Abstract
Background
Previous studies have indicated that standing may be beneficially associated with surrogate metabolic markers, whereas more time spent sitting has an adverse association. Studies assessing the dose-response associations of standing, sitting and composite stationary behaviour time with cardiovascular disease (CVD) and orthostatic circulatory disease are scarce and show an unclear picture.
Objective
To examine associations of daily sitting, standing and stationary time with CVD and orthostatic circulatory disease incidence
Methods
We used accelerometer data from 83 013 adults (mean age ± standard deviation = 61.3 ± 7.8; female = 55.6%) from the UK Biobank to assess daily time spent sitting and standing. Major CVD was defined as coronary heart disease, heart failure and stroke. Orthostatic circulatory disease was defined as orthostatic hypotension, varicose vein, chronic venous insufficiency and venous ulcers. To estimate the dose-response hazard ratios (HR) we used Cox proportional hazards regression models and restricted cubic splines. The Fine–Gray subdistribution method was used to account for competing risks.
Results
During 6.9 (±0.9) years of follow-up, 6829 CVD and 2042 orthostatic circulatory disease events occurred. When stationary time exceeded 12 h/day, orthostatic circulatory disease risk was higher by an average HR (95% confidence interval) of 0.22 (0.16, 0.29) per hour. Every additional hour above 10 h/day of sitting was associated with a 0.26 (0.18, 0.36) higher risk. Standing more than 2 h/day was associated with an 0.11 (0.05, 0.18) higher risk for every additional 30 min/day. For major CVD, when stationary time exceeded 12 h/day, risk was higher by an average of 0.13 (0.10, 0.16) per hour. Sitting time was associated with a 0.15 (0.11, 0.19) higher risk per extra hour. Time spent standing was not associated with major CVD risk.
Conclusions
Time spent standing was not associated with CVD risk but was associated with higher orthostatic circulatory disease risk. Time spent sitting above 10 h/day was associated with both higher orthostatic circulatory disease and major CVD risk. The deleterious associations of overall stationary time were primarily driven by sitting. Collectively, our findings indicate increasing standing time as a prescription may not lower major CVD risk and may lead to higher orthostatic circulatory disease risk.
Keywords: Sitting, standing, sedentary behaviour, cardiovascular disease, orthostatic circulatory disease, accelerometery, wearable sensor
Key Messages.
We investigated the dose-response associations of sitting and standing time with major cardiovascular disease (CVD) incidence (coronary heart disease, stroke and heart failure) and orthostatic circulatory disease in 83 013 UK adults.
Both sitting (above 10 h/day) and standing (above 2 h/day) were directly associated with increased orthostatic circulatory disease incidence risk; standing was not associated with CVD incidence risk.
Standing alone may not be a sufficient strategy for lowering CVD risk, and may lead to a higher risk of circulatory conditions
Introduction
Sitting and standing postures are termed collectively ‘stationary behaviour’, i.e. no ambulatory movement and low energy expenditure.1 Both postures have attracted interest as risk factors for cardiovascular disease (CVD) and premature mortality.2–6 Intervention and cross-sectional studies have suggested decreasing sitting (the main component of sedentary time—combination of sitting or in a reclined position while awake1) and increasing standing time may improve surrogate cardiovascular outcomes such as metabolic markers (e.g. density lipoproteins, total cholesterol, triglycerides).7,8 However, studies assessing clinical endpoints such as CVD hospitalization and mortality risk are very scarce and show an unclear picture for the dose-response of both sitting and standing.9,10
The majority of prospective sitting and standing time studies have relied on self-report measures, known for their inherent biases, e.g. social desirability and recall, leading to imprecise evidence on links with cardiovascular disease incidence.11–13 Importantly, prior studies have not differentiated orthostatic circulatory diseases from other CVD types, when assessing sitting and standing time. Prior evidence suggests different postures, e.g. sitting and standing, may have distinct mechanistic pathways to orthostatic circulatory diseases vs other CVD types such as coronary heart disease and stroke, due to influences on autonomic neuropathy and strain on the vascular (e.g. haemodynamic) and musculoskeletal systems.14–18 Collectively, these limitations may have contributed to the inconclusive evidence regarding the associations of standing time with CVD risk.
Prior studies with mortality and CVD outcomes19–21 have, in some cases, unintentionally examined stationary behaviour by using waist-attached wearable devices that only measured ambulatory activities and cannot differentiate between sitting and standing. Such misclassification in these studies, which were originally aimed at examining sedentary behaviour, may have distorted dose-response estimates of sitting time, since standing often occupies approximately 20% to 30% of adults’ waking times (3–5 h per day22,23) Despite all these limitations of the current literature and the absence of consistent evidence with clinical endpoints,4,9 standing has been recommended as health enhancing by clinicians and public health researchers,7,24–26 primarily in efforts to reduce time spent sitting in workplace environments.
In a large population sample of adults with device-based measures of posture and physical activity, we examined the prospective associations of stationary behaviour and its constituent components (sitting and standing) with major CVD incidence (coronary heart disease, stroke and heart failure) and orthostatic circulatory disease.
Methods
Study participants
Participants were included from the UK Biobank Study, and enrolled between 2006 and 2010. Ethical approval was provided by the UK’s National Health Service, National Research Ethics Service (Ref 11/NW/0382). Participants completed physical examinations by trained staff and touchscreen questionnaires.13
Orthostatic circulatory disease and CVD incidence ascertainment
Participants were followed up through 31 October 31 2021, with deaths obtained through linkage with the National Health Service (NHS) Digital of England and Wales or the NHS Central Register and National Records of Scotland (30 September–31 October 2021). Inpatient hospitalization data were provided by either the Hospital Episode Statistics for England, the Patient Episode Database for Wales or the Scottish Morbidity Record for Scotland (30 September 2021 for England, 31 July 2021 for Scotland and 28 February 2018 for Wales). Primary care data were linked up to 31 March–31 August 2017. We defined orthostatic circulatory disease events as orthostatic hypotension, varicose vein, chronic venous insufficiency and venous ulcers.27,28 Major CVD was defined as coronary heart disease, stroke and heart failure. Full methods for the assessment of orthostatic circulatory disease and CVD events and International Classification of Diseases (ICD-10) codes are provided in Supplementary Table S1 (available as Supplementary data at IJE online).
