Hospital of Philadelphia, Philadelphia, Pa.
Virus propagation and purification.
Rotavirus strains SA11, NCDV, and UK were propagated in MA104 (rhesus monkey kidney) cells in the presence of trypsin (7). Briefly, cells grown in Earle minimal essential medium (MEM) containing 10% fetal calf serum were washed with phosphate-buffered saline (pH 7.5) (PBS) before the infection. Rotaviruses were treated for 30 min at 37°C with 25 μg of porcine trypsin (Biokrom KG) per ml before addition to the cells. After 1 h of adsorption at 37°C, M199 medium containing 10% tryptose phosphate (Bioconcept) and 10 μg of trypsin per ml (infection medium) was added to the viral inoculum.
After complete destruction of the cell monolayer, rotavirus particles were recovered from the cell-free culture supernatant by ultracentrifugation (90,000 × g for 2 h at 4°C) through a 20% sucrose cushion. The virus pellet, resuspended in TNC buffer (50 mM Tris, 150 mM NaCl, 10 mM CaCl2 [pH 7.5]) containing sufficient CsCl to achieve a homogeneous density of 1.368 g/ml, was then centrifuged for 15 h at 190,000 × g. Bands located at 1.36 and 1.38 g/ml, which correspond to TLP and DLP, respectively, were recovered from the CsCl gradients, diluted in TNC buffer, and pelleted by a further high-speed centrifugation step (190,000 × g for 2 h at 4°C). The purity of the viral particles was estimated using electron microscopy (6), by direct counting of the number of TLP and DLP present in each fraction, and by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis (43)
Virus radiolabeling.
The purified TLP and DLP were radiolabeled with 125I using the Iodogen method (1). The labeled particles were purified on a PD-10 column (Amersham Pharmacia Biotech AB, Uppsala, Sweden) and eluted with bovine serum albumin (BSA) (20 mg/ml) in PBS (PBS-BSA). An aliquot of CsCl-purified TLP or DLP corresponding to approximately 100 μg of protein was taken for each labeling reaction, and the specific activity obtained was typically around 2 μCi/μg of protein. The protein content was estimated by bicinchoninic acid protein assay (Pierce) with BSA as a standard (58). The radiolabeled virus particles were stored at 4°C in PBS-BSA and were used within a week after labeling. Under these conditions the integrity of TLP and DLP was maintained for at least 1 week after labeling (see Results and Fig. 1D).
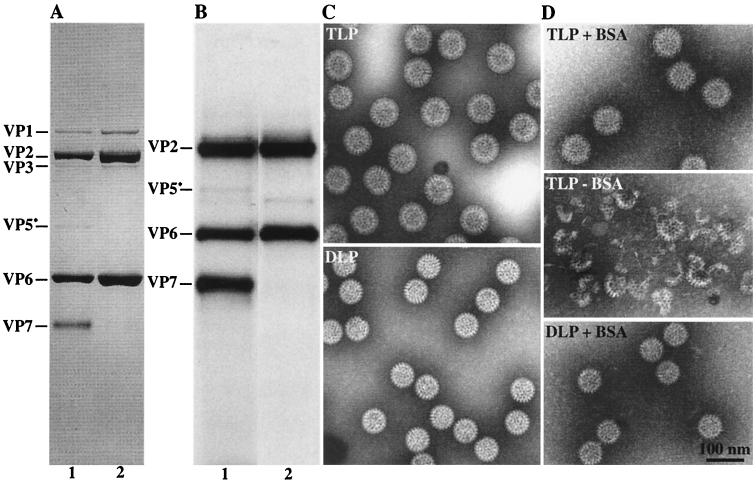
FIG. 1.
Analysis of CsCl-purified rotavirus particles. (A and B) Polypeptide composition of purified rotavirus particles obtained before (A) and after (B) 125I labeling. CsCl-purified viral particles were separated by SDS-PAGE on a 12% acrylamide gel. The proteins were visualized by either Coomassie blue staining (A) or autoradiography (B). Each lane contained 5 μg of proteins (A) or 0.2 μg of 125I-labeled proteins (B). Lanes 1, purified TLP; lanes 2, purified DLP. (C and D) Electron micrographs of CsCl-purified SA11 particles obtained before (C) and after (D) 125I labeling, in the presence (+) or absence (−) of BSA. Electron microscopy was performed as described previously, using ammonium molybdate/bacitracin negative staining (6).
Virus infectivity assay.
The virus infectivity assay was adapted from the neutralization assay described previously (10). Briefly, MA104 cells grown in 96-well microtiter plates were inoculated with twofold serial dilutions of the viral suspension. After overnight incubation at 37°C, the amount of infected cells was determined by an immunoperoxidase staining (8). For neuraminidase treatment, MA104 cells were incubated for 2 h at 37°C with increasing concentrations of neuraminidase from A. ureafaciens or V. cholerae (from 0.5 to 25 mU/ml) in MEM. Control cells were incubated with MEM alone. After two washes with MEM, the cells were infected with the viral suspension. Infectivity in neuraminidase-treated cells is expressed as the percentage of infectivity in infected cells in the absence of neuraminidase treatment (100%). For the competition experiments, the relevant competitive substance at concentrations of 0, 0.1, 0.3, and 1 mg/ml was incubated with the viral dilution for 1 h at 37°C before addition to the cells. The experiment was performed twice in triplicate wells.
TLC.
TLC was performed on glass- or aluminum-backed silica gel plates using chloroform-methanol-water (60:35:8 by volume) and chloroform–methanol–0.25% KCl in water (50:40:10 by volume) as the solvent systems for the separation of glycosphingolipid mixtures and pure gangliosides, respectively. Chemical detection was accomplished with anisaldehyde (65).
TLC binding assay.
The chromatogram binding assay was performed as described previously (26). Briefly, chromatograms with separated mixtures of glycosphingolipids (about 40 μg/lane) or pure glycosphingolipids (about 2 μg/lane) were dipped in 0.5% (wt/vol) polyisobutylmethacrylate (Sigma-Aldrich) in diethylether–n-hexane (1:5 vol/vol). After blocking of the plate for 2 h at room temperature with PBS containing 2% (wt/vol) BSA and 0.1% NaN3, the chromatogram was incubated for 2 h at room temperature with 5 ml of 125I-labeled virus diluted 1:50 to 1:100 in PBS-BSA. The amount of viral protein applied to each TLC plate was around 2 to 5 μg, which corresponds to about 107 cpm. After the plates were washed four times with PBS and dried, the TLCs were autoradiographed for 12 to 48 h using X-Omat AR film (Eastman Kodak, Rochester, N.Y.).
Microtiter plate binding assay.
Serial dilutions of pure gangliosides in methanol were applied to microtiter wells (Falcon microtiter plates; Becton Dickinson). After solvent evaporation, the wells were blocked for 2 h with 200 μl of PBS-BSA. After one wash with PBS-BSA, 50 μl of 125I-labeled viral particles (TLP or DLP), diluted 1:100 in PBS-BSA, was added to each well (approximately 105 cpm), followed by incubation for 4 h at room temperature. The plates were then washed six times with PBS. Following drying, the wells were cut out and the radioactivity was counted in a gamma counter. The data are means of triplicate determinations.
Reference glycosphingolipids.
Total nonacid and acid glycosphingolipid fractions from the sources given in the legends to Fig. 1 and 3 were isolated as described previously (37). The term acid glycosphingolipid refers to both gangliosides and sulfatides. These negatively charged glycosphingolipids were separated from the nonacid glycosphingolipids by ion-exchange chromatography on a DEAE-cellulose column (37). The pure glycosphingolipids used in the binding studies were isolated by repeated chromatography of native glycosphingolipids or acetylated derivatives (25) on silicic acid columns and were characterized by mass spectrometry (57), proton nuclear magnetic resonance spectroscopy (41), and degradation studies (61, 68).
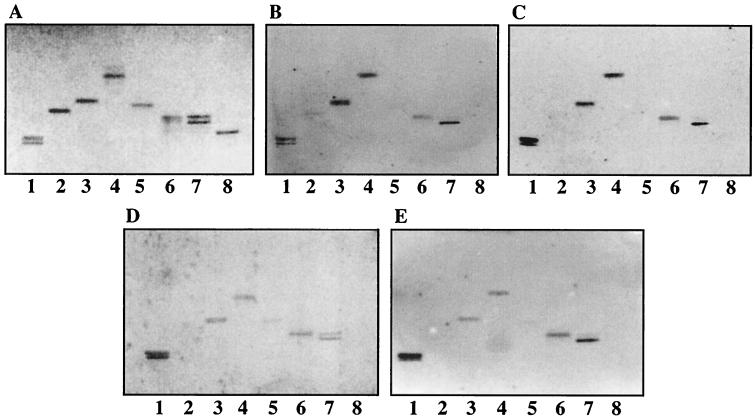
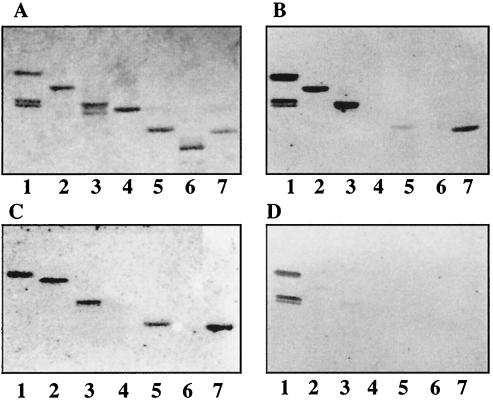
FIG. 3.
