Abstract
Background
Pneumococcal conjugate vaccines (PCVs) provide strong direct protection in children, while limited data are available on their indirect effect on mortality among older age groups. This multicountry study aimed to assess the population-level impact of pediatric PCVs on all-cause pneumonia mortality among children ≥5 years of age, and invasive pneumococcal disease (IPD) cases in Chile.
Methods
Demographic and mortality data from Argentina, Brazil, Chile, Colombia, and Mexico were collected considering the ≥ 5-year-old population, from 2000 to 2019, with 1 795 789 deaths due to all-cause pneumonia. IPD cases in Chile were also evaluated. Time series models were employed to evaluate changes in all-cause pneumonia deaths during the postvaccination period, with other causes of death used as synthetic controls for unrelated temporal trends.
Results
No significant change in death rates due to all-cause pneumonia was detected following PCV introduction among most age groups and countries. The proportion of IPD cases caused by vaccine serotypes decreased from 29% (2012) to 6% (2022) among people aged ≥65 years in Chile.
Discussion
While an effect of PCV against pneumonia deaths (a broad clinical definition that may not be specific enough to measure indirect effects) was not detected, evidence of indirect PCV impact was observed among vaccine-type–specific IPD cases.
Keywords: all-cause pneumonia, mortality, national registry, Latin American countries, interrupted time series, pneumococcal conjugate vaccines, pneumococcus, synthetic control model, vaccine impact, indirect effects
This multicountry study evaluates the population-level impact of PCVs on pneumonia mortality in ≥5 year olds and IPD in Chile. PCV introduction showed no significant change in pneumonia death rates but reduced vaccine-type IPD cases in Chile, highlighting indirect effects.
Pneumococcus is among the leading causes of severe bacterial disease and death, with children <5 years of age and older adults carrying the largest burden worldwide [1, 2]. The most severe form of disease, known as invasive pneumococcal disease (IPD), can lead to pneumonia, meningitis, and septicemia [3]. Pneumococcal infections cause about 300 000 deaths in children <5 years of age globally each year, and a large, but poorly characterized, burden in adults [4].
Pneumococcal conjugate vaccines (PCVs) have now been introduced in >140 countries, including in many low- and middle-income settings, with countries in the Americas being among the first lower- and middle-income countries (LMICs) to introduce PCVs into their national immunization programs for infants [1, 5, 6]. This widespread introduction of PCVs for use in infants resulted in a substantial decrease in the incidence of IPD due to PCV-targeted serotypes in children [7]. These vaccines also reduced transmission of pneumococcus from the upper respiratory tract [8]. Therefore, vaccination of children with PCVs resulted in indirect benefits for unvaccinated groups, especially in older adults [9, 10]. For example, after the introduction of PCV7 in 2000 in the United States, a substantial decline was observed across all age groups for IPD as well as pneumococcal pneumonia hospitalizations and deaths [11]. In South Africa, PCV introduction was associated with reductions in deaths due to all-cause pneumonia in children and teenagers but not in adults [12]. The magnitude of the reduction in rates of IPD varied depending on population demographics, the proportion of cases caused by vaccine-targeted serotypes, the degree to which the incidence of nonvaccine serotypes increased following vaccine introduction (serotype replacement), and contact patterns in the population [13, 14].
There are various practical and technical challenges when estimating the population-level impact of PCVs. In many settings, particularly in LMICs, there is a lack of systematic surveillance for pneumococcal diseases. Specific bacterial or viral causes of disease or death are often not recorded in administrative records. Evaluations of PCV impact, therefore, often focus on nonspecific outcomes, such as all-cause pneumonia, instead of pneumococcal pneumonia [1]. Detecting changes in all-cause pneumonia associated with introduction of PCVs is challenging because pneumococcus is just one of many etiologies of pneumonia [1], and thus the fraction of all-cause pneumonia mortality that could be prevented by PCVs is relatively small. Furthermore, the overall impact of PCVs might be masked due to the replacement of serotypes or counterbalancing trends in mortality. Previous studies employed various statistical methods to overcome these challenges and detect changes in all-cause pneumonia in children during the post-PCV period [15, 16]. To date, nearly all countries in the Latin America and Caribbean Region (n = 25) have introduced PCV in their routine immunization schedules [17].
