Abstract
Abstract
Background
China’s plasmapheresis donation policy differs from that of Western countries. The association between regular plasmapheresis donation and donor health in China is still unknown.
Objectives
To investigate the association of regular plasmapheresis donation with serum protein and electrolyte levels and provide scientific evidence for policy improvement.
Design
Multicentre cross-sectional study.
Setting and participants
A total of 767 regular and 726 new donors from the provinces of Sichuan, Hunan, Henan and Yunnan were recruited from September 2021 to October 2022.
Primary and secondary outcome measures
Our primary outcome focused on measuring the levels of serum protein and electrolyte levels, including total serum protein (TSP), IgG, albumin (Alb), haemoglobin (Hb), calcium, potassium (K+) and magnesium (Mg2+). The secondary outcome assessed their abnormal rates.
Results
Male and female donors in the high donation frequency group (>16 donations per year) exhibited lower IgG levels compared with new donors (p=0.008 for male donors and p=0.007 for female donors). Additionally, female donors with high donation frequency and a high total number of lifetime donations (>100 donations) had significantly lower Hb concentrations than new donors. However, no significant changes were observed in TSP, Alb, calcium, K+ and Mg2+ levels. There were also no statistically significant differences in the rates of abnormal protein and electrolyte values below the respective threshold levels between new and regular donors.
Conclusions
Plasmapheresis donation is not associated with an increased risk of abnormalities in the analysed parameters. However, the results provide preliminary evidence supporting the routine inclusion of IgG screening for donors, as plasmapheresis donation is associated with a decrease in IgG levels. Particular attention should be paid to the Hb levels of female donors, especially those who donate frequently. Testing of TSP at each donation may not be necessary.
Keywords: China, blood bank & transfusion medicine, health services administration & management, health surveys
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Enhances the understanding of real-world heterogeneity through a multicentre approach with a larger sample size, making the findings more generalisable.
The cross-sectional design of our study limits our ability to establish causality between plasmapheresis donation and serum protein and electrolyte levels.
In our study, we attempted to control for key confounding variables, but some residual confounding may still be present.
Variability in assay conditions across laboratories could affect reliability, especially for haemoglobin measurements.
Introduction
Blood and blood products are essential medicines for clinical use, saving millions of lives annually and being included in the Model List of Essential Medicines of the WHO.1 Plasma-derived medicinal products (PDMPs), such as albumin, coagulation factors and immunoglobulins, are prepared from human plasma and are crucial in preventing and treating a variety of life-threatening diseases.2 Source plasma (SP) is a vital raw material for PDMP production and is exclusively used for further manufacturing into final therapies through fractionation. In China, all SP is obtained through apheresis plasma donation. Plasma donors are required to undergo a health assessment and blood tests, and only those who meet the criteria are eligible to donate (online supplemental table 1).3
During the process of plasmapheresis donation, plasma is separated and collected while the blood cells are returned to the donors. Citrate, which serves as an anticoagulant, can lead to a decrease in electrolyte levels in the blood, such as total calcium (CaT),4,6 Mg2+,7 K+.5 8 Continuous plasma loss and anticoagulant use may impact donor physiology and protein levels. Therefore, the health of regular plasmapheresis donors is closely monitored. Several trials conducted in the USA have indicated that the ability of donors to regenerate lost plasma proteins can be a limiting factor in plasmapheresis donation.9 10 A small-scale study observed that regular plasmapheresis donors had lower levels of IgG and total serum protein (TSP), but the levels remained within normal ranges.11 The public, including those in China, is concerned that plasmapheresis might lower haemoglobin (Hb) levels. This concern has led to the strict enforcement of Hb testing. However, a study conducted in the USA found no difference in Hb levels between regular and new plasma donors.12
China has implemented different standards and guidelines for plasmapheresis donation compared with other countries. In China, the minimum time interval between plasmapheresis donations is set at 14 days, and the maximum number of donations allowed per year is limited to 24,3 while plasma donors in the USA may be eligible to donate twice a week.13 In Australia, plasmapheresis donors can contribute up to 26 times per year.14 Additionally, the volume of plasma that Chinese donors can provide per donation is limited to 600 mL, including the anticoagulant,3 whereas in the USA, donors can donate between 625 and 800 mL without anticoagulant.13 It is important to recognise that previous studies conducted in foreign settings cannot be directly extrapolated to China due to differences in collection criteria, ethnicity and dietary habits. The health outcomes of the Chinese plasmapheresis population have not been well studied.
In recent years, there has been a growing demand for plasma-derived products and apheresis plasma. China had 3.667 million registered plasmapheresis donors and 25.654 million donations in 2019.15 Donation serves the demand for plasma, but there are concerns among potential donors and the public about the impact of continuous blood loss and anticoagulant exposure during plasmapheresis process on physical health. In order to further understand the potential impacts and risks associated with regular plasma donation, we launched a series of multicentre studies focusing on the health outcomes, donation behaviour and risk interventions of Chinese plasma donors. This subproject focused on the protein and electrolyte metabolism of regular plasma donors.
Materials and methods
Study design and population
We conducted a multicentre cross-sectional study from September 2021 to October 2022 to evaluate TSP, IgG, Alb, Hb, CaT, K+ and Mg2+ in plasmapheresis donors from Sichuan, Hunan, Henan and Yunnan provinces. The study included two groups: a control group consisting of new donors with no prior plasmapheresis history and an investigator group consisting of regular donors who had donated plasma in the past years. Due to the allowance of up to 24 donations per year in China, we categorised regular donors based on their donation frequency in the previous 12 months into three groups: low (1–8 donations), medium (9–16 donations) and high (17–24 donations). Additionally, divided regular donors into low (1–50 donations), medium (51–100 donations) and high (more than 100 donations) total number of lifetime donations groups based on a combination of the distribution within our study cohort and expert consensus to ensure a balanced distribution of participants across categories. Our primary outcome focused on measuring the levels of serum protein and electrolytes, while the secondary outcome assessed their abnormal rates. Analyses were stratified by gender. According to the National Guide to Clinical Laboratory Procedures, the low values of TSP, Alb, IgG, calcium, Mg2+ and K+ were assigned as less than 65 g/L, 40 g/L, 7.0 g/L, 2.11 mmol/L, 0.75 and 3.5 mmol/L, respectively.16
Inclusion and exclusion criteria
All participants in this study were within the age range of 18–60 years, following the Chinese donor criteria.3 Specifically, donors who had donated whole blood or platelets in the past 12 months were excluded. Participants were asked whether they had taken protein and/or electrolyte supplements in the past year prior to enrolment. Those who had taken such supplements were excluded from the study. Since calcium status is influenced by vitamin D or parathyroid hormone, donors with vitamin D deficiency and hyperparathyroidism were excluded. Additionally, donors with a self-reported chronic inflammatory syndrome or a history of metabolic diseases, including abnormal blood lipids, uric acid and cholesterol, were also excluded. These exclusions were implemented because these conditions have the potential to affect the target parameters and introduce confounding variables.