Binding of 125I-labeled SA11 rotavirus to pure nonacid glycosphingolipids on TLC plates. Glycosphingolipids were separated on TLC plates using chloroform-methanol-water (60:35:8 by volume). One chromatogram (A) was stained with anisaldehyde. Other chromatograms were incubated with the following purified radiolabeled viral particles: TLP from SA11 (B), DLP from SA11 (C), TLP from NCDV (D), and DLP from NCDV (E). Each lane contained 2 μg of pure glycosphingolipids. Lanes 1, GA1; lanes 2, globoside; lanes 3, GA2; lanes 4, lactosylceramide t18:0-h16:0-h24:0 (Galβ4Glcβ1Cer); lanes 5, isoglobotriaosylceramide; lanes 6, lactotetraosylceramide; lanes 7, neolactotetraosylceramide; lanes 8, B5 glycosphingolipid.
Derivatives of NeuGc-GM3 ganglioside.
Modifications of the C-1 carboxyl group of NeuGc-GM3 to amides and primary alcohol were achieved as described previously (44, 50). Cleavage of the glycerol tail of NeuGc-GM3 or NeuAcα3-neolactotetraosylceramide was performed by treating the ganglioside with sodium metaperiodate under mild conditions, followed by borohydride reduction as described before (48, 64). The derivatives were purified by ion-exchange chromatography on DEAE-Sepharose CL-6B (Pharmacia Amersham Biotech, Little Chalfont, United Kingdom) or by high-performance liquid chromatography (HPLC) (silica gel column) and analyzed by negative-ion fast atom bombardment (FAB) mass spectrometry.
Preparation of glycosphingolipids from bovine small intestines.
Pathogen-free bovine samples were obtained from the Central Veterinary Institute, Lelystad, The Netherlands. The newborn Jersey calf was 2 days old and the adult bovine was more than 6 months of age (63). We could not verify whether the newborn calf was colostrum deprived. However, although colostrum has a high content of NeuGc-containing gangliosides (51), the ganglioside profile of bovine colostrum (52) is very different from the pattern found in the calf intestinal epithelium. Indeed, the major ganglioside of bovine colostrum is GD3 (60 to 70%), which was not found in the calf small intestinal epithelium (63). The total acid glycosphingolipid fractions of bovine small intestines were prepared from intestinal mucosa using standard procedures (37).
Of the total acid fraction from adult bovine intestine, 10 mg was further purified by HPLC on a 1- by 25-cm silica gel column (Kromasil 5 Silica, 5-μm particles; Phenomex, Torrance, Calif.). The sample dissolved in 500 μl of chloroform-methanol-water (80:20:1 by volume; solvent A) was applied to the column, which had previously been equilibrated with solvent A. The column was then eluted with a linear gradient of chloroform-methanol-water (40:40:12 by volume; solvent B) in solvent A. Aliquots of each 2-ml fraction were analyzed by TLC and anisaldehyde staining. Glycosphingolipid-containing fractions were tested for rotavirus binding activity using the chromatogram binding assay. Fractions were pooled according to the TLC migration and rotavirus binding. The pooled fractions were characterized by negative-ion FAB mass spectrometry.
RESULTS
Purity of rotavirus probes.
Radioiodinated CsCl-purified TLP from simian SA11 and bovine NCDV rotaviruses were investigated for their glycosphingolipid binding specificities on TLC plates. The purity and the integrity of each rotavirus fraction were verified by SDS-PAGE analysis and by electron microscopy before and after radiolabeling (Fig. 1). If BSA was added immediately after the radioiodination reaction, no disintegration of labeled TLP was observed. A small contamination of TLP by DLP could not be excluded even by repeated CsCl gradient centrifugation, while DLP could be prepared with high purity. Therefore, all TLP binding experiments were carried out with DLP as negative controls. Two different experiments argued against a possible physiological role of DLP in the infection process. CsCl-purified DLP were treated with EDTA to remove any contaminating TLP, as checked by electron microscopy. It was shown with the virus infectivity assay that EDTA-treated DLP lacked any infectivity for MA104 cells. In addition, DLP did not bind to a membrane fraction of MA104 cells (data not shown).
Both DLP and TLP bind to nonacid glycosphingolipids.
Among several total nonacid fractions coming from different sources (more than 30 fractions tested), TLP of SA11 bound only to few glycosphingolipids (Fig. 2). At least three independent viral particle preparations were used for each binding experiment. However, the binding patterns of noninfectious DLP from rotaviruses SA11 and NCDV (Fig. 2C and D) were identical to that of infectious TLP from rotavirus SA11 (Fig. 2B). Binding of both TLP and DLP to nonacid glycosphingolipids was also detected when using pure glycosphingolipid fractions (the results are summarized in Table 1). TLP (Fig. 3B) and DLP of rotavirus SA11 (Fig. 3C) bound to GA1, GA2, lactosylceramide with phytosphingosine and hydroxy fatty acids, lactotetraosylceramide, and neolactotetraosylceramide (Fig. 3, lanes 1, 3, 4, 6, and 7, respectively). Not all nonacid glycosphingolipids bound rotavirus particles; e.g., globoside and B5 glycosphingolipid were not recognized by DLP or TLP (lanes 2 and 8, respectively). Rotavirus NCDV did not differ from rotavirus SA11 with respect to nonacid glycosphingolipid binding (Fig. 3C and E, respectively). A subgroup I monoclonal antibody (antibody 255) directed against VP6 (45) did not prevent the binding of SA11 DLP to the nonacid glycosphingolipid GA1 separated on a TLC plate (data not shown), raising doubts about the specificity of the interaction.
FIG. 2.
Binding of 125I-labeled SA11 and NCDV rotaviruses to mixtures of nonacid glycosphingolipids on TLC plates. Glycosphingolipids were separated on TLC plates using chloroform-methanol-water (60:35:8 by volume). One chromatogram (A) was stained with anisaldehyde. Other chromatograms were incubated with the following purified radiolabeled viral particles: TLP from SA11 (B), DLP from SA11 (C), and DLP from NCDV (D). Each lane contained 40 μg of glycosphingolipid mixture. Lanes 1, nonacid glycosphingolipids of human erythrocytes, blood group AB; lanes 2, nonacid glycosphingolipids of guinea pig erythrocytes; lanes 3, nonacid glycosphingolipids of bovine intestine; lanes 4, nonacid glycosphingolipids of bovine erythrocytes; lanes 5, partially purified nonacid glycosphingolipids of bovine erythrocytes; lanes 6, nonacid glycosphingolipids of mouse small intestine; lanes 7, nonacid glycosphingolipids of human large intestinal adenocarcinoma; lanes 8, nonacid glycosphingolipids of guinea pig small intestine.
TABLE 1.