Sitting, standing and non-stationary physical activity assessment
Between 2013 and 2015, 103 684 participants wore an Axivity AX3 accelerometer (Axivity, Newcastle upon Tyne, UK) on their dominant wrist for 7 days.24 Devices were calibrated with sleep and non-wear periods were identified according to standard procedures.29–31 Monitoring days were considered valid if wear time was greater than 16 h. Participants were required to have at least 4 days of valid wear time with at least one of those days being a weekend day. Primary exposures were daily time spent stationary (i.e. sitting and standing combined), sitting, and standing and were all classified with an accelerometer-based activity machine learning scheme that has been previously validated under free-living conditions.32,33 Briefly, this activity scheme uses features in the raw acceleration signal to identify and quantify waking time spent in sitting, standing (standing still and standing with subtle movement), and walking/running in 60-s windows. Under free-living conditions, the activity classifier had a balanced accuracy (combination of sensitivity and specificity) of 88% for sitting time and 80% for standing time).33 We provide additional independent validation results in Supplementary Text 1 (available as Supplementary data at IJE online) with overall balanced accuracy of 84%.
Covariates
In line with previous analogous studies9,34 and known correlates of posture,23 covariates in our analyses included (see Supplementary Figure S1, available as Supplementary data at IJE online for the directed acyclic graph): age (continuous; years), sex (female/male), body mass index35 (continuous), smoking status (never, past, current), alcohol consumption (never, ex-drinker, within guidelines, above guidelines), fruit and vegetable consumption (continuous; kg/m2), education (college/university; A/AS level; O levels; CSE; NVQ/HND/HNC; other), self-reported parental history of CVD (yes/no), prevalent major CVD (in the orthostatic circulatory disease analysis) or orthostatic circulatory disease (in the CVD analysis) events, and cholesterol (yes/no), anti-hypertensive (yes/no) or diabetes (yes/no) medication use. All analyses were also adjusted for accelerometer-measured time spent walking/running. We also included mutual adjustment for sitting time and standing time in the corresponding models. Complete covariate definitions are provided in Supplementary Table S2 (available as Supplementary data at IJE online).
Analyses
We excluded participants with prevalent orthostatic circulatory disease and major CVD as appropriate, ascertained through self-report, hospital admission and primary care linkage, as well as participants who were underweight (body mass index <18.5 kg/m2), had missing covariate data or had an event within the first 12 months following the accelerometry measurements (Supplementary Figure S2, available as Supplementary data at IJE online).
We calculated the adjusted dose-response absolute risk using Poisson regression, age- and sex-adjusted incidence rate ratios and crude risk percent. We used Cox proportional hazards regression models to estimate the dose-response hazard ratios (HR) with 95% confidence intervals (CI) for orthostatic circulatory disease and CVD events, with restricted cubic splines and knots placed at the 10th, 50th and 90th percentiles for stationary, standing and sitting time distributions. The Fine–Gray subdistribution method was used with non-orthostatic circulatory disease or non-CVD mortality events treated as a competing risk. The proportional hazards assumption was assessed using Schoenfeld residuals, and no violations were observed. Due to an absence of prior studies assessing clinical endpoints, we used the adjusted absolute risk curves to determine reference values for the three exposures, using the data point where orthostatic circulatory disease and CVD risk became pronounced (Figures 1–2). The reference points were 12 h/day, 10 h/day and 2 h/day for stationary, sitting and standing time, respectively. Departure from linearity was assessed by a Wald test examining the null hypothesis that the coefficient of the second spline was equal to zero. We calculated E-values to estimate the plausibility of bias from unmeasured confounding. To provide conservative E-value point estimates, we assessed the minimal dose, defined as the duration of each exposure associated with 50% of the highest HR (‘minimum harmful dose’).
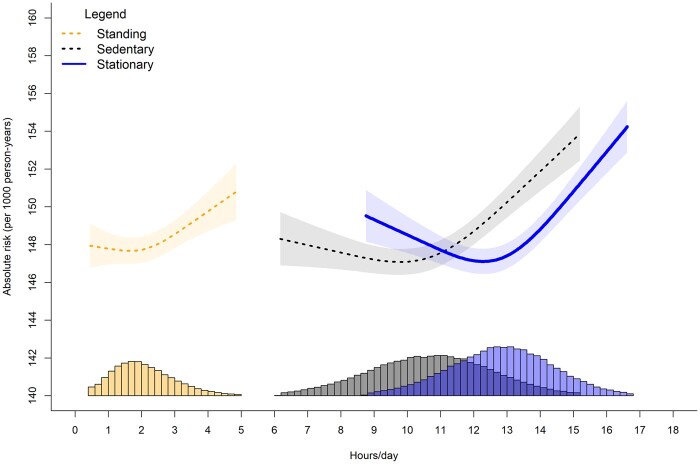
Figure 1.