Samples and laboratory testing
Once a donor was found eligible for the study, a 1 mL sample of predonation blood was collected using sterile tubes for Hb detection (Automated haematology analyzer, Matenu, China) at local laboratories. An additional 2 mL of predonation blood was centrifuged at 3000 r/min for 10 min. The separated serum samples were stored at −20°C until analysis. The same batch of serum samples was tested for TSP, Alb, IgG, CaT, Mg2+ and K+ (Beckman Coulter Chemistry Analyzer AU5800 Serie, USA). It is worth noting that Alb affects CaT. Therefore, the albumin-corrected calcium (ACCA) obtained by adjusting Alb can better reflect the true calcium level. ACCA (mg/dL)=CaT (mg/dL)+0.8 [4−Albumin (g/dL)].17 Plasmapheresis donation was carried out using the Nigale Plasma Separator (NGL XJC 2000, Sichuan Nigale Biotechnology Co., China).
Data collection
The number of donations in the prior 12 months and the total number of lifetime donations were retrieved from each plasmapheresis donation centre by the Donor Management System. Demographic information (living place, sex, age, female menstrual history), socioeconomic information (education, annual household income), and lifestyle variables (smoking, drinking, meat intake, physical activity) were collected through face-to-face interviews conducted by staff at each plasma donation centre using paper questionnaires. Annual household income, initially recorded in Chinese yuan (CNY), was uniformly converted to US dollars (USD) using the exchange rates from September 2021 (US$1=CNY6.4599). Subsequently, the annual household income was categorised into three intervals: low (<US$4644), moderate (US$4644–US$12 384) and high (>US$12 384). Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ)18 and categorised as low (<600 MET-minutes per week), moderate (600–3000 MET-minutes per week) and high (>3000 MET-minutes per week). Body mass index (BMI)=weight (kg)/height (m) squared.
Quality control
Preparatory measures were taken prior to information and sample collection involving uniform and standardised training materials. These materials were then used to train the staff in the technical requirements, technical operations and other pertinent aspects of the study. Additionally, surveys, barcodes and questionnaires were dispatched to each collection centre. On completion of the collection process, serum samples were promptly chilled and repeated freeze-thaw cycles were avoided. During sample testing, real-time monitoring strategies were employed to meticulously assess the accuracy of the standard curve and maintain strict adherence to rigorous quality control measures.
Statistical analysis
We summarised continuous measurements using the mean and SD if they were normally distributed. For intergroup comparisons, we conducted independent-sample t-test; otherwise, the continuous variables were represented as IQR and used the Wilcoxon rank-sum test for group comparisons. Categorical observations were presented as frequencies and percentages. The donation categories were classified and ordered. Text and figure legends indicated whether p values retained statistical significance at the p=0.0167 (p=0.05/3) level after Bonferroni adjustment. Additionally, we employed univariate logistic regression to compare the rates of low protein and electrolyte between the regular donor groups and the new donors.
We considered confounding factors that could affect the serum protein and electrolyte levels for more accurate results. Covariates were selected based on factors reported in previous literature as potentially related to protein and electrolyte metabolism.19,21 Multiple linear regression was used to compare protein and electrolyte levels between regular and new donors. In the adjusted model, confounding factors, including age, BMI, living place, meat intake, education, smoking, drinking, household income and physical activity (female donors additionally had menstrual history adjusted), were adjusted. 540 male donors have 90% power to detect an effect size (f2) of 0.02 attributable to 1 independent variable using an F-test with an alpha of 0.05. The adjusted variables include nine independent variables. 542 female donors have 90% power to detect an effect size (f2) of 0.02 attributable to 1 independent variable using an F-test with alpha=0.05. The adjusted variables include 10 independent variables. Different rates of low protein and electrolytes between each of the regular donor group and the new donor group were compared using multivariate logistic regression with adjusted models for confounding factors. Multiple comparisons were also corrected using the Bonferroni (p<0.05/3=0.0167). A p value of 5% is considered significant unless otherwise indicated.
Patient and public involvement
It was not possible to involve patients or the public in the design, conduct, reporting or dissemination plans of our research.
Results
Demographics of study donors
The study included a total of 1493 donors. Among them, 774 were male donors and 719 were female donors. Among the male donors, 384 were new donors and 390 were regular donors. Among the female donors, 342 were new donors and 377 were regular donors. The regular donors were older than new donors among males, with the median age of male new donors being 36 years (IQR 25–46) and that of regular donors being 41 years (IQR 31–50) (p<0.001). However, there was no significant difference in age between the two groups among females, with the median age of female new donors being 41 years (IQR 32–49) and that of regular donors being 42 years (IQR 32–50) (p=0.102). The BMI of regular donors was higher than that of new donors in both males and females (male: p=0.002; female: p=0.018), with the median BMI of male new donors being 24.48 (IQR 21.82–27.10) compared with 25.57 (IQR 22.65–28.39) for regular donors, and the median BMI of female new donors being 23.76 (IQR 21.48–26.59) compared with 24.60 (IQR 22.06–27.25) for regular donors. Significant differences were observed in annual household income and physical activity between new donors and regular donors (p<0.05) (table 1).