Binding of 125I-labeled NCDV and SA11 rotavirus strains to glycosphingolipids on thin-layer plates
| Trivial name | Structure | Binding on TLC platesa
|
|||
|---|---|---|---|---|---|
| DLP (SA11, NCDV, and UK) | TLP
|
||||
| SA11 | NCDV | UK | |||
| 1. Galactosylceramide | Galβ1Cer | − | − | − | − |
| 2. Glucosylceramide | Glcβ1Cer | − | − | − | − |
| 3. LacCer d18:1-16:0/24:1 | Galβ4Glcβ1Cer | − | − | − | − |
| 4. LacCer t18:0-h16:0-h24:0 | Galβ4Glcβ1Cer | + | + | + | + |
| 5. Isoglobotriaosylceramide | Galα3Galβ4Glcβ1Cer | − | − | − | ND |
| 6. Globoside | GalNAcβ3Galα4Galβ4Glcβ1Cer | − | − | − | − |
| 7. GA2 | GalNAcβ4Galβ4Glcβ1Cer | + | + | + | + |
| 8. GA1 | Galβ3GalNAcβ4Galβ4Glcβ1Cer | ++ | ++ | ++ | ++ |
| 9. Lactotetraosylceramide | Galβ3GlcNAcβ3Galβ4Glcβ1Cer | + | + | + | ND |
| 10. Neolactotetraosylceramide | Galβ4GlcNAcβ3Galβ4Glcβ1Cer | + | + | + | ND |
| 11. B5 | Galα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | − | − | ND |
| 12. NeuAc-GM3 | NeuAcα3Galβ4Glcβ1Cer | − | − | − | + |
| 13. NeuGc-GM3 | NeuGcα3Galβ4Glcβ1Cer | − | ++ | ++ | − |
| 14. NeuAc-GM2 | GalNAcβ4(NeuAcα3)Galβ4Glcβ1Cer | − | + | + | ND |
| 15. NeuGc-GM2 | GalNAcβ4(NeuGcα3)Galβ4Glcβ1Cer | − | + | + | + |
| 16. NeuAc-GM1a | Galβ3GalNAcβ4(NeuAcα3)Galβ4Glcβ1Cer | − | − | − | + |
| 17. NeuGc-GM1a | Galβ3GalNAcβ4(NeuGcα3)Galβ4Glcβ1Cer | − | − | − | + |
| 18. NeuAc-GD1a | NeuAcα3Galβ3GalNAcβ4(NeuAcα3)Galβ4Glcβ1Cer | − | ± | ± | ND |
| 19. NeuGc/Ac-GD1a | NeuGcα3Galβ3GalNAcβ4(NeuAcα3)Galβ4Glcβ1Cer | − | + | + | + |
| 20. NeuGc-GD1a | NeuGcα3Galβ3GalNAcβ4(NeuGcα3)Galβ4Glcβ1Cer | − | + | + | ND |
| 21. NeuAc-GD1b | Galβ3GalNAcβ4(NeuAcα8NeuAcα3)Galβ4Glcβ1Cer | − | − | − | ND |
| 22. NeuAc-GT1b | NeuAcα3Galβ3GalNAcβ4(NeuAcα8NeuAcα3)Galβ4Glcβ1Cer | − | − | − | ND |
| 23. NeuAcα3-neolactotetraosylceramide | NeuAcα3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | + | + | + |
| 24. NeuAcα6-neolactotetraosylceramide | NeuAcα6Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | ± | ND | ND |
| 25. NeuAc-SLex | NeuAcα3Galβ4(Fucα3)GlcNAcβ3Galβ4Glcβ1Cer | − | + | + | ND |
| 26. NeuAc-SLea | NeuAcα3Galβ3(Fucα4)GlcNAcβ3Galβ4Glcβ1Cer | − | − | ± | ND |
| 27. NeuGc-GD2 | GalNAcβ4(NeuGcα8NeuGcα3)Galβ4Glcβ1Cer | − | ND | − | ND |
| 28. NeuAc-GD3 | NeuAcα8NeuAcα3Galβ4Glcβ1Cer | − | − | − | ND |
| 29. NeuAc-GQ1b | NeuAcα8NeuAcα3Galβ3GalNAcβ4(NeuAcα8NeuAcα3)Galβ4Glcβ1Cer | − | − | − | ND |
| 30. None | NeuGcα3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | ++ | ND | ND |
| 31. None | Galβ4GlcNAcβ6(NeuAcα6Galβ4GlcNAcβ3)Galβ4Glcβ1Cer | − | − | − | ND |
| 32. None | Galα3Galβ4GlcNAcβ6(NeuAcα3Galβ4GlcNAcβ3)Galβ4GlcNAcβ3Galβ4Glcβ1Cer | − | + | + | ND |
++, +, and ±, strong, moderate, and weak darkening, respectively, of the autoradiogram when 2 μg of the glycosphingolipid was applied on the thin-layer plate; −, no binding at all. ND, binding was not determined.
TLP bind to ganglioside-containing fractions.
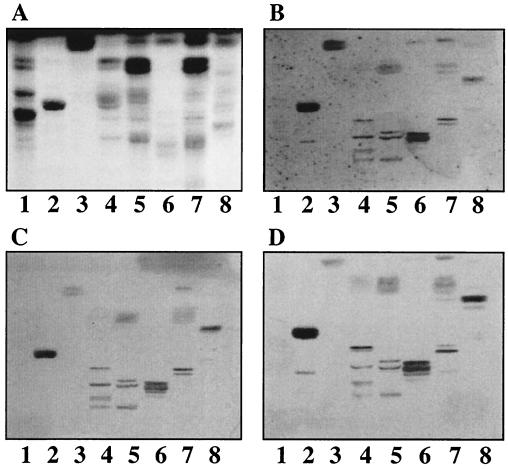
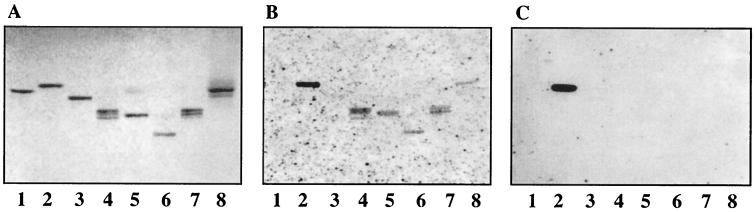
In the next step, crude fractions containing acid glycosphingolipids (both gangliosides and sulfatides) were examined for binding of rotavirus. TLP, but not DLP, of rotaviruses UK and SA11 bound to some acid fractions, although the labeled DLP bound to the reference nonacid glycosphingolipid GA2 (exemplified in Fig. 4 with SA11). SA11 TLP bound to the GM3 ganglioside region of adult and newborn pig intestinal fractions (Fig. 4, lanes 4 and 5) and to slow-migrating gangliosides of the proximal and middle parts of calf intestine (Fig. 4, lanes 6 and 7). Notably, the neuraminidase-insensitive bovine strain UK also bound to gangliosides. In the case of UK the binding was to slow-migrating gangliosides present in acid fractions of human kidney cancer, sheep intestine, calf brain, human liver metastatis from colon cancer, and human meconium (results not shown).
FIG. 4.
Binding of 125I-labeled SA11 rotavirus to mixtures of acid glycosphingolipids on TLC plates. Glycosphingolipids were separated on TLC plates using chloroform-methanol-water (60:35:8 by volume). One chromatogram (A) was stained with anisaldehyde. Other chromatograms were incubated with the following purified radiolabeled viral particles: TLP from SA11 (B) and DLP from SA11 (C). Each lane contained 40 μg of glycosphingolipid mixture. Lanes 1, nonacid glycosphingolipids of human erythrocytes, blood group AB; lanes 2, nonacid glycosphingolipids of guinea pig erythrocytes; lanes 3, acid glycosphingolipids of human meconium; lanes 4, acid glycosphingolipids of adult pig intestine; lanes 5, acid glycosphingolipids of newborn pig intestine; lanes 6, acid glycosphingolipids of the proximal part of calf small intestine; lanes 7, acid glycosphingolipids of the middle part of calf small intestine; lanes 8, acid glycosphingolipids of the distal part of calf small intestine.
Ganglioside binding specificity of TLP from rotaviruses SA11 and NCDV.
Pure gangliosides were subsequently used to analyze the binding specificity of TLP from rotaviruses SA11 and NCDV. The structures of these gangliosides and the binding results are summarized in Table 1. TLP from rotavirus SA11 bound strongly to NeuGc-GM3 and NeuGc-GM2 (Fig. 5B, lanes 2 and 3) as well as NeuAc-GD1a and NeuGc-GD1a (Fig. 5B, lanes 5 and 7). For GD1a ganglioside the binding to the N-acetyl isomer was much weaker than to the N-glycolyl isomer and was observed only with freshly labeled particles. No binding to NeuAc-GM1 or NeuAc-GD1b was detected (Fig. 5B, lanes 4 and 6, respectively). Similar binding results were obtained with TLP from rotavirus NCDV (Fig. 5C).
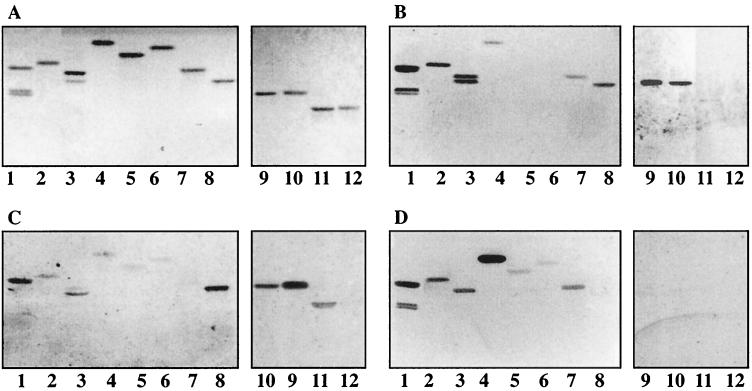
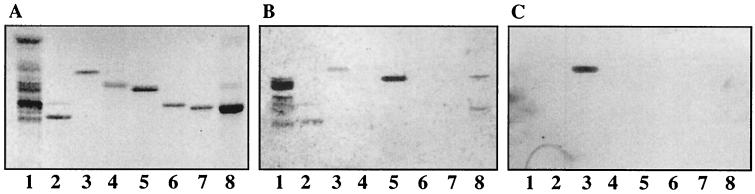
FIG. 5.