In this multicountry study, we aimed to investigate the population-level impact of PCVs against all-cause pneumonia mortality among people ≥5 years of age and against IPD cases in Chile, following our previous study evaluating the impact among children <5 years of age. We used standardized data from 5 middle-income countries in Latin America and employed several complementary analytic approaches to overcome data challenges and increase the robustness of our findings.
METHODS
Mortality Data
Mortality data from Argentina, Brazil, Colombia, Chile, and Mexico were obtained from national mortality registries in each country, considering all cases of death, and the following age strata: 5–19, 20–39, 40–64, 65–79, and 80+ years. All countries conducted standardized data cleaning and quality control [1]. Mortality data were extracted from the national mortality information systems in each country [18].
We extracted the month and year of birth and death for each death record, age of the individual at death, and the primary cause of death, as coded in the International Classification of Diseases, Tenth Revision (ICD-10), similarly to our previous analysis [1]. To assess data quality, we obtained standardized indicators for each country, including the proportions of underreporting, completeness of death registration with cause of death, reported deaths with “ill-defined” causes of death (ICD-10 codes R00–R99), and the proportion of deaths in which “garbage codes” are indicated as causes of death [18].
Study Periods
We considered 3 immunization periods in our analysis: (1) prevaccine period; (2) transition period, defined as the first 12 months after PCV introduction to allow vaccine uptake to stabilize; and (3) evaluation or postvaccine period, during which the impact of PCV was evaluated. Study periods for each country were defined depending on the timing of universal introduction of PCVs in each country (Table 1).
Table 1.
Study Periods in Participating Countries
| Country | Prevaccine Period | Date of PCV Effective Start of Vaccination | Product (Schedule) | Vaccine Coverage, % With at Least 3 Doses | Transition Period | Evaluation Period |
|---|---|---|---|---|---|---|
| Argentina | Jan 2005 to Jan 2012 | Jan 2012 | PCV13 (2 + 1) | 85.3 as of 2013 | Feb 2012 to Dec 2012 | Jan 2013 to Dec 2019 |
| Brazil | Jan 2005 to Feb 2010 | Mar 2010 | PCV10 (3 + 1) in Mar 2010 PCV13 (2 + 1) in Jan 2017 |
81.7 as of 2011 | Apr 2010 to Mar 2011 | Apr 2011 to Dec 2019 |
| Chile | Jan 2005 to Jan 2011 | Jan 2011 | PCV10 (3 + 1) in Jan 2011 PCV13 (2 + 1) in Nov 2017 |
82.2 as of 2013 | Feb 2011 to Jan 2012 | Feb 2012 to Dec 2018 |
| Colombia | Jan 2005 to Jan 2011 | Jan 2011 | PCV10 (2 + 1) | 84 as of 2012 | Feb 2011 to Jan 2012 | Feb 2012 to Dec 2019 |
| Mexico | Jan 1999 to Jan 2008 | Jan 2008 | PCV7 (2 + 1) in Jan 2008 PCV13 (2 + 1) in Feb 2011 |
92 as of 2009 | Feb 2008 to Jan 2009 | Feb 2009 to Dec 2019 |
Abbreviation: PCV, pneumococcal conjugate vaccine.
Data on Pneumococcal Serotypes of IPD Cases in Chile
We obtained data on pneumococcal serotypes from the national surveillance for IPD cases in Chile from 2012 (1 year after the introduction of PCV) to 2022. We analyzed data for those aged ≥65 years and calculated the proportion of IPD cases caused by (1) PCV10 serotypes; (2) serotype 3, 6A, and 19A (additional serotypes included in PCV13); and (3) nonvaccine-type serotypes for each year [19]. We only analyzed data for older adults, because the serotype information was frequently missing for some of the other age groups.
Statistical Approaches
The goal of our analysis was to evaluate the population-level impact of PCVs in age groups ≥5 years old following universal introduction of PCV in infants. The outcome of interest was death due to all-cause pneumonia, defined as having an ICD-10 code for the primary cause of death in the range of J12–J18. The age group of 5–19 years captured both direct and indirect effects in many of these countries due to the long follow-up period, while for the remaining age groups (20+ years), our analysis captured indirect effects only.