Adjusted absolute risk of stationary, standing, and sitting time with orthostatic circulatory disease incidence. Adjusted for age, sex, ethnicity, smoking history, alcohol consumption, body mass index, time spent walking/running, mutual adjustment for time spent standing and sitting, education, diet, family history of cardiovascular disease (CVD), prevalent CVD incidence and medication use. Histogram represents sample distribution
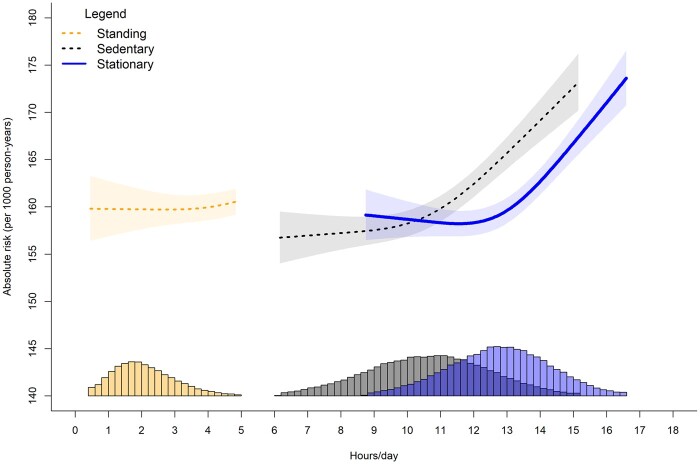
Figure 2.
Adjusted absolute risk of stationary, standing and sitting time with major cardiovascular disease incidence. Adjusted for age, sex, ethnicity, smoking history, alcohol consumption, body mass index, time spent walking/running, mutual adjustment for time spent standing and sitting, education, diet, family history of cardiovascular disease (CVD), prevalent orthostatic incidence and medication use. Histogram represents sample distribution
To assess the influence of residual confounding, we used a negative control outcome of deaths and hospitalization from accidents (excluding accidents that may be associated with physical activity, i.e. cycling, and falls incidence or self-harm), an outcome that does not have a mechanistic link to stationary behaviour. Negative controls can improve causal inference by illustrating pervasive bias and confounding. If the negative control has a similar association pattern as the primary outcomes, then it is more plausible that associations are due to bias and confounding than causal mechanisms.36 We conducted sensitivity analyses to minimize bias attributable to reverse causation by: (i) exclusion of participants who were obese (body mass index >30 kg/m2); (ii) exclusion of participants reporting fair or poor health; or (iii) those with an event within the first 24 months of follow-up. In Supplementary analyses, we assessed how covariate relationships with both the exposure and outcome might influence associations and present sequential modelling for: (i) Model 1: age and sex; (ii) Model 2: Model 1 + lifestyle factors, body mass index (BMI) and education; (iii) Model 3: Model 2 + medication and prevalent disease.
We performed all analyses using R statistical software. We reported this study as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline (see STROBE Statement in the Supplementary Material, available as Supplementary data at IJE online).
Results
Our analytical sample for orthostatic circulatory disease incidence included 83 013 participants [average age (SD) = 61.3 (7.8) years; 55.6% female] followed up for an average of 6.9 ± 0.9 years with 2042 events. Our CVD incidence sample included 75 897 participants with 6829 events. Mean (SD) time spent stationary, standing and sitting was 12.8 (1.6) h/day, 2.1 (0.9) h/day and 10.7 (1.9) h/day, respectively. Participants spent an average of 71.3 min/day walking/running and 64.0% of the participants were not smokers. Participant characteristics by stationary behaviour daily duration are provided in Table 1. Participant characteristics by standing and sitting daily duration are presented in Supplementary Tables S3 and S4 (available as Supplementary data at IJE online). Participants in the higher quartiles of sitting were more likely to be male and have tertiary education, whereas participants in higher quartiles of standing were more likely to be female and less educated.
Table 1.
Participant descriptive characteristics by stationary time
| H/day stationary quartiles | <12 | 12 to <13 | 13 to <14 | ≥14 | Overall |
|---|---|---|---|---|---|
| Participants | 24 843 | 19 901 | 19 257 | 19 012 | 83 013 |
| Follow up, years | 6.9 (0.8%) | 6.9 (0.9%) | 6.9 (0.9%) | 6.8 (1.0%) | 6.9 (0.9%) |
| Age | 60.3 (7.6%) | 61.0 (7.8%) | 61.6 (7.9%) | 62.7 (7.8%) | 61.3 (7.8%) |
| Male | 8244 (33.2%) | 8313 (41.8%) | 9432 (49.0%) | 10 888 (57.3%) | 36 877 (44.4%) |
| Stationary, h/day | 10.9 (0.9%) | 12.5 (0.3%) | 13.5 (0.3%) | 14.9 (0.8%) | 12.8 (1.6%) |
| Standing, h/day | 2.1 (0.9%) | 2.1 (0.9%) | 2.1 (0.9%) | 2.0 (1.0%) | 2.1 (0.9%) |
| Sitting, h/day | 8.8 (1.2%) | 10.4 (1.0%) | 11.4 (1.0%) | 12.8 (1.3%) | 10.7 (1.9%) |
| Walking, min/day | 98.6 (66.8%) | 73.9 (48.1%) | 60.2 (41.0%) | 44.1 (33.1%) | 71.3 (54.4%) |