Binding of 125I-labeled SA11 and NCDV rotaviruses to pure gangliosides on TLC plates. Glycosphingolipids were separated on TLC plates using chloroform–methanol–0.25% KCl in water (50:40:10 by volume). One chromatogram (A) was stained with anisaldehyde. Other chromatograms were incubated with the following purified radiolabeled viral particles: TLP from SA11 (B), TLP from NCDV (C), and DLP from SA11 (D). Each lane contained 2 μg of pure glycosphingolipids. Lanes 1, GA2 and/or GA1; lanes 2, NeuGc-GM3; lanes 3, NeuGc-GM2+NeuGc-GM1 [GalNAcβ4(NeuGcα3)Galβ4Glcβ1Cer plus Galβ3GalNAcβ4(NeuGcα3)Galβ4Glcβ1Cer]; lanes 4, NeuAc-GM1; lanes 5, NeuAc-GD1a; lanes 6, NeuAc-GD1b; lanes 7, NeuGc-GD1a.
Several types of experiments suggested that the interaction of TLP with gangliosides was specific. DLP from rotavirus SA11 did not bind to any of these gangliosides (Fig. 5D, compare lanes 2 to 7 with lane 1). Preincubation of rotavirus SA11 TLP with a guinea pig antiserum, at a dilution that inhibited infection of MA104 cells with rotavirus SA11, completely blocked the binding of TLP to NeuGc-GM3 and NeuGc-GM2 gangliosides on TLC plates (data not shown). Moreover, treatment of TLP from rotavirus SA11 with EDTA resulted in the loss of the outer shell proteins, in the loss of infectivity, and in a substantially decreased NeuGc-GM3 binding on TLC compared to that of mock-incubated TLP (data not shown).
Bovine rotavirus UK shows a distinct ganglioside binding specificity.
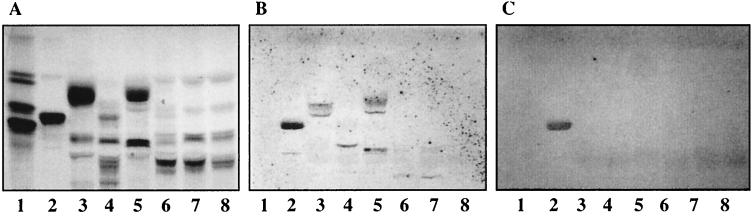
TLP of UK bound to gangliosides NeuGc-GM2, NeuGc-GM1, NeuAc-GM1, NeuGc/Ac-GD1a, NeuAcα3-neolactotetraosylceramide, and NeuAc-GM3 (Fig. 6B, lanes 4 to 8), whereas no binding to NeuGc-GM3 or to globoside was observed (Fig. 6B, lanes 3 and 1, respectively). DLP from UK did not bind to any of these gangliosides, while they bound to GA2 (Fig. 6C, lane 2).
FIG. 6.
Binding of 125I-labeled UK rotavirus to pure gangliosides on TLC plates. Glycosphingolipids were separated on TLC plates using chloroform–methanol–0.25% KCl in water (50:40:10 by volume). One chromatogram (A) was stained with anisaldehyde. Other chromatograms were incubated with the following purified radiolabeled viral particles: TLP from UK (B) and DLP from UK (C). Each lane contained 2 μg of pure glycosphingolipids. Lanes 1, globoside; lanes 2, GA2; lanes 3, NeuGc-GM3; lanes 4, NeuGc-GM2 plus NeuGc-GM1 [GalNAcβ4(NeuGcα3)Galβ4Glcβ 1Cer plus Galβ3GalNAcβ4(NeuGcα3)Galβ4Glcβ1Cer]; lanes 5, NeuAc-GM1; lanes 6, NeuGc-GD1a or NeuAc-GD1a; lanes 7, NeuAcα3-neolactotetraosylceramide; lanes 8, NeuAc-GM3.
Thus, the UK strain bound to the GM1 ganglioside and NeuAc-GM3, which were not recognized by either SA11 or NCDV. Conversely, UK did not recognize NeuGc-GM3, which was strongly bound by SA11 and NCDV.
SA11 and NCDV bind to gangliosides containing the terminal sialyl-Galβ sequence.
A total of 21 pure gangliosides were tested for binding of TLP and DLP from rotaviruses SA11 and NCDV using the chromatogram binding assay in order to determine a common structural element involved in the binding process. The structures of these gangliosides and the binding results are presented in Table 1. In summary, the infectious particles of both strains bound to gangliosides with a terminal NeuGc- or NeuAcα3Galβ sequence (lines 13, 18, 19, 20, 23, 25, 30, and 32 in Table 1), except the GT1b ganglioside (line 22) and NeuAc-GM3 (line 12). The only difference between the two strains was a weak binding of rotavirus NCDV to the sialyl-Lea hexaglycosylceramide (line 26), which was never observed with rotavirus SA11. No binding of either strain to gangliosides with a terminal NeuAcα8NeuAcα3Galβ sequence, such as the GD3 ganglioside (line 28), was obtained. The infectious particles of both strains also bound to the NeuAc- and NeuGc-GM2 gangliosides (lines 14 and 15), while no binding to the NeuAc- or NeuGc-GM1 gangliosides (lines 16 and 17) was found. When the binding of rotaviruses SA11 and NCDV to NeuGc-GM3 and NeuAc-GM3 with identical ceramides (d18:1–24:0) was examined, only NeuGc-GM3 was recognized by TLP of both strains. Finally, a strong binding of the TLP of the SA11 strain to NeuAcα3-neolactotetraosylceramide (line 23) was found, while the binding to NeuAcα6-neolactotetraosylceramide (line 24) was much weaker. This binding difference was even more marked with gangliosides immobilized in microtiter wells (data not shown).
Binding of SA11 and NCDV to chemical derivatives of NeuGc-GM3. (i) Modifications of the carboxyl group.
The importance of the carboxyl group at position C-1 of the sialic acid for the binding was evaluated by binding studies with derivatives of NeuGc-GM3 substituted at the C-1 position by different chemical groups (see Table 2 for the structures). TLP of SA11 bound to NeuGc-GM3 amide and methylamide, whereas the binding to the alcohol derivative was much weaker (Fig. 7B, compare lanes 2 and 3 with lane 7). No binding or very weak binding was observed when bulkier groups, such as ethylamide, propylamide, and benzylamide, were added to C-1 (Fig. 7B, lanes 5, 6, and 4). Similar results were obtained with TLP of NCDV (Fig. 7C). Notably, DLP of either SA11 (Fig. 7D, lanes 2 to 7) or NCDV (data not shown) bound more or less to all C-1 derivatives of NeuGc-GM3, while no binding was detected with native NeuGc-GM3 (Fig. 7D, lane 8).
TABLE 2.
Structures of the sialic acid parts of chemical derivatives of NeuGc-GM3 and NeuAcα3-neolactotetraosylceramide
| Name of derivative | Modification of C-1–COOH group | Modification of C-7–glycerol side chain- (CHOH)2-CH2OH | Binding on TLC plates
|
||
|---|---|---|---|---|---|
| TLP
|
DLP (SA11 and NCDV) | ||||
| SA11 | NCDV | ||||
| NeuGc-GM3 alcohol | -CH2OH | ± | − | + | |
| NeuGc-GM3 amide | -CONH2 | + | ± | + | |
| NeuGc-GM3 methylamide | -CONHCH3 | + | ± | + | |
| NeuGc-GM3 ethylamide | -CONHCH2CH3 | − | ± | ± | |
| NeuGc-GM3 propylamide | -CONHCH2CH2CH3 | − | ± | ± | |
| NeuGc-GM3 benzylamide | -CONHCH2C6H5 | ± | ± | + | |
| C-8–NeuAcα3–neolactotetraosylceramide | -CHOH-CH2OH | − | − | − | |
| C-7–NeuAcα3–neolactotetraosylceramide | -CH2OH | − | − | − | |
FIG. 7.
Binding of 125I-labeled SA11 and NCDV rotaviruses to chemical derivatives of NeuGc-GM3 and NeuAcα3-neolactotetraosylceramide on TLC plates. Glycosphingolipids were separated on TLC plates using chloroform–methanol–0.25% KCl in water (50:40:10 by volume). One chromatogram (A) was stained with anisaldehyde. Other chromatograms were incubated with the following purified radiolabeled viral particles: TLP from SA11 (B), TLP from NCDV (C), and DLP from SA11 (D). The solvent system used was chloroform–methanol–0.25% KCl in water (50:40:10 by volume). Each lane contained 2 μg of glycosphingolipids. Lanes 1, GA2 and/or GA1; lanes 2, NeuGc-GM3 methylamide; lanes 3, NeuGc-GM3 amide; lanes 4, NeuGc-GM3 benzylamide; lanes 5, NeuGc-GM3 ethylamide; lanes 6, NeuGc-GM3 propylamide; lanes 7, NeuGc-GM3 alcohol; lanes 8, NeuGc-GM3; lanes 9, NeuGc-GM3; lanes 10, periodate-oxidized NeuGc-GM3; lanes 11, NeuAcα3-neolactotetraosylceramide; lanes 12, periodate-oxidized NeuAcα3-neolactotetraosylceramide. See Table 2 for the structures of these derivatives.