We used synthetic control (SC) models as our primary analysis for all age groups and countries to reduce biases and to isolate changes in all-cause pneumonia mortality caused by PCV as opposed to those caused by other factors, such as changes in underlying health of the population and reporting of mortality records. This approach uses other causes of death not affected by the vaccine to adjust for shared temporal trends. Advantages of using the SC approach have been shown previously [7]. Among the most important, the SC approach is more robust to unmeasured confounding and biases than other methods (eg, interrupted time series [ITS]) because it uses information on control conditions that are affected by common unrelated factors but not by the intervention of interest (ie, PCV in this study). Additionally, SC models select an appropriate set of controls based on the prevaccine data; this step is performed in a data-driven way, without requiring users to select controls a priori. Finally, unlike ITS models, the SC method does not need to assume that trends in the prevaccine period continue linearly in the postvaccine period or assume that changes happen at specified time points. All analyses were performed in R.
Synthetic Control Model
The version of the SC model used in this study is a negative binomial regression model where the outcome was the observed number of all-cause pneumonia deaths (ICD-10 code J12–J18) per month. The covariates or control diseases were monthly time series for other ICD-10 chapters or subchapters of death per month that are not likely to be influenced by PCVs, including disease of the circulatory system (I00–I99), skin (L00–L99), musculoskeletal system (M00–M99), and genitourinary system (N00–N99) (see Supplementary Material for the full list of control diseases). We fitted the SC model to the pre-PCV vaccine data from each age group in each country separately, using the rjags package [20]. Regularization prior distribution, similar to those used in ridge regression, was assigned for the control variable regression parameters to deal with the potentially large number of control variables under consideration and correlation between them. These selected controls were combined into a composite used to generate a counterfactual of the number of all-cause pneumonia deaths that would have occurred without the intervention. This prediction was then compared with observed all-cause pneumonia deaths during the evaluation period for each age group in each country. We quantified the impact of PCV by computing a rate ratio (RR) for each age group in each country, which is the total cumulative number of observed all-cause pneumonia deaths divided by the counterfactual cumulative number of predicted all-cause pneumonia deaths during the evaluation period. Posterior medians and 2.5th and 97.5th posterior percentiles were reported as point estimates and 95% credible intervals (CrIs) of RR, respectively. The model used here differs from SC models used in previous studies, which used a quasi-Poisson model together with a spike-and-slab prior as the regularization prior distribution [1]. This version of the model did not converge well, leading us to explore alternative structures.
Sensitivity Analysis
In addition to the SC models, we applied a more commonly used approach, ITS models [21]. We fit a negative binomial regression model to the monthly time series data on all-cause pneumonia deaths over the entire study period, with an offset being all-cause mortality other than those caused by respiratory illness (ICD-10 J chapter). Separate models were fit for each age group in each country. A linear trend was included throughout the entire study period; also, we included 2 changes in intercept and slope to model the impact of the intervention. The first change in intercept and slope represented the transition period; while the second change in intercept and slope represented the evaluation period. The counterfactual number of all-cause pneumonia deaths was then generated by removing those slopes and intercepts. The impact of PCV was quantified based on an RR, this time using the model fitted to the observed time series of pneumonia deaths divided by the expected number of pneumonia deaths if the intervention had not been introduced for the evaluation period. As a second sensitivity model, we combined the SC and ITS approach together into the SC + ITS model. We included all the control variables as in the SC model and we used the ridge regression style prior distributions to provide robust estimation results. Additionally, we included 2 slopes and intercepts to quantify the impact of the intervention, and we fitted the model to the entire time series of data. The impact of PCV was quantified following the approach described for the ITS model.
Ethical Considerations
The study protocol was reviewed and approved by institutional review boards in each country and also by the Pan American Health Organization Ethical Research Committee.
Availability of Code and Data
The aggregated time series data and code can be found in the following github repository: https://github.com/weinbergerlab/Paho_project_PCV_adult.