| Ethnicity | |||||
| Asian | 217 (0.9%) | 188 (0.9%) | 220 (1.1%) | 300 (1.6%) | 925 (1.1%) |
| Black | 130 (0.5%) | 135 (0.7%) | 144 (0.7%) | 262 (1.4%) | 671 (0.8%) |
| Mixed | 118 (0.5%) | 99 (0.5%) | 114 (0.6%) | 117 (0.6%) | 448 (0.5%) |
| Other | 160 (0.6%) | 157 (0.8%) | 152 (0.8%) | 185 (1.0%) | 654 (0.8%) |
| White | 24 218 (97.5%) | 19 322 (97.1%) | 18 627 (96.7%) | 18 148 (95.5%) | 80 315 (96.7%) |
| Smoking history | |||||
| Current | 1383 (5.6%) | 1203 (6.0%) | 1346 (7.0%) | 1718 (9.0%) | 5650 (6.8%) |
| Never | 14 881 (59.9%) | 11 612 (58.3%) | 10 887 (56.5%) | 10 196 (53.6%) | 47 576 (57.3%) |
| Previous | 8579 (34.5%) | 7086 (35.6%) | 7024 (36.5%) | 7098 (37.3%) | 29 787 (35.9%) |
| Alcohol consumption | |||||
| Ex-drinker | 579 (2.3%) | 485 (2.4%) | 504 (2.6%) | 662 (3.5%) | 2230 (2.7%) |
| Never | 683 (2.7%) | 499 (2.5%) | 486 (2.5%) | 658 (3.5%) | 2326 (2.8%) |
| Within guidelines | 14 448 (58.2%) | 11 300 (56.8%) | 10 799 (56.1%) | 10 702 (56.3%) | 47 249 (56.9%) |
| Above guidelines | 9133 (36.8%) | 7617 (38.3%) | 7468 (38.8%) | 6990 (36.8%) | 31 208 (37.6%) |
| Education | |||||
| College/university | 10 083 (40.6%) | 8742 (43.9%) | 8868 (46.1%) | 8575 (45.1%) | 36 268 (43.7%) |
| A levels | 3314 (13.3%) | 2711 (13.6%) | 2418 (12.6%) | 2498 (13.1%) | 10 941 (13.2%) |
| O levels | 5416 (21.8%) | 4142 (20.8%) | 3856 (20.0%) | 3549 (18.7%) | 16 963 (20.4%) |
| CSE | 1255 (5.1%) | 747 (3.8%) | 637 (3.3%) | 621 (3.3%) | 3260 (3.9%) |
| NVQ/HND/HNC | 1288 (5.2%) | 1012 (5.1%) | 1021 (5.3%) | 1141 (6.0%) | 4462 (5.4%) |
| Other | 3487 (14.0%) | 2547 (12.8%) | 2457 (12.8%) | 2628 (13.8%) | 11 119 (13.4%) |
| Diet, servings/daya | 8.4 (4.5%) | 8.2 (4.4%) | 7.9 (4.4%) | 7.8 (4.4%) | 8.1 (4.4%) |
| History of CVD | 1423 (5.7%) | 1590 (8.0%) | 1883 (9.8%) | 2544 (13.4%) | 7440 (9.0%) |
| Family history of CVD | 13 465 (54.2%) | 10 848 (54.5%) | 10 645 (55.3%) | 10 707 (56.3%) | 45 665 (55.0%) |
| Medication use | |||||
| Cholesterol | 2425 (9.8%) | 2529 (12.7%) | 2954 (15.3%) | 4046 (21.3%) | 11 954 (14.4%) |
| Blood pressure | 2913 (11.7%) | 2932 (14.7%) | 3491 (18.1%) | 4699 (24.7%) | 14 035 (16.9%) |
| Insulin | 106 (0.4%) | 108 (0.5%) | 119 (0.6%) | 225 (1.2%) | 558 (0.7%) |
| Body mass index | 25.5 (3.8%) | 26.4 (4.1%) | 27.1 (4.3%) | 28.5 (5.1%) | 26.7 (4.5%) |
| Self-rated health | |||||
| Excellent | 6292 (25.3%) | 4713 (23.7%) | 4127 (21.4%) | 3193 (16.8%) | 18 325 (22.1%) |
| Good | 15 225 (61.3%) | 12 154 (61.1%) | 11 660 (60.5%) | 10 909 (57.4%) | 49 948 (60.2%) |
| Fair | 2958 (11.9%) | 2676 (13.4%) | 3055 (15.9%) | 4074 (21.4%) | 12 763 (15.4%) |
| Poor | 368 (1.5%) | 358 (1.8%) | 415 (2.2%) | 836 (4.4%) | 1977 (2.4%) |
Values represent means (SD%) unless noted otherwise.
CVD, cardiovascular disease; A levels, Advanced level qualifications; O levels, Ordinary level qualifications; CSE, Certificate of Secondary Education; NVQ, National Vocational Qualification; HND, Higher National Diploma.
Servings of fruits and vegetables per day.
Absolute risk
Supplementary Tables S5 and S6 (available as Supplementary data at IJE online) present the crude risk and partially adjusted incidence rate ratios for orthostatic circulatory disease and major CVD events by quartiles of each exposure. For orthostatic circulatory disease, participants who had <12 h/day of stationary time had a crude risk of 2.49% (95% CI= 2.46%, 2.52%), whereas ≥14 h/day had a crude risk of 5.22% (5.14%, 5.29%). Corresponding values for CVD are 2.70% (2.67%, 2.74%) for <12 h/day and 5.13% (5.05%, 5.20%) for ≥14 h/day. The adjusted absolute risk dose-responses per 1000 person-years are shown in Figures 1–2. For CVD and orthostatic circulatory disease incidence, the dose-response was non-linear for stationary and sitting time, with risk becoming more pronounced after approximately 12 h/day and 10.5 h/day, respectively. For standing time, the risk became more pronounced after 2 h/day for orthostatic circulatory disease; however we observed no changes in CVD incidence risk between 1 h/day [e.g. 160 995% CI 157, 163) events/1000 person-years) through 4 h/day [160 (158, 162) events/1000 person-years]. For CVD incidence, more standing time was not associated with higher absolute risk.