(ii) Cleavage of the glycerol side chain.
Cleavage of the glycerol side chain of NeuGc-GM3 neuraminic acid by periodate oxidation followed by borohydride reduction gave rise to a mixture of uncleaved NeuGc-GM3 and C-8 analog of NeuGc-GM3 (see Table 2 for the structure). Neither SA11 nor NCDV TLP bound to the C-8 analog of NeuGc-GM3, while they bound to the residual NeuGc-GM3 (Fig. 7, lane 10). Neither strain bound to a mixture of C-7 and C-8 analogs of NeuAcα3-neolactotetraosylceramide, obtained after periodate oxidation of NeuAcα3-neolactotetraosylceramide (Fig. 7, lane 12).
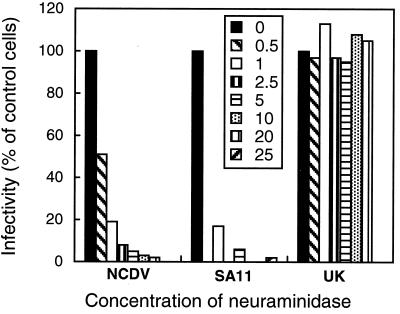
Inhibition of rotavirus infection of MA104 cells.
Treatment of MA104 cells with neuraminidase from either A. ureafaciens or V. cholerae decreased the susceptibility of the cells to rotaviruses NCDV and SA11 in a dose-dependent way, while no such effect was seen for UK (Fig. 8). However, the soluble trisaccharide α2,3-sialyllactose, which bears the carbohydrate moiety present at the terminal part of recognized gangliosides, did not significantly decrease the number of infected cells compared to control cells (≤30% reduction in infected cells at the highest trisaccharide concentration, 1.7 mM). No inhibition was obtained with α2,6-sialyllactose. In addition, incubation of virus with soluble sialyloligosaccharide peptides derived from hen egg yolk failed to inhibit viral infection at a maximum concentration of 3 mg/ml.
FIG. 8.
Effect of neuraminidase treatment of MA104 cells on infectivity of SA11 and NCDV strains. MA104 cells were treated with neuraminidase from A. ureafaciens (from 0.5 to 25 mU/ml) for 2 h at 37°C, and the virus infectivity assay was carried out as described in Materials and Methods. The results are expressed as the percentage of infected cells in the absence of neuraminidase treatment (100%) and are the means of two independent experiments with triplicate values.
Gangliosides from bovine small intestinal epithelium.
Acid glycosphingolipids were isolated from adult bovine small intestinal epithelium. The fractions were characterized by negative-ion FAB mass spectrometry and tested for binding with the NCDV bovine strain (Fig. 9B and C, lanes 8). The glycosphingolipid content and the binding activity were compared to that of a newborn calf small intestinal sample (Fig. 9B and C, lanes 1) characterized previously (63). The results are summarized in Table 3. The main gangliosides found in the epithelium from the small intestine of a 2-day-old calf were NeuAc- and NeuGc-GM3, NeuGc-GM2, NeuGc-GM1, and NeuGc-GD1a (Fig. 9A, lane 1). Acid glycosphingolipids isolated from adult bovine intestinal epithelium showed a clearly distinct composition (Fig. 9A, lane 8). NeuAc- and NeuGc-GM3 gangliosides were purified from the adult intestinal fraction (Fig. 9A, lanes 4 and 5, respectively), but in contrast to the newborn calf sample, they were present in much smaller amounts (Table 3). The major ganglioside compound in the adult bovine intestine was NeuGc-GD2, which was devoid of rotavirus binding activity (Fig. 9, lanes 7, and Table 3).
FIG. 9.
Comparison of the binding of 125I-labeled NCDV rotavirus to acid glycosphingolipids of newborn and adult bovine small intestinal epithelia. Glycosphingolipids were separated on TLC plates using chloroform–methanol–0.25% KCl in water (50:40:10, by volume). One chromatogram (A) was stained with anisaldehyde. Other chromatograms were incubated with the following purified radiolabeled viral particles: TLP from NCDV (B) and DLP from NCDV (C). Each lane contained 2 μg of purified glycosphingolipids or 40 μg of glycosphingolipid mixture. Lanes 1, acid fraction of newborn calf small intestinal epithelium; lanes 2, NeuGc-GD1a; lanes 3, GA2; lanes 4, NeuAc-GM3; lanes 5, NeuGc-GM3; lanes 6, NeuGc-GM1; lanes 7, NeuGc-GD2; lanes 8, acid fraction of adult bovine small intestinal epithelium.
TABLE 3.
Amounts of pure glycosphingolipids obtained from a 10-mg total acid fraction of adult bovine small intestinal epithelium
| Glycosphingolipid | Newborna | Adult |
|---|---|---|
| Sulfatide | 0.90 | |
| NeuAc-GM3 or NeuGc-GM3 | 1.50 | 0.38 |
| GM2b | 0.18 | 0.10 |
| GM1c | 0.08 | 0.70 |
| NeuGc-GD1a | 0.08 | |
| NeuGc-GD2 | 8.00 |
The amounts of pure glycosphingolipids previously obtained from a newborn calf small intestinal sample (63) have been included for comparison.
The GM2 fraction of the newborn calf intestine contained only NeuGc-GM2, while the GM2 fraction of the adult bovine intestine was a mixture of NeuAc- and NeuGc-GM2.
The GM1 fraction of the newborn calf intestine contained only NeuGc-GM1, while the GM1 fraction of the adult bovine intestine was a mixture of NeuAc- and NeuGc-GM1.
DISCUSSION
A substantial part of current rotavirus research is focused on the cellular receptor(s) for the virus. Several data indicate that rotavirus attachment and entry into cells constitute a multistep process (34, 47). Recent models suggest that rotavirus interacts first with a sialic acid receptor and then with a sialic acid-independent receptor(s) (34, 47). The first contact could involve sialic acid present on either glycoproteins or glycosphingolipids. Recent cell biology experiments using specific metabolic inhibitors involved N-glycosylated glycoproteins, glycosphingolipids, and cholesterol in the early steps of the rotavirus infection process. Therefore, it was suggested that the rotavirus receptor(s) on MA104 cells might be part of lipid microdomains (rafts) in the cell membrane (22). Five proteins, which inhibit rotavirus infection and are potential receptor candidates, have been isolated from MA104 cells after detergent extraction (22). Overlay protein blot assays with solubilized MA104 proteins identified further potential cellular receptor proteins for rotavirus (34). These proteins have not been characterized yet, and there is no evidence that they correspond to the integrins that have been implicated in rotavirus infection (15, 28). Clearly, more work is needed to develop a consensus on these proteins.
In the present work, glycosphingolipid-rotavirus interaction was studied to investigate the carbohydrate binding specificity of rotaviruses. Previous work had established that GM3 gangliosides are relevant cell surface receptors for the porcine rotavirus OSU (53, 54). We reexamined the glycosphingolipid binding specificity of rotavirus SA11, since conflicting results had been published for this virus (60, 62, 66). We included two bovine rotaviruses in the study for several reasons. First, bovine rotaviruses are important veterinary pathogens causing calf scour, which has been linked to substantial economical losses (59). Second, relatively detailed epidemiological information is available for bovine strains (3, 9, 23), while glycosphingolipid binding data are completely lacking. Third, within bovine rotaviruses we could select two strains, NCDV and UK, that share the G serotype (both are G6) and most genes (21) but which differ in P serotype (17) and neuraminidase sensitivity. NCDV is P1 type, neuraminidase sensitive, and hemagglutinating, while UK is P5 type, neuraminidase insensitive, and nonhemagglutinating (17).
In the present study we used extensively the TLC binding assay to investigate the carbohydrate binding specificities of these three rotavirus strains (38). Glycosphingolipids were separated on thin-layer plates and virus binding was evaluated with radioiodinated CsCl gradient-purified viral particles. With this assay mainly the carbohydrate part of the glycosphingolipid is exposed on the chromatogram (38). This technique is therefore most suitable for the detection and characterization of carbohydrate binding epitopes of bacteria, bacterial toxins, and viruses (39). The multivalent presentation of glycosphingolipids on TLC allows detection of binding to low-affinity binding sites, which is not possible when using oligosaccharides in solution. A further advantage of TLC assay is the analytical resolution on the chromatogram that allows the detection of minor carbohydrate components with virus binding activity in complex glycosphingolipid mixtures.