RESULTS
Descriptive Results
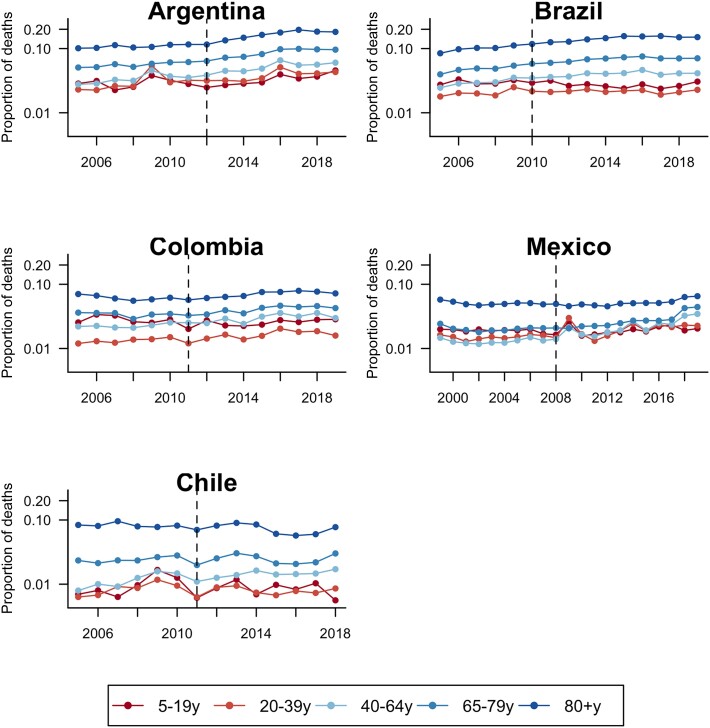
We analyzed a total of 4 600 987 deaths in Argentina, 17 540 909 in Brazil, 1 359 206 in Chile, 11 099 274 in Mexico, and 2 806 045 in Colombia. The proportion of all deaths caused by all-cause pneumonia (ICD-10 code J12–J18 as the primary cause of death) varied by country and age group (Figure 1). In general, the proportion was highest among the oldest age groups (65–80, 80+ years), ranging from 5% to 10%, and lowest among younger age groups, ranging from 1% to 5%. The proportion gradually increased in some age groups in some countries, especially older age groups (eg, Argentina and Brazil).
Figure 1.
Annual time series for the proportion of all deaths recorded as pneumonia (International Classification of Diseases, Tenth Revision [ICD-10] codes J12–J18) as primary cause of death, by age group in 5 Latin American countries. Vertical dashed lines represent the timing of universal pneumococcal conjugate vaccine introduction.
Changes in All-Cause Pneumonia Mortality Across Age Groups and Countries
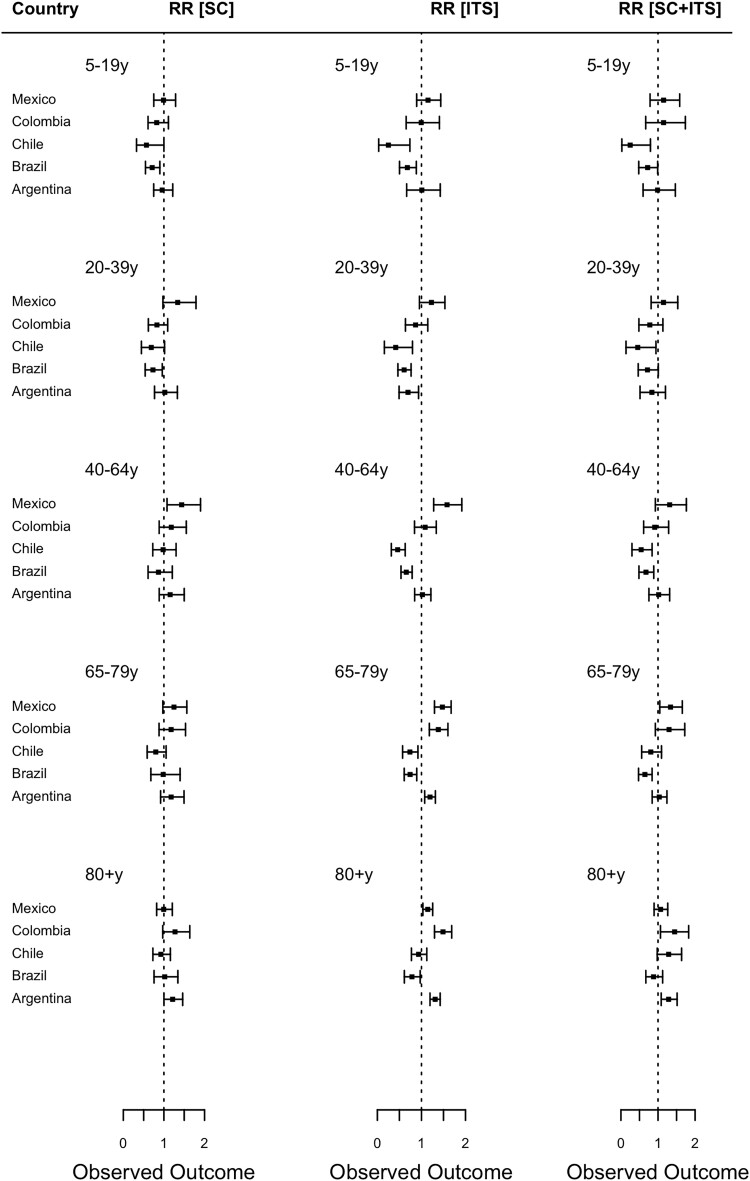
In general, there was not a detectable change in rates of death due to all-cause pneumonia following the introduction of PCV among most age groups in the majority of the countries (Figure 2, left, and Supplementary Figures 1–5). The estimates of the RRs were generally close to 1, or with wide 95% CrIs, suggesting that there was no detectable difference between the observed and expected number of all-cause pneumonia deaths during the evaluation period, and/or that the uncertainty in the estimates was too large to draw firm conclusions.
Figure 2.