Table 1. Baseline data of repeat donors and new donors.
| Variables | Male | Female | ||||
| New donors | Regular donor | P value | New donors | Regular donor | P value | |
| Total number | 384 | 390 | 342 | 377 | ||
| Living place | 0.601 | 0.991 | ||||
| Hunan | 78 (20.3) | 89 (22.8) | 62 (18.1) | 101 (26.8) | ||
| Yunnan | 90 (23.4) | 100 (25.6) | 97 (28.4) | 71 (18.8) | ||
| Henan | 109 (28.4) | 98 (25.1) | 92 (26.9) | 101 (26.8) | ||
| Sichuan | 107 (27.9) | 103 (26.4) | 91 (26.6) | 104 (27.6) | ||
| Age(year) | 36 (25–46) | 41 (31–50) | <0.001 | 41 (32–49) | 42 (32–50) | 0.102 |
| BMI (kg/m2) | 24.48 (21.82–27.10) | 25.57 (22.65–28.39) | 0.002 | 23.76 (21.48–26.59) | 24.60 (22.06–27.25) | 0.018 |
| Education | 0.001 | 0.62 | ||||
| Elementary school | 50 (13.0) | 71 (18.2) | 98 (28.7) | 121 (32.1) | ||
| Junior high school | 170 (44.3) | 190 (48.7) | 143 (41.8) | 156 (41.4) | ||
| High school | 147 (38.7) | 100 (25.6) | 90 (26.3) | 92 (24.4) | ||
| Universities | 17 (4.4) | 29 (7.4) | 11 (3.2) | 8 (2.1) | ||
| Meat intake | 0.302 | 0.019 | ||||
| None | 3 (0.8) | 4 (1) | 7 (2) | 5 (1.3) | ||
| Occasionally | 161 (41.9) | 184 (47.2) | 192 (56.1) | 250 (66.3) | ||
| Frequently | 220 (57.3) | 202 (51.8) | 143 (41.8) | 122 (32.4) | ||
| Smoking | 0.012 | 0.304 | ||||
| None | 112 (29.2) | 126 (32.3) | 328 (95.9) | 369 (97.9) | ||
| Occasionally | 136 (35.4) | 100 (25.6) | 11 (3.2) | 6 (1.6) | ||
| Frequently | 136 (35.4) | 164 (42.1) | 3 (0.9) | 2 (0.5) | ||
| Drinking | 0.129 | 0.058 | ||||
| None | 126 (32.8) | 147 (37.7) | 330 (96.5) | 352 (93.4) | ||
| Occasionally | 245 (63.8) | 223 (57.2) | 12 (3.5) | 25 (6.6) | ||
| Frequently | 13 (3.4) | 20 (5.1) | 0 (0) | 0 (0) | ||
| Annual household income | 0.008 | 0.004 | ||||
| Low | 99 (25.8) | 73 (18.7) | 137 (40.1) | 125 (33.2) | ||
| Moderate | 178 (46.4) | 223 (57.2) | 140 (40.9) | 200 (53.1) | ||
| High | 107 (27.9) | 94 (24.1) | 65 (19) | 52 (13.8) | ||
| Physical activity | <0.001 | 0.005 | ||||
| Low | 144 (37.5) | 77 (19.7) | 167 (48.8) | 145 (38.5) | ||
| Moderate | 171 (44.5) | 233 (59.7) | 140 (40.9) | 200 (53.1) | ||
| High | 69 (18.0) | 80 (20.5) | 35 (10.2) | 32 (8.5) | ||
| Menstrual history | – | 0.612 | ||||
| Premenopausal | – | – | 272 (79.5) | 294 (78) | ||
| Postmenopausal | – | – | 70 (20.5) | 83 (22) | ||
| Total number of lifetime donations | – | 32 (14–73) | – | 34 (12–75) | ||
Continuous measurements are expressed as mean±SD if normally distributed; otherwise, as interquartile range (IQR)IQR. Categorical observations are expressed as n (%).
BMI, body mass index(calculated as weight in kilograms divided by height in metersmetres squared).
BMIbody mass index
Association of recent donation frequency and protein and electrolyte values
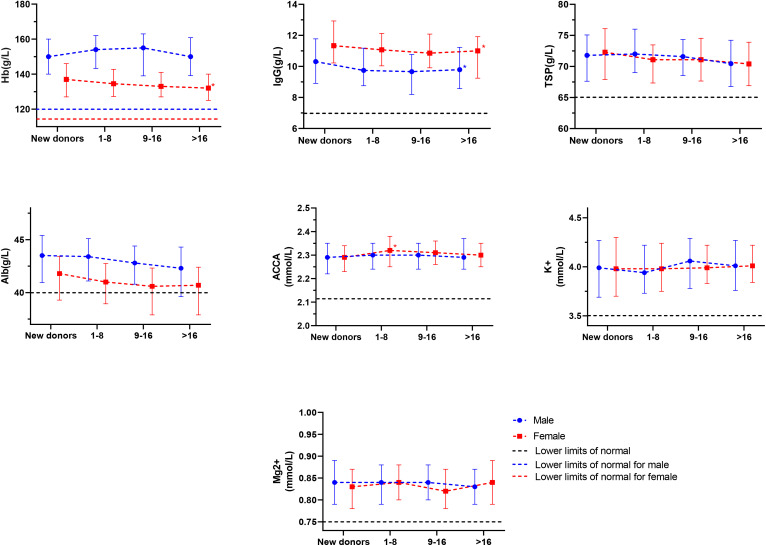
Among male donors, the mean IgG concentration of new donors was 10.31 g/L (IQR: 8.91–11.78 g/L). The mean IgG concentration of the high-frequency group was 9.79 g/L (IQR: 8.57–11.23 g/L). Compared with the new donors, the difference between the two groups was statistically significant after adjusting for confounding factors (p=0.008). The mean concentration of Alb in the new donors was 43.5 g/L (IQR: 40.95–45.4 g/L), and that in the high-frequency group was 42.3 g/L (IQR: 39.62–44.3 g/L). Before adjusting for confounding factors, the difference in mean Alb between the two groups was statistically significant (p=0.005); however, after adjusting for confounding factors, the difference was not significant (p=0.246). The concentrations of mean Hb, TSP, ACCA, K+ and Mg2+ in different donation frequency groups showed no statistical differences compared with the new donors (table 2).