Several important findings were obtained from our TLC binding assays. First, TLP from all three tested rotavirus strains bound specifically to gangliosides. Second, the screening of a large collection of pure and chemically characterized glycosphingolipids allowed us to identify a sialic acid-binding epitope for TLP of rotaviruses SA11 and NCDV. Third, chemical modifications of the sialic acid residue allowed a further dissection of this potential binding epitope. Below we discuss these chemical observations and put them in perspective. Both neuraminidase-sensitive rotaviruses bound to the terminal sequence NeuGc- or NeuAcα3Galβ of gangliosides. However, a number of exceptions to that rule suggested substantial contributions of additional carbohydrate residues of the ganglioside molecule to TLP binding. One apparent exception is the absence of binding of SA11 and NCDV TLP to NeuAc-GM3, while both strongly bound to NeuGc-GM3. This suggests that the NeuGcα3Galβ sequence is preferred to NeuAcα3Galβ, probably because the N-glycolyl group affords additional interactions with the binding site. This preference of the N-glycolyl over the N-acetyl group was also observed in SA11 TLP binding to GD1a ganglioside and has also been reported for the porcine strain OSU with GM3 gangliosides (54). This preference might be critical for the binding to GM3 ganglioside that has only a short carbohydrate chain that might be poorly exposed on the thin-layer plate, while it might be of lesser importance for gangliosides with a longer carbohydrate chain, such as GD1a gangliosides. The absence of binding to the GT1b ganglioside (Table 1, line 22) might be due to the fact that the internal NeuAcα8NeuAcα3 sequence interferes with TLP binding to the external NeuAcα3Gal sequence. The binding to GM2 (Table 1, lines 14 and 15) but not to GM1 ganglioside (Table 1, lines 16 and 17) suggests that the addition of GalNAcβ in position 4 of Gal is tolerated in the binding epitope, while a Galβ3GalNAcβ linked to position 4 of the same Gal can no longer be accommodated in the binding site.
Rolsma et al. (54) showed that the soluble oligosaccharides α2,3-sialyllactose and α2,6-sialyllactose were capable of inhibiting the binding of rotavirus OSU. We observed a similar weak inhibitory effect of α2,3-sialyllactose on the infectivity of rotaviruses SA11 and NCDV. However, millimolar concentrations of oligosaccharides were required to inhibit rotavirus binding by 50%, which calls into question the physiological significance of these observations.
The binding of SA11 to the nonacid glycosphingolipids GA1 and GA2 on TLC plates, but not to gangliosides, has been reported (60, 66). The difference with our results might be more virtual than real. In the previous studies, the viral particles were purified by metrizamide gradients rather than CsCl gradients. However, CsCl gradients give better separation of DLP and TLP than metrizamide gradients (13, 42). A probable substantial DLP contamination in their TLP preparation might account for the strong GA1 and GA2 binding. Furthermore, GA1 has not been detected in the intestinal epithelium of mammalian species (5), including humans (4). The lack of binding to GM3 ganglioside is explained by the fact that NeuAc-GM3 but not NeuGc-GM3 was tested in the binding assays.
Another discrepancy is the lack of correlation between chemical binding and biological inhibition assays. It was reported that GM1 ganglioside inhibited infection of LLC-MK2 cells by SA11 rotavirus (62), while we could not detect GM1 binding of SA11 with the TLC assay. However, Superti and Donelli (62) also showed that neuraminidase treatment of the cells decreased the infectivity of SA11. This excludes a potential role of GM1 ganglioside as a receptor for SA11, since GM1 is not cleaved by neuraminidase under their experimental conditions (24).
Substitution of the carboxyl group of sialic acid by different chemical groups was accompanied by a partial or total loss of SA11 TLP binding and a concomitant gain in SA11 DLP binding. This suggests that the carboxyl group of sialic acid not only is critical for the interaction with SA11 TLP but also is involved in the repulsion of SA11 DLP. Notably, in the alcohol derivative the carboxylate group is replaced by another small group that does not affect the conformation of the molecule (49). The glycerol side chain of sialic acid also contributes in the interaction with TLP, as demonstrated by the lack of binding of chemical derivatives with a shortened glycerol tail.
An important observation is that the neuraminidase-insensitive bovine rotavirus UK also bound to gangliosides, although with different binding specificity than rotaviruses SA11 and NCDV. TLP of UK bound to the GM1 ganglioside and NeuAc-GM3, which were not recognized by TLP from SA11 and NCDV. Conversely, UK did not recognize NeuGc-GM3, which was strongly bound by TLP of SA11 and NCDV. Ganglioside binding has been demonstrated for neuraminidase-insensitive human rotavirus strains. The infectivity of human strains KUN and MO in cell culture was inhibited by GM1 ganglioside (24), although binding of the virus to GM1 could not be demonstrated. The latter observation might reflect technical difficulties. In our hands, GM1 binding by rotavirus UK was weaker than GM3 binding by rotavirus SA11. Also, the human rotavirus Wa was inhibited by gangliosides in MA104 cell culture infections and bound to gangliosides on TLC (42). The widespread concept that neuraminidase-insensitive strains bind in a sialic acid-independent manner is therefore probably misleading. Instead, the neuraminidase-insensitive strains bind to gangliosides with internal sialic acids that are not removed by neuraminidase treatment, as experimentally demonstrated for GM1 ganglioside (24). More data on neuraminidase-insensitive rotavirus strains are clearly needed to substantiate this hypothesis.
We observed that bovine rotaviruses NCDV and UK bound to gangliosides with different specificities. Since both bovine rotaviruses shared a highly related VP7 gene (27) and many other closely related genes (21), it is tempting to associate the distinct VP4 proteins with the distinct ganglioside binding specificities. In fact, a number of publications associated VP4 or its tryptic cleavage products with ganglioside binding (18, 29, 55). However, this argument should be used with caution since bovine rotavirus NCDV and simian rotavirus SA11, which clearly differ in VP4, showed nearly identical ganglioside binding specificities. This observation suggests that the ganglioside binding specificity of a given virus plays only a minor role in the host range determination. One should also realize that the concepts of host and age range specificity were derived from epidemiological observations of rotavirus disease. Rotavirus infection, the necessary but not sufficient condition for rotavirus disease, is much less host and age restricted than rotavirus disease. The lack of correlation between ganglioside binding pattern and host specificity, as seen in our experiments, is therefore not an argument against a potential physiological role of gangliosides in the establishment of a first virus-cell contact. However, further rotavirus-cell surface interactions are apparently needed to confer host specificity and still other rotavirus-host interactions are needed to cause disease.
For the same reasons one should be cautious in suggesting parallels between age development of ganglioside patterns in the gut of mammalians and the age dependence of rotavirus disease susceptibility. Rolsma et al. (54) observed an age-related decline of GM3 gangliosides in the intestine of pigs. These GM3 gangliosides were identified as possible receptors for the porcine strain OSU (54). We saw a similar relative decline of gangliosides, bound by TLP of NCDV, in the gut from newborn to adult cattle. However GM1, a potential ganglioside receptor for bovine rotavirus UK, did not show an age-related decrease in the bovine intestine (Table 3). In Europe, rotavirus UK is one of the major bovine rotavirus serotypes isolated from symptomatic calves, while NCDV-like bovine rotaviruses have not been isolated (12). The age development of gangliosides in the bovine intestine thus does not explain the decreased susceptibility of older cattle to a major bovine rotavirus serotype.
Before addressing possible links between rotavirus receptors and host, age, and tissue tropism of rotavirus infection, we need a careful characterization of the early events of rotavirus-cell interaction in a cell culture model. This analysis should combine chemical, biochemical, and cell biological approaches to integrate the available observations in this intriguing research area.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Medical Research Council (no. 12628, 3967, and 10435), the Swedish Cancer Foundation, and the Wallenberg Foundation. N.R. was funded by the program “Glycoconjugates in Biological Systems” sponsored by the Swedish Foundation for Strategic Research.
We thank Marie-Lise Dillmann and Marie-France Clerc for their contribution to the electron microscopy experiments.