Estimated impact of pneumococcal conjugate vaccine among age groups 5–19, 20–39, 40–64, 65–79, and 80+ years using the SC, ITS, and SC + ITS modeling approaches, by country. Rate ratios were calculated by dividing the cumulative number of observed pneumonia deaths by the cumulative number of predicted pneumonia deaths during the evaluation period. Black squares represent the point estimates of rate ratio and bars represent their 95% credible intervals. A rate ratio < 1 indicates evidence of indirect protection effect of the vaccine; a rate ratio = 1 indicates no evidence of indirect effect of the vaccine; while a rate ratio > 1 indicates evidence of indirect detrimental effect of the vaccine. Abbreviations: ITS, interrupted time series model; SC, synthetic control model.
Changes in all-cause pneumonia deaths in the evaluation period were observed in a few age groups in certain countries. The SC model estimated that after the introduction of PCV, all-cause pneumonia mortality in Brazil declined in the 5–19 years age group (RR, 0.71; 95% CrI, 0.55–0.90) and 20–39 years age group (RR, 0.73; 95% CrI, 0.54–0.96) (Figure 2 and Supplementary Figures 1–5). On the other hand, all-cause pneumonia mortality was estimated to have increased by 44% (RR, 1.44; 95% CrI, 1.08–1.90) among adults aged 40–64 years in Mexico and by 21% (RR, 1.21; 95% CrI, 1.00–1.46) among those aged ≥80 years in Argentina.
Distribution of Pneumococcus Serotype in IPD Cases in Chile
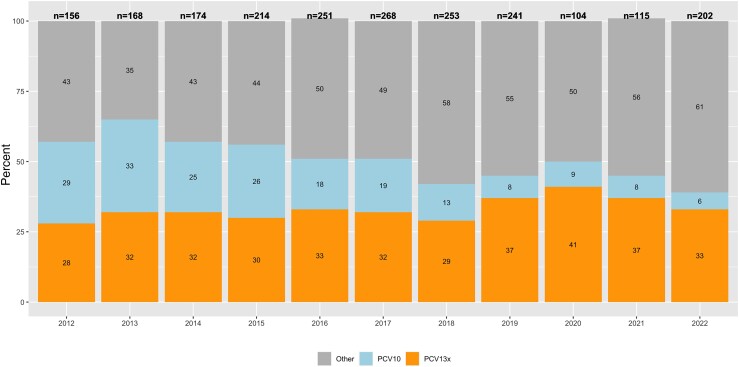
Among IPD cases aged ≥65 years in Chile, we found that vaccine-type pneumococcus, especially those included in PCV10, decreased every year after PCV introduction, ranging from 29% in 2012 to 6% in 2022 (Figure 3).