Table 2. Influence of recent donation frequency on protein and electrolyte values of plasmapheresis donors.
| Measurements | Male | Female | |||||||
| New donors* | Recent donation frequency (times per year) | New donors* | Recent donation frequency (times per year) | ||||||
| 1–8 | 9–16 | >16 | 1–8 | 9–16 | >16 | ||||
| Hb (g/L) | Mean (IQR) | 150 (140–160) | 154 (143.2–162) | 155 (139–163) | 150 (139.15–160.85) | 137 (127–146) | 134.5 (127.25–142.75) | 133 (127–141) | 132 (125–140) |
| P (unadjusted model) | Reference group | 0.049 | 0.102 | 0.911 | Reference group | 0.161 | 0.014 | <0.001 | |
| P (adjusted model)† | Reference group | 0.188 | 0.171 | 0.273 | Reference group | 0.059 | 0.023 | 0.002 | |
| IgG (g/L) | Mean (IQR) | 10.31 (8.91–11.78) | 9.74 (8.76–11.18) | 9.66 (8.2–10.77) | 9.79 (8.57–11.23) | 11.34 (10.22–12.93) | 11.07 (10.04–12.13) | 10.86 (9.91–12.08) | 11 (9.24–11.92) |
| P (unadjusted model) | Reference group | 0.031 | 0.001 | 0.036 | Reference group | 0.037 | 0.021 | 0.001 | |
| P (adjusted model)† | Reference group | 0.125 | 0.03 | 0.008 | Reference group | 0.178 | 0.12 | 0.007 | |
| TSP (g/L) | Mean (IQR) | 71.80 (67.62–75.07) | 72.00 (69.00–76.00) | 71.60 (68.55–74.35) | 70.45 (66.77–74.22) | 72.30 (67.90–76.10) | 71.10 (67.35–73.47) | 71.10 (67.65–74.55) | 70.40 (66.90–73.90) |
| P (unadjusted model) | Reference group | 0.207 | 0.91 | 0.113 | Reference group | 0.745 | 0.334 | 0.489 | |
| P (adjusted model)† | Reference group | 0.759 | 0.555 | 0.856 | Reference group | 0.526 | 0.909 | 0.335 | |
| Alb (g/L) | Mean (IQR) | 43.5 (40.95–45.4) | 43.4 (41.12–45.1) | 42.8 (40.8–44.4) | 42.3 (39.62–44.3) | 41.8 (39.3–43.4) | 40.9 (38.52–42.65) | 40.9 (39.1–42.5) | 40.7 (38.2–42.7) |
| P (unadjusted model) | Reference group | 0.815 | 0.205 | 0.005 | Reference group | 0.027 | 0.059 | 0.011 | |
| P (adjusted model)† | Reference group | 0.261 | 0.275 | 0.246 | Reference group | 0.481 | 0.91 | 0.495 | |
| ACCA(mmol/L) | Mean (IQR) | 2.29 (2.22–2.35) | 2.30 (2.24–2.35) | 2.3 (2.24–2.35) | 2.29 (2.24–2.37) | 2.29 (2.23–2.34) | 2.32 (2.25–2.38) | 2.31 (2.26–2.36) | 2.3 (2.25–2.35) |
| P (unadjusted model) | Reference group | 0.133 | 0.209 | 0.316 | Reference group | 0.001 | 0.027 | 0.176 | |
| P (adjusted model)† | Reference group | 0.024 | 0.374 | 0.073 | Reference group | 0.015 | 0.056 | 0.185 | |
| K+ (mmol/L) | Mean (IQR) | 3.99 (3.69–4.27) | 3.94 (3.73–4.22) | 4.06 (3.78–4.29) | 4.01 (3.76–4.27) | 3.98 (3.70–4.30) | 3.98 (3.75–4.24) | 3.99 (3.83–4.22) | 4.01 (3.84–4.22) |
| P (unadjusted model) | Reference group | 0.485 | 0.223 | 0.282 | Reference group | 0.611 | 0.488 | 0.51 | |
| P (adjusted model)† | Reference group | 0.904 | 0.135 | 0.083 | Reference group | 0.532 | 0.819 | 0.947 | |
| Mg2+(mmol/L) | Mean (IQR) | 0.84 (0.79–0.89) | 0.84 (0.79–0.88) | 0.84 (0.80–0.88) | 0.83 (0.79–0.87) | 0.83 (0.78–0.87) | 0.84 (0.80–0.88) | 0.82 (0.78–0.87) | 0.84 (0.79–0.89) |
| P (unadjusted model) | Reference group | 0.708 | 0.898 | 0.553 | Reference group | 0.574 | 0.182 | 0.322 | |
| P (adjusted model)† | Reference group | 0.963 | 0.646 | 0.175 | Reference group | 0.35 | 0.601 | 0.172 | |
Continuous measurements are expressed as mean±SD if normally distributed; otherwise, as median (IQR). Adjusted for multiple comparisons by Bonferroni correction.
New donors served as the control group and each experimental group was compared with the control group.
Adjusted for age, BMI, living place, meat intake, education, smoking and drinking, annual household income, physical activity (female donors additionally adjusted menstrual history).
ACCAalbumin-corrected calciumAlbalbuminBMIbody mass indexHbhaemoglobinTSPtotal serum protein
Among the female donors, the mean Hb concentration of the new donors was 137 g/L (IQR: 127–146 g/L). The mean Hb concentration of the high donation frequency group was 132 g/L (IQR: 125–140 g/L). Compared with the new donors, before adjusting for confounding factors, the difference in mean Hb between the two groups was statistically significant (p<0.001). Results were unchanged after adjusting for confounding factors (p=0.002). The mean IgG concentration of new donors was 11.90 g/L (IQR: 11.63–12.17 g/L), and that of high donation frequency donors was 11.00 g/L (IQR: 9.24–11.92 g/L). After adjusting for confounding factors, the difference between the two groups was statistically significant (p=0.007). The mean concentration of Alb in the new donors was 41.8 g/L (IQR: 39.3–43.4 g/L), and that in the high-frequency group was 40.7 g/L (IQR: 38.2–42.7 g/L). Before adjusting for confounding factors, the difference between the two groups was statistically significant (p=0.011), but after adjusting for confounding factors, the difference was not statistically significant (p=0.495). The mean ACCA concentration of new donors was 2.29 mmol/L (IQR: 2.23–2.34 mmol/L), and that of the low donation frequency group was 2.32 mmol/L (IQR: 2.25–2.38 mmol/L). After adjusting for confounding factors, the difference was statistically significant (p=0.015). However, there was no statistical difference between the mean ACCA concentrations of the medium and high donation frequency groups and those of the new donors. The mean concentrations of TSP, K+ and Mg2+ in different donation frequency groups showed no statistical difference compared with those of new donors (table 2, figure 1).