REFERENCES
- 1.Aggarwal B B, Eessalu T E, Hass P E. Characterizations of receptors for human tumor necrosis factor and their regulation by γ-interferon. Nature. 1985;318:665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- 2.Bass D M, Mackow E R, Greenberg H B. Identification and partial characterization of a rhesus rotavirus binding glycoprotein on murine enterocytes. Virology. 1991;183:602–610. doi: 10.1016/0042-6822(91)90989-o. [DOI] [PubMed] [Google Scholar]
- 3.Bellinzoni R C, Blackhall J O, Mattion N M, Estes M K, Snodgrass D R, La Torre J L, Scodeller E A. Serological characterization of bovine rotaviruses isolated from dairy and beef herds in Argentina. J Clin Microbiol. 1989;27:2619–2623. doi: 10.1128/jcm.27.11.2619-2623.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Björk S, Breimer M E, Hansson G C, Karlsson K-A, Leffler H. Structures of blood group glycosphingolipids of human small intestine. A relation between the expression of fucolipids of epithelial cells and the ABO, Le and Se phenotype of the donor. J Biol Chem. 1987;262:6758–6765. [PubMed] [Google Scholar]
- 5.Breimer E M, Hansson G C, Karlsson K-A, Leffler H. Blood group type glycosphingolipids from the small intestine of different animals analyzed by mass spectrometry and thin-layer chromatography. A note on species diversity. J Biochem. 1981;90:589–609. doi: 10.1093/oxfordjournals.jbchem.a133513. [DOI] [PubMed] [Google Scholar]
- 6.Brüssow H, Walther I, Fryder V, Sidoti J, Bruttin A. Cross-neutralizing antibodies induced by single serotype vaccination of cows with rotavirus. J Gen Virol. 1988;69:1647–1658. doi: 10.1099/0022-1317-69-7-1647. [DOI] [PubMed] [Google Scholar]
- 7.Brüssow H, Bruttin A, Marc-Martin S. Polypeptide composition of rotavirus empty capsids and their possible use as subunit vaccine. J Virol. 1990;64:3635–3642. doi: 10.1128/jvi.64.8.3635-3642.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brüssow H, Offit P A, Gerna G, Bruttin A, Sidoti J. Polypeptide specificity of antiviral serum antibodies in children naturally infected with human rotavirus. J Virol. 1990;64:4130–4136. doi: 10.1128/jvi.64.9.4130-4136.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brüssow H, Eichhorn W, Rohwedder A, Snodgrass D, Sidoti J. Cattle develop neutralizing antibodies to rotavirus serotypes which could not be isolated from faeces of symptomatic calves. J Gen Virol. 1991;72:1559–1567. doi: 10.1099/0022-1317-72-7-1559. [DOI] [PubMed] [Google Scholar]
- 10.Brüssow H, Offit P A, Sidoti J. Neutralizing antibodies to heterologous animal rotavirus serotypes 5, 6, 7, and 10 in sera from Ecuadorian children. J Clin Microbiol. 1991;29:869–873. doi: 10.1128/jcm.29.5.869-873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brüssow H, Nakagomi O, Gerna G, Eichhorn W. Isolation of an avian-like group A rotavirus from a calf with diarrhea. J Clin Microbiol. 1992;30:67–73. doi: 10.1128/jcm.30.1.67-73.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brüssow H, Rohwedder A, Nakagomi O, Sidoti J, Eichhorn W. Bovine rotavirus V1005 a P5, not a P12, type like all viruses in a German survey. J Clin Microbiol. 1994;32:2876–2879. doi: 10.1128/jcm.32.11.2876-2879.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, Ramig R F. Determinants of rotavirus stability and density during CsCl purification. Virology. 1992;186:228–237. doi: 10.1016/0042-6822(92)90077-3. [DOI] [PubMed] [Google Scholar]
- 14.Ciarlet M, Estes M K. Human and most animal rotavirus strains do not require the presence of sialic acid on the cell surface for efficient infectivity. J Gen Virol. 1999;80:943–948. doi: 10.1099/0022-1317-80-4-943. [DOI] [PubMed] [Google Scholar]
- 15.Coulson B S, Londrigan S L, Lee D J. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc Natl Acad Sci USA. 1997;94:5389–5394. doi: 10.1073/pnas.94.10.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn S J, Greenberg H B, Ward R L, Nakagomi O, Burns J W, Vo P T, Pax K A, Das M, Gowda K, Rao C D. Serotypic and genotypic characterization of human serotype 10 rotaviruses from asymptomatic neonates. J Clin Microbiol. 1993;31:165–169. doi: 10.1128/jcm.31.1.165-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estes M K. Rotavirus replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 1625–1655. [Google Scholar]
- 18.Fiore L, Greenberg H B, Mackow E R. The VP8 fragment of VP4 is the rhesus rotavirus hemagglutinin. Virology. 1991;181:553–563. doi: 10.1016/0042-6822(91)90888-i. [DOI] [PubMed] [Google Scholar]
- 19.Fukudome K, Yoshie O, Konno T. Comparison of human, simian and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology. 1989;172:196–205. doi: 10.1016/0042-6822(89)90121-9. [DOI] [PubMed] [Google Scholar]
- 20.Garbang-Chenon A, Nicolas J C, Bouvier M, Desjouis G, Molinier G, Repiquet D, Baptista-Lourenco M H, Gomant J P, Bricout F, Huraux J J. Epidemiologic and genomic study of rotavirus strains infecting young children and calves in the same rural environment. Eur J Epidemiol. 1986;2:108–111. doi: 10.1007/BF00157020. [DOI] [PubMed] [Google Scholar]
- 21.Gerna G, Sarasini A, Parea M, Arista S, Miranda P, Brüssow H, Hoshino Y, Flores J. Isolation and characterization of two distinct human rotavirus strains with G6 specificity. J Clin Microbiol. 1992;30:9–16. doi: 10.1128/jcm.30.1.9-16.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerrero C A, Zarate S, Corkidi G, Lopez S, Arias C F. Biochemical characterization of rotavirus receptors in MA104 cells. J Virol. 2000;74:9362–9371. doi: 10.1128/jvi.74.20.9362-9371.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gulati B R, Nakagomi O, Koshimura Y, Nakagomi T, Pandey R. Relative frequencies of G and P types among rotaviruses from Indian diarrheic cow and buffalo calves. J Clin Microbiol. 1999;37:2074–2076. doi: 10.1128/jcm.37.6.2074-2076.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo C T, Nakagomi O, Mochizuki M, Ishida H, Kiso M, Ohta Y, Suzuki T, Miyamoto D, Hidari K I, Suzuki Y. Ganglioside GM(1a) on the cell surface is involved in the infection by human rotavirus KUN and MO strains. J Biochem (Tokyo) 1999;126:683–688. doi: 10.1093/oxfordjournals.jbchem.a022503. [DOI] [PubMed] [Google Scholar]
- 25.Handa S. Blood group active glycolipid from human erythrocytes. Jpn J Exp Med. 1963;33:347–360. [PubMed] [Google Scholar]
- 26.Hansson G C, Karlsson K-A, Larson G, Strömberg N, Thurin J. Carbohydrate-specific adhesion of bacteria to thin-layer chromatograms: a rationalized approach to the study of host cell glycolipid receptors. Anal Biochem. 1985;146:158–163. doi: 10.1016/0003-2697(85)90410-5. [DOI] [PubMed] [Google Scholar]
- 27.Hardy E M, Woode G N, Xu Z, Gorziglia M. Comparative amino acid sequence analysis of VP4 for VP7 serotype 6 bovine rotavirus strains NCDV, B641, and UK. J Virol. 1991;65:5535–5538. doi: 10.1128/jvi.65.10.5535-5538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hewish J M, Takada Y, Coulson B S. Integrins α2β1 and α4β1 can mediate SA11 rotavirus attachment and entry into cells. J Virol. 2000;74:228–236. doi: 10.1128/jvi.74.1.228-236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iša P, López S, Segovia L, Arias C F. Functional and structural analysis of the sialic acid-binding domain of rotaviruses. J Virol. 1997;71:6749–6756. doi: 10.1128/jvi.71.9.6749-6756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.IUB-IUPAC Joint Commission on Biochemical Nomenclature (JCNB) Abbreviated terminology of oligosaccharide chains. Recommendations 1980. J Biol Chem. 1982;257:3347–3351. [PubMed] [Google Scholar]
- 31.IUPAC-IUB Commission on Biochemical Nomenclature (CNB) The nomenclature of lipids. Recommendations 1976. Eur J Biochem. 1977;79:11–21. [Google Scholar]
- 32.IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCNB) Nomenclature of glycoproteins, glycopeptides and peptidoglycans. Recommendations 1985. J Biol Chem. 1987;262:13–18. doi: 10.1111/j.1432-1033.1986.tb09825.x. [DOI] [PubMed] [Google Scholar]
- 33.IUPAC-IUBMB Joint Commission on Biochemical Nomenclature (JCNB) Nomenclature of carbohydrates. Recommendations 1996. Eur J Biochem. 1997;243:9. [Google Scholar]