Multivariable adjusted associations for orthostatic circulatory disease
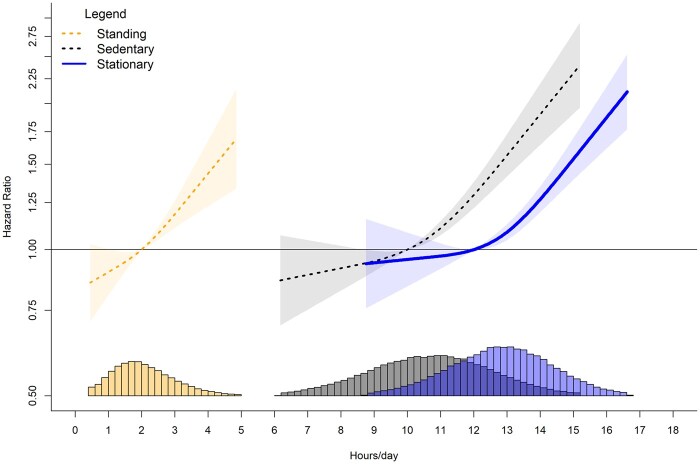
When stationary time exceeded the 12 h/day referent data point, risk increased by an average HR of 0.22 (95% CI 0.16, 0.29) with every 1-h increment (Figure 3). Similarly, every 1-h increment of sitting time above the reference data point of 10 h/day (referent) was associated with an average HR increase of 0.26 (0.18, 0.36). For standing time, compared with the referent 2 h/day, every 30-min increment above 2 h/day was associated with an average HR increase of 0.11 (0.05, 0.18). For all three exposures, we observed a null to weak protective association for time spent below the referent value. For example, 9 h/day sitting and 1.5 h/day standing were associated with an HR of 0.96 (0.93, 0.99) and 0.94 (0.90, 0.99), respectively.
Figure 3.
Dose-response associations of stationary, standing and sitting time with orthostatic circulatory disease incidence. Adjusted for age, sex, ethnicity, smoking history, alcohol consumption, body mass index, time spent walking/running, mutual adjustment for time spent standing and sitting, education, diet, family history of cardiovascular disease (CVD), prevalent CVD incidence and medication use. Histogram represents sample distribution
Multivariable adjusted associations for major cardiovascular disease incidence
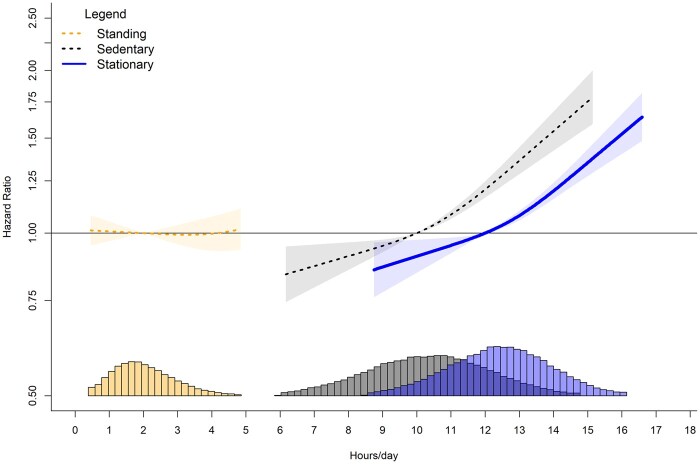
When stationary time exceeded 12 h/day, we observed higher CVD risk in a linear fashion (Figure 4). Every 1-h increment in stationary time above 12 h/day was associated with an average HR increase of 0.13 (0.10, 0.16). We observed lower CVD risk when stationary time was below 12 h/day. For example, at 9 h/day we observed an HR of 0.87 (0.78, 0.96). We also observed a linear association for sitting time when the daily duration was above 10 h/day. Every 1-h increment above 10 h/day associated with an average HR increase of 0.15 (0.11, 0.19). When sitting time was below 10 h/day, we observed lower CVD risk [e.g. 9 h/day of sitting time had an HR of 0.95 (0.93, 0.97)]. Contrary to sitting time, more time spent standing was not associated with a higher CVD risk. Overall, there was no association for higher or lower CVD risk throughout the range of standing duration.
Figure 4.
Dose-response association of stationary, standing and sitting time with major cardiovascular disease incidence. Adjusted for age, sex, ethnicity, smoking history, alcohol consumption, body mass index, time spent walking/running, mutual adjustment for time spent standing and sitting, education, diet, family history of cardiovascular disease (CVD), prevalent orthostatic incidence, and medication use. Histogram represents sample distribution
Additional and sensitivity analyses
The association pattern for orthostatic circulatory disease and CVD incidence remained consistent after exclusion of participants who were: (i) obese; (ii) reported fair or poor health; (iii) or had an event within the first 24 months of follow-up (Supplementary Figures S3 and S4, available as Supplementary data at IJE online). Negative control and E-value analyses for the minimum harmful dose indicated that residual and unmeasured confounding had minimal impact on the findings. For the negative control, associations for standing were non-significant with wide 95% CIs, and sitting behaviour point estimates were inconsistent with the main analysis showing a U-shaped association (Supplementary Figure S5, available as Supplementary data at IJE online). The E-values suggest a substantial degree of unmeasured confounding would be required to reduce our observed associations at the minimum harmful dose for orthostatic circulatory disease (e.g. an association of 2.01 to 2.79) and CVD incidence (1.97 to 2.13) to null (Supplementary Table S7, available as Supplementary data at IJE online). In our sequential adjustment modelling, crude analysis (age and sex) suggested standing was associated with lower CVD risk. This association was eliminated after adjustment for lifestyle factors, BMI and education. The null association remained consistent after additional adjustment for medication and prevalent disease. Crude analysis also showed a U-shaped association for standing with orthostatic circulatory disease. However after further adjustments, the association became linear (Supplementary Figures S6 and S7, available as Supplementary data at IJE online).