Figure 1. The impact of donation frequency on serum protein and electrolyte level. Mean (IQR) of serum protein and electrolyte level. The horizontal dotted line indicates the lower limit of normal; new donors served as the control group. *Significant after adjusting for age, BMI, living place, meat intake, education, smoking and drinking, annual household income, physical activity (female donors additionally adjusted menstrual history). ACCA, albumin-corrected calcium; BMI, body mass index; Hb, haemoglobin; TSP, total serum protein.
Association of the total number of lifetime donation and protein and electrolyte values
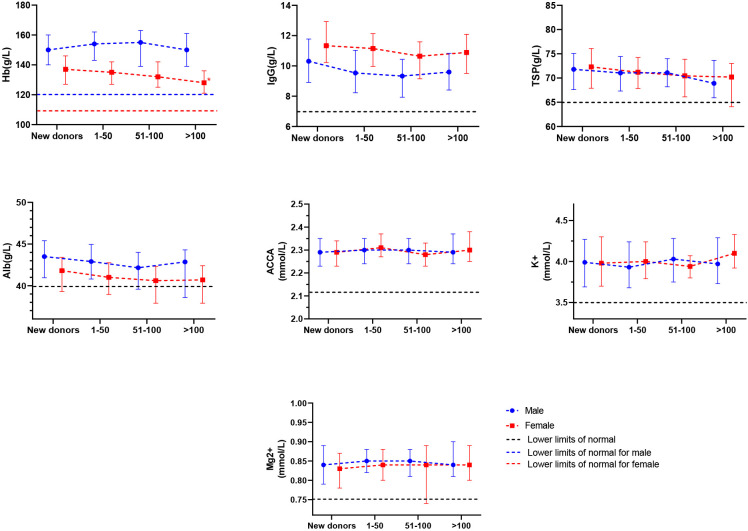
Among male donors, the mean concentrations of Hb, IgG, Alb, TSP, ACCA, K+ and Mg2+ showed no statistical difference between groups with different total numbers of donations and new donors. For the female donors, mean Hb concentration in the medium and high total number of lifetime donations group was significantly lower than that in the new group before adjusting for confounding factors, and mean Hb concentration in the high total number of lifetime donation group was still significantly lower than that in the new group after adjusting for confounding factors (p=0.001). The mean ACCA in the group of low total number of lifetime donation groups was significantly higher than that in the group of new donors (p<0.001), and the difference was still significant after adjusting for confounding factors (p<0.001). However, the mean ACCA in the group of medium and high total number of lifetime donations showed no statistical difference compared with that of new donors (table 3, figure 2).
Table 3. Influence of total number of lifetime donations on protein and electrolyte values of plasmapheresis donors.
| Measurements | Male | Female | |||||||
| New donors* | Total number of lifetime donations | New donors* | Total number of lifetime donations | ||||||
| 1–50 | 51–100 | >100 | 1–50 | 51–100 | >100 | ||||
| Hb (g/L) | Mean (IQR) | 150 (140–160) | 154 (143.2–162) | 155 (139–163) | 150 (139.15–160.85) | 137 (127–146) | 135 (127–142.5) | 132 (125–141.75) | 128 (121–136) |
| P (unadjusted model) | Reference group | 0.014 | 0.206 | 0.037 | Reference group | 0.126 | 0.002 | <0.001 | |
| P (adjusted model)† | Reference group | 0.096 | 0.115 | 0.652 | Reference group | 0.233 | 0.526 | 0.001 | |
| IgG (g/L) | Mean (IQR) | 10.31 (8.91–11.78) | 9.54 (8.23–11.03) | 9.33 (7.93–10.44) | 9.60 (8.41–10.83) | 11.34 (10.22–12.93) | 11.15 (9.97–12.13) | 10.64 (9.15–11.59) | 10.89 (9.50–12.09) |
| P (unadjusted model) | Reference group | <0.001 | 0.197 | 0.677 | Reference group | 0.002 | 0.001 | 0.117 | |
| P (adjusted model)† | Reference group | 0.021 | 0.028 | 0.632 | Reference group | 0.507 | 0.568 | 0.017 | |
| TSP (g/L) | Mean (IQR) | 71.80 (67.62–75.07) | 71.05 (67.32–74.45) | 71.10 (68.20–74.00) | 68.90 (65.92–73.65) | 72.30 (67.90–76.10) | 71.20 (67.85–74.25) | 70.45 (66.12–73.87) | 70.20 (64.10–73.00) |
| P (unadjusted model) | Reference group | 0.977 | 0.743 | 0.453 | Reference group | 0.406 | 0.511 | 0.22 | |
| P (adjusted model)† | Reference group | 0.315 | 0.986 | 0.607 | Reference group | 0.693 | 0.516 | 0.629 | |
| Alb (g/L) | Mean (IQR) | 43.50 (40.95–45.40) | 42.90 (40.80–44.95) | 42.15 (39.58–44.00) | 42.85 (38.58–44.30) | 41.8 (39.30–43.40) | 41.00 (38.95–42.75) | 40.60 (37.90–42.32) | 40.70 (37.90–42.40) |
| P (unadjusted model) | Reference group | 0.25 | 0.015 | 0.081 | Reference group | 0.028 | 0.009 | 0.029 | |
| P (adjusted model)† | Reference group | 0.622 | 0.5 | 0.744 | Reference group | 0.558 | 0.265 | 0.019 | |
| ACCA(mmol/L) | Mean (IQR) | 2.29 (2.23–2.35) | 2.30 (2.24–2.35) | 2.30 (2.24–2.35) | 2.29 (2.24–2.37) | 2.29 (2.23–2.34) | 2.31 (2.27–2.37) | 2.28 (2.23–2.33) | 2.30 (2.25–2.38) |
| P (unadjusted model) | Reference group | 0.73 | 0.501 | 0.543 | Reference group | <0.001 | 0.987 | 0.267 | |
| P (adjusted model)† | Reference group | 0.023 | 0.113 | 0.252 | Reference group | <0.001 | 0.71 | 0.892 | |
| K+(mmol/L) | Mean (IQR) | 3.99 (3.69–4.27) | 3.93 (3.68–4.24) | 4.03 (3.75–4.28) | 3.97 (3.73–4.29) | 3.98 (3.7–4.30) | 4.00 (3.79–4.24) | 3.94 (3.80–4.07) | 4.10 (3.92–4.33) |
| P (unadjusted model) | Reference group | 0.683 | 0.829 | 0.126 | Reference group | 0.818 | 0.406 | 0.042 | |
| P (adjusted model)† | Reference group | 0.018 | 0.041 | 0.078 | Reference group | 0.782 | 0.606 | 0.822 | |
| Mg2+(mmol/L) | Mean (IQR) | 0.84 (0.79–0.89) | 0.85 (0.82–0.88) | 0.85 (0.81–0.88) | 0.84 (0.81–0.90) | 0.83 (0.78–0.87) | 0.84 (0.80–0.88) | 0.84 (0.74–0.89) | 0.84 (0.80–0.89) |
| P (unadjusted model) | Reference group | 0.627 | 0.598 | 0.049 | Reference group | 0.735 | 0.737 | 0.482 | |
| P (adjusted model)† | Reference group | 0.122 | 0.739 | 0.158 | Reference group | 0.145 | 0.23 | 0.112 | |
Continuous measurements are expressed as mean±SD if normally distributed; otherwise, as median (IQR). Adjusted for multiple comparisons by Bonferroni correction.