Figure 3.
Serotype distribution of Streptococcus pneumoniae strains isolated from invasive pneumococcal disease among older adults ≥65 years of age, in Chile, 2012–2022. On top of each stacked bar plot, the total number of isolates is shown. PCV13x includes serotypes 3, 6A, and 19A. Abbreviation: PCV, pneumococcal conjugate vaccine. Source: Laboratorio de Referencia de Agentes de Meningitis Bacteriana, Departamento de Laboratorio Biomedico, ISP.
Sensitivity Analyses
Changes in all-cause pneumonia mortality following the introduction of PCV estimated by the ITS approach varied across age groups and countries (Figure 2 and Supplementary Figures 6–10). RRs estimated by the ITS approach were qualitatively different from those estimated by the SC approach, with estimated declines or increases in some age groups and countries, where a change was not estimated with the SC method. For example, the ITS approach predicted an estimated decline in deaths in all age groups ≤ 79 years in Brazil and Chile (approximately 25% to 80%). The SC + ITS results were in line with the ITS model in most age groups and countries with a few exceptions (Supplementary Figure 11). In Argentina, the SC + ITS seemed to correctly adjust for the bias among those aged 64–79 years (RR, 1.04; 95% CrI, 0.83–1.25). Also, the RRs from the SC + ITS were close to 1 in a few age groups in Brazil and Chile, confirming the SC model's results (Figure 2 and Supplementary Figure 11).
DISCUSSION
This multicountry study represents the first comprehensive, multinational effort to measure the indirect effects of PCV on all-cause pneumonia mortality across wide age ranges in the Latin American region. Our analyses relied on national mortality registries in each country and used different methodological approaches, finding no consistent evidence of a decline in all-cause pneumonia deaths among individuals ≥5 years of age following PCV introduction in most settings. In our previous study, where we employed similar approaches to evaluate the impact of PCVs among children ≤5 years of age in 10 Latin American and Caribbean countries, we found detectable declines in all-cause pneumonia deaths in Colombia (RR, 0.76; 95% CrI, 0.65–0.97) and Mexico (RR, 0.89; 95% CrI, 0.82–0.97) but not in Argentina (RR, 0.92; 95% CrI, 0.74–1.11) nor in Brazil (RR, 0.98; 95% CrI, 0.92–1.04) (Chile was not included) [1]. Given that the impact of PCV against all-cause pneumonia mortality was modest or null among directly vaccinated age groups, it was not surprising to see no impact among individuals ≥5 years of age.
Several studies have shown evidence of indirect effects of pediatric vaccination against IPD and morbidity due to pneumonia in older age groups [11, 22]. Furthermore, real-world evidence of the direct impact of PCVs on children's mortality are largely from countries with low infant and child mortality, with a few exceptions [1, 12, 23]. In contrast, investigations into the indirect effects of PCV on pneumonia mortality in older age groups in middle-income countries remain limited [12, 23]. Our study investigates the indirect effects of PCV on pneumonia mortality in unvaccinated age groups across 5 middle-income countries. We believe that one of the strengths of our analyses lies in testing several methods with different assumptions about the underlying trends in the data.