Discussion
In one of the largest wearables-based studies of stationary time and its constituent components of standing and sitting time in >83 000 adults, we observed a linear association for higher orthostatic circulatory disease risk from increased standing time with no protective association for CVD risk. After approximately 10 h/day, we observed a deleterious association of increased sitting time with higher risk of both orthostatic circulatory disease and CVD risk. This calls into question current intervention strategies that focus on only replacing sitting with standing time without increasing physical activity.37
Orthostatic circulatory disease risk
Stationary time, as well as its constituent postures sitting and standing behaviours, were all associated with increased risk of orthostatic circulatory disease. For stationary time, risk increased by an average 22% with every 1-h increment above 12 h/day. For sitting time above 10 h/day, risk increased by an average 26% with every 1 h. For standing time, risk increased by an average of 11% with every 30-min increment above 2 h/day. The pattern of the dose-response relationship appears similar for sitting and standing (Figure 1), suggesting that a common aspect of sitting and standing, i.e. absence of ambulatory movement, is likely to be important in the mechanistic pathway for orthostatic circulatory disease. The lack of muscle movement during stationary time may result in a reduced venous return by skeletal muscle contraction and pumps contributing to venous pooling, causing orthostatic circulatory problems.38 Therefore, a key implication of our finding is that non-stationary movement (e.g. walking, cycling or other physical activities involving some degree of movement) is important to reduce orthostatic circulatory disease risk, aligning with current public health messaging to ‘move more’.39 If confirmed to be causal in future randomized control trials, our findings would have implications for patient care among those at high risk of CVD. Public health strategies that promote standing as a sufficient substitute to overcome the cardiovascular health risks of sitting (e.g. as seen by common advice to adopt standing desks in office environments) may not achieve their goal.
Our findings also suggest the dose-risk association between stationary time and orthostatic circulatory disease is non-linear, with no association for lower risk below inflexion points of approximately 10 h of sitting/day and 2 h of standing/day. This suggests simple messaging to ‘sit less’ may not be optimal, as that would not lower the risk for those currently accumulating less than 10 h a day and may even increase risk of musculoskeletal14 and circulatory issues by increasing the time spent standing. The non-linear associations we detected suggest that up to a certain level, neither sitting nor standing are harmful for orthostatic conditions, suggesting that a there may be a healthy balance between these two behaviours. This balance likely varies among individuals, depending on comorbidities, overall health status and daily physical activity levels.40–42
Cardiovascular disease risk
Stationary and sitting behaviours were both associated with increased risk of CVD above certain thresholds. For stationary time, risk increased by an average of 13% with every 1-h increment above the reference 12 h/day. For sitting time, risk increased by an average of 15% with every 1-h increment above 10 h/day. Standing time was not associated with CVD risk. The higher CVD risk we observed with sitting time is similar in magnitude to the associations reported in prior studies of sitting and CVD outcomes.2,34,43,44 The pattern of dose-risk relationship appears similar for sitting and overall stationary behavioursbut not for standing (Figures 2 and 4), suggesting that the sitting component is driving these associations, rather than the absence of movement. There are additional possible mechanisms that are unique to sitting. For example, the lower cumulative energy expenditure of sitting and the muscular and musculoskeletal system engagement during standing45 may partly explain the differential effects of the two postures. Although standing time was not associated with higher CVD risk, we did not observe a protective association. Collectively, the implications of our findings for public health messaging are supportive of current messages39 encouraging sitting reductions for CVD health; however, they do not support increasing standing time alone as a mitigation strategy cited in some guidelines.46
Strengths and limitations
A key strength of this study was that, unlike previous device-based studies, we were able to separately examine the components of stationary behaviours, enabling the estimation of risks associated with sitting vs standing, two postures that are underpinned by different mechanisms, and both have public health an clinical importance. We used the currently largest wearables-based data resource in the world, with rich contextual associations and linkage to health outcomes information. The wrist placement may improve translation of our findings into public health messaging and immediate uptake by users of consumer-level wrist wearables among the general public that track and provide feedback on time spent sitting, standing and activity throughout a day.
Limitations of our study included the potential misclassification of posture and movement which is inherent to wrist-worn devices, although our daily estimates are similar to sitting and standing time assessed from the gold standard of wearables postural assessment, thigh-worn devices, in other UK cohorts.23,47 The observational design of our study precludes us from making causal interpretations. We cannot rule out the presence of unmeasured confounding, although our E-values indicated that unmeasured confounders would need to have to have a very strong association with the exposure and outcomes for the observed associations to be null. There was a median lag of 5.5 years between the UK Biobank baseline when covariates measurements were taken and the accelerometry study, although covariates were stable over time with the exception of medication.48 The UK Biobank had a low response rate; however, previous work has shown that poor representativeness does not materially influence the associations between lifestyle risk factors and non-communicable disease risk.49
Conclusion
The deleterious associations of stationary time with CVD and orthostatic circulatory disease we observed were primarily a consequence of time spent sitting. More time spent standing was not associated with CVD risk but was associated with substantially higher risk of orthostatic circulatory disease. Collectively, our findings are supportive of clinical and public health strategies to curtail excessive sitting time as an important risk factor for major CVD. However, standing time alone may not be a sufficient mitigation strategy for lower CVD risk, and may lead to a higher risk of circulatory conditions.
Ethics approval
Ethical approval was provided by the UK’s National Health Service, National Research Ethics Service (Ref 11/NW/0382).
Supplementary Material
Acknowledgements
This research was conducted using the UK Biobank Resource (Application No. 25813). The authors thank all the participants and professionals contributing to the UK Biobank.
Contributor Information
Matthew N Ahmadi, Mackenzie Wearables Research Hub, Charles Perkins Centre, University of Sydney, Camperdown, NSW, Australia; School of Health Sciences, Faculty of Medicine and Health, University of Sydney, Camperdown, NSW, Australia.
Pieter Coenen, Department of Public and Occupational Health, Amsterdam UMC, Vrije Universiteit, Amsterdam, The Netherlands; Societal Participation and Health, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Leon Straker, School of Allied Health, Curtin University, Perth, WA, Australia.
Emmanuel Stamatakis, Mackenzie Wearables Research Hub, Charles Perkins Centre, University of Sydney, Camperdown, NSW, Australia; School of Health Sciences, Faculty of Medicine and Health, University of Sydney, Camperdown, NSW, Australia.