New donors served as the control group and each experimental group was compared with the control group.
Adjusted for age, BMI, living place, meat intake, education, smoking and drinking, annual household income, physical activity (female donors additionally adjusted menstrual history).
ACCAalbumin-corrected calciumAlbalbuminBMIbody mass indexHbhaemoglobinTSPtotal serum protein
Figure 2. The impact of lifetime donation on serum protein and electrolyte level. Mean (IQR) of serum protein and electrolyte level. The horizontal dotted line indicates the lower limit of normal; new donors served as the control group. *Significant after adjusting for age, BMI, living place, meat intake, education, smoking and drinking, annual household income, physical activity (female donors additionally adjusted menstrual history). ACCA, albumin-corrected calcium; BMI, body mass index; Hb, haemoglobin; TSP, total serum protein.
Association of plasmapheresis donation and the incidence of protein and electrolyte levels below cut-off values
The incidence of protein and electrolyte levels below cut-off values showed no statistically significant differences between the new donors and regular donors after adjusting for confounding factors (p>0.0167) (online supplemental tables 2 and 3).
Discussion
This study found that mean TSP levels were not significantly associated with either the frequency of plasmapheresis donations or the total number of lifetime donations. Before each donation, the donor’s TSP must be 60 g/L or higher in the US and Europe.22 In China, serum TSP must be 65 g/L or higher.3 Donors with low TSP were not allowed to donate plasma. Previous studies have shown results similar to ours. A study of 2467 regular plasma donors in Sichuan, China, found no difference in plasma protein concentration compared with new donors (p>0.05).23 A retrospective study found no significant differences in TSP mean values by donation frequency.24 In a large prospective study, 923 donors who switched from a moderate to an intensive plasmapheresis programme showed no significant difference between their initial and final TSP.23 Therefore, TSP detection is not necessary at each plasma donation since there was no significant reduction even at high-frequency and high total lifetime donations.
One US study found that plasma donation reduced Alb level.25 In another study, intensive donors had lower Alb levels than moderate donors. Notably, the study did not adjust for age as a confounding factor.26 In our study, before adjusting for confounding factors, regular male and female donors had lower Alb levels than new donors. However, after adjusting for confounding factors, the difference was not significant. Using logistic and linear regression analysis, we found a negative correlation between Alb level and age (online supplemental table 4). Previous studies found the same results.27 According to the age distribution in our survey, age is positively correlated with the total number of lifetime donations and the frequency of donations. According to Chinese guidelines,3 Alb should be measured every 12 months, and serum/plasma electrophoresis should be ≥50%, with no significant change from the previous time. Given the negative correlation between Alb levels and age, Alb monitoring before plasma donation should be adjusted based on the donor’s age. For older donors, it is recommended to increase the frequency of Alb testing.
According to the study, IgG levels decreased as donation frequency and the total number of lifetime donations increased for female donors. Previous studies showed that removing 750–1600 mL of plasma per week reduced IgG to the lower normal range.9 A German study found that plasmapheresis reduced donors’ IgG levels. The drop was 13% from the IgG baseline value when 800 mL of plasma was collected. Intensive plasmapheresis donors have lower IgG levels than moderate donors.26 A 5-month Australian study monitored the impact of monthly plasmapheresis on donor serum IgG levels in 127 new and 124 regular plasma donors. IgG levels in regular and new donors fell longitudinally, though they fluctuated.28 In Germany, IgG is determined at every 15th donation and may not be less than 5.8 g/L, while Chinese donors were not tested for IgG.3 Although this study found no significant association between plasmapheresis donation and the risk of IgG abnormality, it did find that IgG levels significantly decreased with increased donation frequency and the total number of donations. Therefore, regular monitoring of IgG levels in donors is still necessary.
As previous studies showed, apheresis devices led to significant loss of red blood cells during the procedure. 30 mL of blood was lost in the harness and during plasmapheresis.12 This study found that plasma donation affected Hb differently by sex. Male donor Hb level was not affected by donation frequency or total number of lifetime donations. For female donors, Hb levels decreased with donation frequency and the total number of lifetime donations. We surmise that this may be due to the additive effect of the higher iron stores in men and menstruation in women. Ferritin was significantly associated with Hb concentration.29 Mean serum ferritin levels were significantly lower in women than men, and lower levels of ferritin in women resulted in slower Hb recovery.30 One Japanese study31 found that serum ferritin levels in the male group remained almost constant with increasing frequency of apheresis donation. According to our previous study on iron deficiency, a higher donation frequency has been associated with reduced ferritin levels and an increased risk of iron deficiency in women.32 Even though an Hb threshold (male donors need 120 g/L and female donors 115 g/L) was set to ensure the donor’s Hb level,3 plasmapheresis donation caused women’s Hb level to drop close to threshold value with no effect on men, according to the results. High-frequency female donors are advised to regularly take iron supplements to prevent and treat iron deficiency anaemia. A diet rich in iron and vitamin C, including red meat, leafy green vegetables and citrus fruits, is also encouraged to enhance iron absorption. Extending the interval between donations in female plasma donors with low Hb values is recommended. Additionally, regular monitoring of Hb levels is essential for timely interventions.