- 34.Jolly C L, Beisner B M, Holmes I H. Rotavirus infection of MA104 cells is inhibited by Ricinus lectin and separately expressed single binding domains. Virology. 2000;275:89–97. doi: 10.1006/viro.2000.0470. [DOI] [PubMed] [Google Scholar]
- 35.Kapikian A Z, Flores J, Hoshino Y, Glass R I, Midthun K, Garziglia M, Chanock R. Rotavirus: the major etiological agent of severe infantile diarrhea may be controllable by a “Jennerian” approach to vaccination. J Infect Dis. 1986;153:815–822. doi: 10.1093/infdis/153.5.815. [DOI] [PubMed] [Google Scholar]
- 36.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1657–1708. [Google Scholar]
- 37.Karlsson K-A. Preparation of total non acid glycolipids for overlay analysis of receptor for bacteria and viruses and for other studies. Methods Enzymol. 1987;138:212–220. doi: 10.1016/0076-6879(87)38018-8. [DOI] [PubMed] [Google Scholar]
- 38.Karlsson K-A. Overlay and solid phase analysis of glycolipid receptors for bacteria and viruses. Methods Enzymol. 1987;138:220–232. doi: 10.1016/0076-6879(87)38019-x. [DOI] [PubMed] [Google Scholar]
- 39.Karlsson K-A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- 40.Keljo D J, Smith A K. Characterization of binding of simian rotavirus to cultured epithelial cells. J Pediatr Gastroenterol Nutr. 1988;7:249–256. doi: 10.1097/00005176-198803000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Koerner T A W, Jr, Prestegard J H, Demou P C, Yu R K. High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry. 1983;22:2676–2687. doi: 10.1021/bi00280a014. [DOI] [PubMed] [Google Scholar]
- 42.Kuhlenschmidt T B, Hanafin W P, Gelberg H B, Kuhlenschmidt M S. Sialic acid dependence and independence of group A rotaviruses. Adv Exp Med Biol. 1999;473:309–317. doi: 10.1007/978-1-4615-4143-1_33. [DOI] [PubMed] [Google Scholar]
- 43.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 44.Lanne B, Uggla L, Stenhagen G, Karlsson K-A. Enhanced binding of enterotoxigenic Escherichia coli K99 to amide derivatives of the receptor ganglioside NeuGc-GM3. Biochemistry. 1995;34:1845–1850. doi: 10.1021/bi00006a004. [DOI] [PubMed] [Google Scholar]
- 45.Lopez S, Espinosa R, Greenberg H B, Arias C F. Mapping the subgroup epitopes of rotavirus protein VP6. Virology. 1994;204:153–162. doi: 10.1006/viro.1994.1519. [DOI] [PubMed] [Google Scholar]
- 46.Mebus C A, Wyatt R G, Kapikian A Z. Intestinal lesions induced in gnotobiotic calves by the virus of human infantile gastroenteritis. Vet Pathol. 1977;14:273–282. doi: 10.1177/030098587701400310. [DOI] [PubMed] [Google Scholar]
- 47.Mendez E, López S, Cuadras M A, Romero P, Arias C F. Entry of rotaviruses is a multistep process. Virology. 1999;263:450–459. doi: 10.1006/viro.1999.9976. [DOI] [PubMed] [Google Scholar]
- 48.Miller-Podroza H, Larsson T, Nilsson J, Teneberg S, Matrosovitch M, Johansson L. Epitope dissection of receptor-active gangliosides with affinity for Helicobacter pylori and influenza virus. Acta Biochim Pol. 1998;45:439–449. [PubMed] [Google Scholar]
- 49.Moreno E, Lanne B, Vázquez A M, Kawashima I, Tai T, Fernández L E, Karlsson K-A, Ångström J, Pérez R. Delineation of the epitope recognized by an antibody specific for N-glycolylneuraminic acid-containing gangliosides. Glycobiology. 1998;8:695–705. doi: 10.1093/glycob/8.7.695. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura K, Handa S. Biochemical properties of N-methylamides of sialic acid in gangliosides. J Biochem (Tokyo) 1986;99:219–226. doi: 10.1093/oxfordjournals.jbchem.a135462. [DOI] [PubMed] [Google Scholar]
- 51.Puente R, Hueso P. Lactational changes in the N-glycolylneuraminic acid content of bovine gangliosides. Biol Chem Hoppe-Seyler. 1993;374:475–478. doi: 10.1515/bchm3.1993.374.7-12.475. [DOI] [PubMed] [Google Scholar]
- 52.Puente R, Garcia-Parlo L A, Hueso P. Gangliosides in bovine milk. Changes in content and distribution of individual ganglioside levels during lactation. Biol Chem Hoppe-Seyler. 1992;373:283–288. doi: 10.1515/bchm3.1992.373.1.283. [DOI] [PubMed] [Google Scholar]
- 53.Rolsma D M, Gelberg H B, Kuhlenschmidt M S. Assay for evaluation of rotavirus-cell interactions: identification of an enterocyte ganglioside fraction that mediates group A porcine rotavirus recognition. J Virol. 1994;68:258–268. doi: 10.1128/jvi.68.1.258-268.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rolsma D M, Gelberg H B, Kuhlenschmidt M S. Structure and function of a ganglioside receptor for porcine rotavirus. J Virol. 1998;72:9079–9091. doi: 10.1128/jvi.72.11.9079-9091.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruggeri F M, Greenberg H B. Antibodies to the trypsin cleavage peptide VP8 neutralize rotavirus by inhibiting binding of virions to target cells in culture. J Virol. 1991;65:2211–2219. doi: 10.1128/jvi.65.5.2211-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryder R W, Yolken R H, Reeves W C, Sack R B. Enzootic bovine rotavirus is not a source of infection in Panamanian cattle ranchers and their families. J Infect Dis. 1986;153:1139–1144. doi: 10.1093/infdis/153.6.1139. [DOI] [PubMed] [Google Scholar]
- 57.Samuelsson B E, Pimlott W, Karlsson K-A. Mass spectrometry of mixtures of intact glycosphingolipids. Methods Enzymol. 1990;193:623–646. doi: 10.1016/0076-6879(90)93442-n. [DOI] [PubMed] [Google Scholar]
- 58.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 59.Snodgrass D R, Terzolo N H, Sherwood D, Campbell I, Menzies J L, Synge B A. Aetiology of diarrhoea in young calves. Vet Rec. 1986;119:31–34. doi: 10.1136/vr.119.2.31. [DOI] [PubMed] [Google Scholar]
- 60.Srnka C A, Tiemeyer M, Gilbert J H, Moreland M, Schweingruber H, de Lappe B W, James P G, Gant T, Willoughby R E, Yolken R H, Nashed M A, Abbas S A, Laine R A. Cell surface ligand for rotavirus: mouse intestinal glycolipids and synthetic carbohydrate analogs. Virology. 1992;190:794–805. doi: 10.1016/0042-6822(92)90917-e. [DOI] [PubMed] [Google Scholar]
- 61.Stellner K, Saito H, Hakomori S I. Determination of aminosugar linkages in glycolipids by methylation. Aminosugar linkages of ceramide pentasaccharides of rabbit erythrocytes and of Forssman antigen. Arch Biochem Biophys. 1973;155:464–472. doi: 10.1016/0003-9861(73)90138-0. [DOI] [PubMed] [Google Scholar]
- 62.Superti F, Donelli G. Gangliosides as binding sites in SA11 rotavirus infection of LLC-MK2 cells. J Gen Virol. 1991;72:2467–2474. doi: 10.1099/0022-1317-72-10-2467. [DOI] [PubMed] [Google Scholar]
- 63.Teneberg S, Willemsen P T J, de Graaf F K, Stenhagen G, Pimlott W, Jovall P Å, Ångström J, Karlsson K-A. Characterization of gangliosides of epithelial cells of calf small intestine, with special reference to receptor-active sequences for enteropathogenic Escherichia coli K99. J Biochem (Tokyo) 1994;116:560–574. doi: 10.1093/oxfordjournals.jbchem.a124562. [DOI] [PubMed] [Google Scholar]
- 64.Veh R W, Corfield A P, Sander M, Schauer R. Neuraminic acid-specific modification and tritium labelling of gangliosides. Biochim Biophys Acta. 1976;486:145–160. doi: 10.1016/0005-2760(77)90079-0. [DOI] [PubMed] [Google Scholar]
- 65.Waldi D. Sprühreagentien für die Dünnschicht-chromatographie. In: Stahl E, editor. Dünnschicht-Chromatographie. Berlin, Germany: Springer-Verlag; 1962. pp. 496–515. [Google Scholar]
- 66.Willoughby R E, Yolken R H, Schnaar R L. Rotaviruses specifically bind to the neutral glycosphingolipid asialo-GM1. J Virol. 1990;64:4830–4835. doi: 10.1128/jvi.64.10.4830-4835.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willoughby R E. Rotaviruses preferentially bind O-linked sialylglycoconjugates and sialomucins. Glycobiology. 1993;3:437–445. doi: 10.1093/glycob/3.5.437. [DOI] [PubMed] [Google Scholar]
- 68.Yang H J, Hakomori S I. A sphingolipid having a novel ceramide and lacto-N-fucopentose III. J Biol Chem. 1971;246:1192–1200. [PubMed] [Google Scholar]
- 69.Yolken R H, Willoughby R, Wee S B, Miskuff R, Vonderfecht S. Sialic acid glycoproteins inhibit in vitro and in vivo replication of rotaviruses. J Clin Investig. 1987;79:148–154. doi: 10.1172/JCI112775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yolken R H, Peterson J A, Vonderfecht S L, Fouts E T, Midthun K, Newburg D S. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Investig. 1992;90:1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]