Data availability
The UK Biobank is an open access resource. Researchers can apply to use the UK Biobank dataset by registering and applying at [http://ukbiobank.ac.uk/register-apply/].
Supplementary data
Supplementary data are available at IJE online.
Author contributions
M.N.A. and E.S. conceptualized the research and analysed the dataset. MNA wrote the first draft of the manuscript. M.N.A., E.S., P.C., L.S. interpreted the results, revised the paper and provided critical intellectual content.
Use of artificial intelligence (AI) tools
AI tools were not used in the analysis or writing of the manuscript.
Funding
M.N.A. is funded by the National Heart Foundation (APP 107158). E.S. is funded by the National Health and Medical Research Council (APP 1194510).
Conflict of interest
None declared.
References
- 1. Tremblay MS, Aubert S, Barnes JD. et al. Sedentary Behavior Research Network (SBRN) – terminology consensus project process and outcome. Int J Behav Nutr Phys Activity 2017;14:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pandey A, Salahuddin U, Garg S. et al. Continuous dose-response association between sedentary time and risk for cardiovascular disease. JAMA Cardiol 2016;1:575–83. [DOI] [PubMed] [Google Scholar]
- 3. Lavie CJ, Ozemek C, Carbone S, Katzmarzyk PT, Blair SN.. Sedentary behavior, exercise, and cardiovascular health. Circ Res 2019;124:799–815. [DOI] [PubMed] [Google Scholar]
- 4. Smith P, Ma H, Glazier RH, Gilbert-Ouimet M, Mustard C.. The relationship between occupational standing and sitting and incident heart disease over a 12-year period in Ontario, Canada. Am J Epidemiol 2018;187:27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Visseren FLJ, Mach F, Smulders YM. et al. ; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. [DOI] [PubMed] [Google Scholar]
- 6. Arnett DK, Khera A, Blumenthal RS.. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: part 1, lifestyle and behavioral factors. JAMA Cardiol 2019;4:1043–44. [DOI] [PubMed] [Google Scholar]
- 7. Healy GN, Winkler EA, Owen N, Anuradha S, Dunstan DW.. Replacing sitting time with standing or stepping: associations with cardio-metabolic risk biomarkers. Eur Heart J 2015;36:2643–49. [DOI] [PubMed] [Google Scholar]
- 8. Torbeyns T, Bailey S, Bos I, Meeusen R.. Active workstations to fight sedentary behaviour. Sports Med 2014;44:1261–73. [DOI] [PubMed] [Google Scholar]
- 9. van der Ploeg HP, Chey T, Ding D, Chau JY, Stamatakis E, Bauman AE.. Standing time and all-cause mortality in a large cohort of Australian adults. Prev Med 2014;69:187–91. [DOI] [PubMed] [Google Scholar]
- 10. Shockey TM, Luckhaupt SE, Groenewold MR, Lu M-L.. Frequent exertion and frequent standing at work, by industry and occupation group—United States, 2015. MMWR Morb Mortal Wkly Rep 2018;67:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reis JP, DuBose KD, Ainsworth BE, Macera CA, Yore MM.. Reliability and validity of the occupational physical activity questionnaire. Med Sci Sports Exerc 2005;37:2075–83. [DOI] [PubMed] [Google Scholar]
- 12. Chastin S, Culhane B, Dall P.. Comparison of self-reported measure of sitting time (IPAQ) with objective measurement (activPAL). Physiol Meas 2014;35:2319–28. [DOI] [PubMed] [Google Scholar]
- 13. Prince SA, Cardilli L, Reed JL. et al. A comparison of self-reported and device measured sedentary behaviour in adults: a systematic review and meta-analysis. Int J Behav Nutr Phys Act 2020;17:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coenen P, Willenberg L, Parry S. et al. Associations of occupational standing with musculoskeletal symptoms: a systematic review with meta-analysis. Br J Sports Med 2018;52:176–83. [DOI] [PubMed] [Google Scholar]
- 15. Coenen P, Parry S, Willenberg L. et al. Associations of prolonged standing with musculoskeletal symptoms—a systematic review of laboratory studies. Gait & Posture 2017;58:310–18. [DOI] [PubMed] [Google Scholar]
- 16. Xin W, Mi S, Lin Z, Wang H, Wei W.. Orthostatic hypotension and the risk of incidental cardiovascular diseases: a meta-analysis of prospective cohort studies. Prev Med 2016;85:90–97. [DOI] [PubMed] [Google Scholar]
- 17. Benvenuto LJ, Krakoff LR.. Morbidity and mortality of orthostatic hypotension: implications for management of cardiovascular disease. Am J Hypertens 2011;24:135–44. [DOI] [PubMed] [Google Scholar]
- 18. Juraschek SP, Daya N, Appel LJ. et al. Orthostatic hypotension and risk of clinical and subclinical cardiovascular disease in middle‐aged adults. J Am Heart Assoc 2018;7:e008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vasankari V, Husu P, Vähä-Ypyä H. et al. Association of objectively measured sedentary behaviour and physical activity with cardiovascular disease risk. Eur J Prev Cardiol 2017;24:1311–18. [DOI] [PubMed] [Google Scholar]
- 20. Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N.. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J 2011;32:590–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ekelund U, Tarp J, Fagerland MW. et al. Joint associations of accelerometer-measured physical activity and sedentary time with all-cause mortality: a harmonised meta-analysis in more than 44 000 middle-aged and older individuals. Br J Sports Med 2020;54:1499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. VAN DER Velde JHPM, Koster A, VAN DER Berg JD. et al. Sedentary behavior, physical activity, and fitness-the Maastricht study. Med Sci Sports Exerc 2017;49:1583–91. [DOI] [PubMed] [Google Scholar]
- 23. Hamer M, Stamatakis E.. The descriptive epidemiology of standing activity during free-living in 5412 middle-aged adults: the 1970 British cohort study. J Epidemiol Community Health 2020;74:757–60. [DOI] [PubMed] [Google Scholar]