According to our findings, plasma donation did not seem to affect mineral metabolism. Short-term calcium homoeostasis imbalances did not appear to cause long-term calcium deficiency. Leukapheresis and plateletpheresis consume more anticoagulants, according to most studies.33,35 Conversely, the whole blood/anticoagulant ratio was low in plasmapheresis, resulting in low citrate consumption. Although the effect of citrate on minerals has been confirmed,7 lower plasmapheresis citrate levels did not affect electrolyte levels. Evers and Taborski reported 130±12 mL citrate consumption in plasmapheresis corresponding to 3341 mg citrate on average. As 84.6% of the anticoagulant load was in collected plasma, only a very low concentration of 514 mg citrate ion reached donors’ blood circulation.36 The mean citrate infusion rate in our donors was 0.49±0.08 mg/kg/min, which was less than half of the citrate infusion rate in plateletpheresis.37 In spite of the low anticoagulant consumption, citrate-associated side effects were still observed in our donors and needed to be concerned.
Multicentre studies with larger sample sizes may better represent real-world heterogeneity and produce more generalisable findings than single-centre studies. This is the first study to investigate changes in the serum protein and electrolyte metabolism of regular donors in China, providing a scientific basis for protecting donor health and improving plasmapheresis donation policies. There are some limitations to this study. First, the cross-sectional design of our study limits our ability to establish causality between plasmapheresis donation and serum protein and electrolyte levels. Second, the varying assay conditions between participating laboratories could be a confounding factor for Hb. However, we believe that the inclusion of living locations representing collection and testing locations as covariates in the analysis could further reduce the heterogeneity in different laboratories to some extent. Third, the data from this study provided suggestions for current donor screening strategy, but further scientific evidence is still needed for the improvement of policy and guideline.
In conclusion, plasmapheresis donation was negatively correlated with IgG concentration in both male and female donors. It provides preliminary evidence for the inclusion of IgG in routine screening and decision-making for interventions such as short donation delays. TSP levels are quickly restored, so testing at each donation may not be necessary. The Hb of female donors decreased with donation frequency and lifetime total number of donations, while male donors were unaffected. Anticoagulants did not cause long-term calcium deficiency from short-term calcium imbalances. The screening strategy for plasma donors in China has not changed for many years. With the technological innovation of the apheresis process and the improvement of donors’ physical fitness, the strategy may need to be adjusted.
supplementary material
Acknowledgements
The authors thank Toby Simon for his precious assistance with article revision and language editing.
Footnotes
Funding: Chinese Academy of Medical Sciences Initiative for Innovative Medicine (Grant No. 2021-I2M-1-060).
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2024-085786).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Ethics approval: This study involves human participants and the Ethics Committee of the Institute of Blood Transfusion, Chinese Academy of Medical Sciences (IBT) approved this study (NO. 2021042). Participants gave informed consent to participate in the study before taking part.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Guanglin Xiao, Email: xiao285435619@163.com.
Changqing Li, Email: lichangqing268@163.com.
Yongjun Chen, Email: 563528422@qq.com.
Wenfu Song, Email: songwenfu@sinopharm.com.
Hui Yang, Email: 904348470@qq.com.
Yating Yang, Email: 99978464@qq.com.
Yu Zhang, Email: xxzhangyu@126.com.
Zhongping Pu, Email: 361817083@qq.com.
Xiufang Wang, Email: 3504865752@qq.com.
Shina Xie, Email: 2453303188@qq.com.
Shouqiang Yang, Email: 953808084@qq.com.
Jun Zeng, Email: 1970649796@qq.com.
Wan Li, Email: 2971317731@qq.com.
Ya Wang, Email: 175235831@qq.com.
Data availability statement
Data are available on reasonable request.
References
- 1.World Health Organization . WHO model lists of essential medicines. Geneva: 2021. [Google Scholar]
- 2.Grazzini G, Mannucci PM, Oleari F. Plasma-derived medicinal products: demand and clinical use. Blood Transfus. 2013;11 Suppl 4:s2–5. doi: 10.2450/2013.002s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Health Commission of the PRC Technical Operating Procedures for Plasmapheresis Collection Station. http://www.nhc.gov.cn/yzygj/s7658/202207/dd87dc3941b74dda8eb71ec911c2934a.shtml Available.
- 4.Chen Y, Hou J, Chen G, et al. Calcium supplementation attenuates citrate-related changes in bone metabolism: a placebo-controlled crossover study in healthy volunteers. Bone. 2011;49:506–12. doi: 10.1016/j.bone.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Bieglmayer C, Höcker P, et al. Effect of acute citrate load on markers of bone metabolism in healthy volunteers. Vox Sang. 2009;97:324–9. doi: 10.1111/j.1423-0410.2009.01202.x. [DOI] [PubMed] [Google Scholar]
- 6.Amrein K, Katschnig C, Sipurzynski S, et al. Apheresis affects bone and mineral metabolism. Bone. 2010;46:789–95. doi: 10.1016/j.bone.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Das SS, Chaudhary R, Khetan D, et al. Calcium and magnesium levels during automated plateletpheresis in normal donors. Transfus Med. 2005;15:233–6. doi: 10.1111/j.1365-3148.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- 8.Bolan CD, Greer SE, Cecco SA, et al. Comprehensive analysis of citrate effects during plateletpheresis in normal donors. Transfusion. 2001;41:1165–71. doi: 10.1046/j.1537-2995.2001.41091165.x. [DOI] [PubMed] [Google Scholar]
- 9.Shanbrom E, Lundak R, Walford RL. Long-term plasmapheresis: effects on specific plasma proteins. Transfusion. 1972;12:162–7. doi: 10.1111/j.1537-2995.1972.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 10.Friedman BA, Schork MA, Mocniak JL, et al. Short-term and long-term effects of plasmapheresis on serum proteins and immunoglobulins. Transfusion. 1975;15:467–72. doi: 10.1046/j.1537-2995.1975.15576082222.x. [DOI] [PubMed] [Google Scholar]