- 24. Healy GN, Winkler EAH, Eakin EG. et al. A cluster RCT to reduce workers' sitting time: impact on cardiometabolic biomarkers. Med Sci Sports Exerc 2017;49:2032–39. [DOI] [PubMed] [Google Scholar]
- 25. Saeidifard F, Medina-Inojosa JR, Supervia M. et al. The effect of replacing sitting with standing on cardiovascular risk factors: a systematic review and meta-analysis. Mayo Clinic Proc Innov Qual Outcomes 2020;4:611–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aadahl M, Linneberg A, Møller TC. et al. Motivational counseling to reduce sitting time: a community-based randomized controlled trial in adults. Am J Prev Med 2014;47:576–86. [DOI] [PubMed] [Google Scholar]
- 27. Freeman R. Neurogenic orthostatic hypotension. N Engl J Med 2008;358:615–24. [DOI] [PubMed] [Google Scholar]
- 28. Eberhardt RT, Raffetto JD.. Chronic venous insufficiency. Circulation 2014;130:333–46. [DOI] [PubMed] [Google Scholar]
- 29. Ahmadi MN, Clare PJ, Katzmarzyk PT, Del Pozo Cruz B, Lee IM, Stamatakis E.. Vigorous physical activity, incident heart disease, and cancer: how little is enough? Eur Heart J 2022;43:4801–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stamatakis E, Ahmadi MN, Gill JMR. et al. Association of wearable device-measured vigorous intermittent lifestyle physical activity with mortality. Nat Med 2022;28:2521–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ahmadi MN, Nathan N, Sutherland R, Wolfenden L, Trost SG.. Non-wear or sleep? Evaluation of five non-wear detection algorithms for raw accelerometer data. J Sports Sci 2020;38:399–404. [DOI] [PubMed] [Google Scholar]
- 32. Ellis K, Kerr J, Godbole S, Lanckriet G, Wing D, Marshall S.. A random forest classifier for the prediction of energy expenditure and type of physical activity from wrist and hip accelerometers. Physiol Meas 2014;35:2191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ellis K, Kerr J, Godbole S, Staudenmayer J, Lanckriet G.. Hip and wrist accelerometer algorithms for free-living behavior classification. Med Sci Sports Exerc 2016;48:933–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stamatakis E, Gale J, Bauman A, Ekelund U, Hamer M, Ding D.. Sitting time, physical activity, and risk of mortality in adults. J Am Coll Cardiol 2019;73:2062–72. [DOI] [PubMed] [Google Scholar]
- 35. Pedisic Z, Grunseit A, Ding D. et al. High sitting time or obesity: Which came first? Bidirectional association in a longitudinal study of 31,787 Australian adults. Obesity (Silver Spring) 2014;22:2126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lipsitch M, Tchetgen Tchetgen E, Cohen T.. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology 2010;21:383–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buckley JP, Hedge A, Yates T. et al. The sedentary office: an expert statement on the growing case for change towards better health and productivity. Br J Sports Med 2015;49:1357–62. [DOI] [PubMed] [Google Scholar]
- 38. Gutkin M, Stewart JM.. Orthostatic circulatory disorders: from nosology to nuts and bolts. Am J Hypertens 2016;29:1009–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bull FC, Al-Ansari SS, Biddle S. et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 2020;54:1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Straker L, Mathiassen SE, Holtermann A.. The ‘Goldilocks Principle’: designing physical activity at work to be ‘just right’for promoting health. Br J Sports Med 2018;52:818–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Holtermann A, Rasmussen CL, Hallman DM, Ding D, Dumuid D, Gupta N.. 24-hour physical behavior balance for better health for all: “The Sweet-Spot Hypothesis”. Sports Med Open 2021;7:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Straker L, Rasmussen CL, Gupta N, Holtermann A.. What’s your poison? Is sitting always health hindering and moving always health promoting? J Phys Act Health 2024;21:845–46. [DOI] [PubMed] [Google Scholar]
- 43. Wilmot EG, Edwardson CL, Achana FA. et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 2012;55:2895–905. [DOI] [PubMed] [Google Scholar]
- 44. Ahmadi MN, Rezende LF, Ferrari G, Cruz BDP, Lee I-M, Stamatakis E.. Do the associations of daily steps with mortality and incident cardiovascular disease differ by sedentary time levels? A device-based cohort study. Br J Sports Med 2024;58:261–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mansoubi M, Pearson N, Clemes SA. et al. Energy expenditure during common sitting and standing tasks: examining the 1.5 MET definition of sedentary behaviour. BMC Public Health 2015;15:516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ross R, Chaput J-P, Giangregorio LM. et al. Canadian 24-Hour Movement Guidelines for Adults aged 18–64 years and Adults aged 65 years or older: an integration of physical activity, sedentary behaviour, and sleep. Appl Physiol Nutr Metab 2020;45:S57–102. [DOI] [PubMed] [Google Scholar]
- 47. Hamer M, Stamatakis E, Chastin S. et al. Feasibility of measuring sedentary time using data from a thigh-worn accelerometer. Am J Epidemiol 2020;189:963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ahmadi MN, Hamer M, Gill JMR. et al. Brief bouts of device-measured intermittent lifestyle physical activity and its association with major adverse cardiovascular events and mortality in people who do not exercise: a prospective cohort study. Lancet Public Health 2023;8:e800–10. [DOI] [PubMed] [Google Scholar]
- 49. Stamatakis E, Owen KB, Shepherd L, Drayton B, Hamer M, Bauman AE.. Is cohort representativeness passe? Poststratified associations of lifestyle risk factors with mortality in the UK Biobank. Epidemiology 2021;32:179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The UK Biobank is an open access resource. Researchers can apply to use the UK Biobank dataset by registering and applying at [http://ukbiobank.ac.uk/register-apply/].