- 11.Wasi S, Santowski T, Murray SA, et al. The Canadian Red Cross plasmapheresis donor safety program: changes in plasma proteins after long-term plasmapheresis. Vox Sang. 1991;60:82–7. doi: 10.1111/j.1423-0410.1991.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Condon F, Kessler D, et al. Evidence of relative iron deficiency in platelet- and plasma-pheresis donors correlates with donation frequency. J Clin Apher. 2016;31:551–8. doi: 10.1002/jca.21448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Office of the Federal Register, National Archives and Records Administration Requirements for Blood and Blood Components Intended for Transfusion or for Further Manufacturing Use. 2015. https://www.govinfo.gov/app/details/FR-2015-05-22/2015-12228 Available. [PubMed]
- 14.Bove LL, Bednall T, Masser B, et al. Understanding the plasmapheresis donor in a voluntary, nonremunerated environment. Transfusion. 2011;51:2411–24. doi: 10.1111/j.1537-2995.2011.03168.x. [DOI] [PubMed] [Google Scholar]
- 15.National Health Commission of the PRC . China’s report on blood safety 2019. Beijing, China: People’s Medical Publishing House; 2019. [Google Scholar]
- 16.Hong S. National guide to clinical laboratory procedures, 4th Edn. Beijing, China: People’s Medical Publishing House; 2015. [Google Scholar]
- 17.Sava L, Pillai S, More U, et al. Serum calcium measurement: Total versus free (ionized) calcium. Indian J Clin Biochem. 2005;20:158–61. doi: 10.1007/BF02867418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth M. Assessment of physical activity: an international perspective. Res Q Exerc Sport. 2000;71:114–20. doi: 10.1080/02701367.2000.11082794. [DOI] [PubMed] [Google Scholar]
- 19.Jang ES, Jeong S-H, Hwang SH, et al. Effects of coffee, smoking, and alcohol on liver function tests: a comprehensive cross-sectional study. BMC Gastroenterol. 2012;12:145. doi: 10.1186/1471-230X-12-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong TYN, Key TJ, Gaitskell K, et al. Hematological parameters and prevalence of anemia in white and British Indian vegetarians and nonvegetarians in the UK Biobank. Am J Clin Nutr. 2019;110:461–72. doi: 10.1093/ajcn/nqz072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsalman M, Albarak A, Busaleh F, et al. Heavy menstrual bleeding awareness among Saudi female population and clinical implications. Health Sci Rep . 2021;4:e244. doi: 10.1002/hsr2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulzki T, Seidel K, Storch H, et al. A prospective multicentre study on the safety of long-term intensive plasmapheresis in donors (SIPLA) Vox Sang. 2006;91:162–73. doi: 10.1111/j.1423-0410.2006.00794.x. [DOI] [PubMed] [Google Scholar]
- 23.Jang Y, Dong D, Zou L, et al. Investigation of plasma protein concentration and distributions of ratio of albumin to immunoglobulins in plasma donors in Sichuan Province, China. Chin J Biol. 2015;28:1044–7. [Google Scholar]
- 24.Rodell MB. Collection of source material from remunerated donors. Dev Biol Stand. 1993;81:57–64. [PubMed] [Google Scholar]
- 25.Rodell MB, Lee ML. Determination of reasons for cessation of participation in serial plasmapheresis programs. Transfusion. 1999;39:900–3. doi: 10.1046/j.1537-2995.1999.39080900.x. [DOI] [PubMed] [Google Scholar]
- 26.Hellstern P, Bach J, Haubelt H, et al. The impact of the intensity of serial automated plasmapheresis and the speed of deep-freezing on the quality of plasma. Transfusion. 2001;41:1601–5. doi: 10.1046/j.1537-2995.2001.41121601.x. [DOI] [PubMed] [Google Scholar]
- 27.Lampon N, Tutor JC. Apparent clearance of valproic acid in elderly epileptic patients: estimation of the confounding effect of albumin concentration. Ups J Med Sci. 2012;117:41–6. doi: 10.3109/03009734.2011.640412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgin M, Hopkins G, Moore B, et al. Serum IgG and IgM levels in new and regular long-term plasmapheresis donors. Med Lab Sci. 1992;49:265–70. [PubMed] [Google Scholar]
- 29.Park SK, Ryoo JH, Kim MG, et al. Association of serum ferritin and the development of metabolic syndrome in middle-aged Korean men: a 5-year follow-up study. Diabetes Care. 2012;35:2521–6. doi: 10.2337/dc12-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumbele IUN, Samje M, Nkuo-Akenji T. A longitudinal study on anaemia in children with Plasmodium falciparum infection in the Mount Cameroon region: prevalence, risk factors and perceptions by caregivers. BMC Infect Dis. 2013;13:123. doi: 10.1186/1471-2334-13-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuta M, Shimizu T, Mizuno S, et al. Clinical evaluation of repeat apheresis donors in Japan. Vox Sang. 1999;77:17–23. doi: 10.1159/000031069. [DOI] [PubMed] [Google Scholar]
- 32.Xiao G, Li C, Chen Y, et al. Risk prediction of iron deficiency for plasmapheresis donors in China: Development and validation of a prediction model. Vox Sang. 2024;119:144–54. doi: 10.1111/vox.13572. [DOI] [PubMed] [Google Scholar]
- 33.Buchta C, Macher M, Bieglmayer C, et al. Reduction of adverse citrate reactions during autologous large-volume PBPC apheresis by continuous infusion of calcium-gluconate. Transfusion. 2003;43:1615–21. doi: 10.1046/j.1537-2995.2003.00571.x. [DOI] [PubMed] [Google Scholar]
- 34.Dettke M, Buchta C, Short- BC. And longterm effects of citrate on bone metabolism and bone mineral density in healthy plateletpheresis donors. J Clin Apher. 2003:18. [Google Scholar]
- 35.Bialkowski W, Bruhn R, Edgren G, et al. Citrate anticoagulation: Are blood donors donating bone? J Clin Apher. 2016;31:459–63. doi: 10.1002/jca.21438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evers J, Taborski U. Distribution of citrate and citrate infusion rate during donor plasmaphereses. J Clin Apher. 2016;31:59–62. doi: 10.1002/jca.21403. [DOI] [PubMed] [Google Scholar]
- 37.Moog R, Müller N, Goergens D. Platelet collection with the Amicus and the AS.TEC 204 blood cell separators. Transfusion. 1998;38:285–9. doi: 10.1046/j.1537-2995.1998.38398222873.x. [DOI] [PubMed] [Google Scholar]