The SC models estimated that all-cause pneumonia mortality increased compared to the counterfactual scenario among older age groups in Argentina and Mexico, suggesting that the PCVs had negative impact, an implausible result. There are potential factors contributing to this result. We observed an abrupt increase in all-cause pneumonia deaths during the post-PCV period among older adults in Argentina and Mexico. The SC models were unable to fully account for such increasing trends in all-cause pneumonia deaths unrelated to vaccination, likely because the changes in data trends affected respiratory diseases but did not impact control diseases. These trends were likely artifactual and could have various explanations. For example, causes of death in many settings were inconsistently reported, and there was an overuse of the codes for “unspecified” cause of death [1]. Accuracy of reporting likely improved after the H1N1 pandemic, for example in Colombia, with an improvement in the diagnosis of respiratory diseases precision, thus leading to an apparent increase in trends. Conversely, a further result of the H1N1 pandemic was the overuse of classifying symptoms compatible with pneumonia immediately as such. These explanations could likely have impacted Argentina and Mexico as well. The data quality influences the robustness of the results, therefore our results should be interpreted with caution. It is generally challenging to estimate the impact of PCV against all-cause pneumonia because vaccine-type pneumococcus is only a part of the various pathogens causing pneumonia and nonvaccine-type pneumococcus may increase after the reduction of vaccine type following PCV introduction. Previous trials and observational studies reported that the estimated effect of PCV became smaller as the case definition became more nonspecific [7]. Additionally, while national mortality registry data collection, cleaning, and reporting followed standardized procedures and international guidelines [24], we did conduct additional cleaning and quality control procedures for the mortality data obtained from countries for this analysis [1]. However, it is plausible that misclassifications and missing reports of the cause of mortality can occur, potentially influencing our results. We found that, among IPD cases in Chile, vaccine-type pneumococcus decreased every year after PCV introduction, suggesting an indirect effect of the vaccine, while nonvaccine-type pneumococcus increased among older age groups (Figure 3). However, it is unclear why disease rates increased in Argentina and Mexico. While it is technically possible that this is related to serotype replacement, the magnitude of the increase for all-cause pneumonia argues against this. Most likely this increase is unrelated to the vaccine.
The SC model used in this study faced challenges in accurately accounting for nonlinear underlying trends in mortality data over time, particularly in certain age groups and countries. In Argentina and Mexico, for instance, the observed increase in specific age groups can be attributed to changes after vaccine introduction that were not adequately captured by the control diseases, highlighting the limitations of the SC model in those settings. Furthermore, traditional approaches such as ITS also exhibited limitations in addressing nonlinear underlying trends in various instances, resulting in counterintuitive findings among older adults in Argentina, Colombia, and Mexico (Supplementary Figures 6–10). The ITS predicted a positive impact in Chile and Brazil across the majority of age groups. However, these results could be influenced by the linear trend assumption, potentially introducing biases (Supplementary Figures 6–10). To mitigate these biases, we explored the performance of SC + ITS models, which demonstrated the ability to adjust for these limitations in certain settings. This suggests that it might be possible to address these biases through modifications to the existing methods. However, further investigation is required.
In conclusion, we evaluated the indirect effects of PCV on pneumonia mortality among older age groups using routinely collected data from national mortality registries in 5 Latin American countries. The overall evidence suggested no detectable effect of pediatric PCV vaccination on all-cause pneumonia mortality in the unvaccinated population with consistent results across most age groups and countries, although the data quality influenced the robustness of our results in a few settings. On the contrary, our analysis of IPD suggests that PCVs are indeed reducing exposure to vaccine-type pneumococci in older adults. Our findings highlight the importance of using different analytical methods to increase the robustness of the results and to detect biases in the data. Further investigation is needed to understand more robustly the indirect impact of the PCV vaccine on mortality in older adults.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Ottavia Prunas, Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland.
Kayoko Shioda, School of Public Health, Boston University, Boston, Massachusetts, USA.
Cristiana M Toscano, Institute of Tropical Pathology and Public Health, Federal University of Goias, Goiania, Goiania, Brazil.
Magdalena Bastias, Special Program Comprehensive Immunization, Pan-American Health Organization, Santiago, Chile.
Maria Teresa Valenzuela-Bravo, Special Program Comprehensive Immunization, Pan-American Health Organization, Santiago, Chile; Faculty of Health Care Sciences, Universidad San Sebastian, Santiago, Chile.
Janepsy Diaz Tito, Departamento Agencia Nacional de Dispositivos Medicos, Innovacion y Desarollo, Public Health Institute of Chile, Santiago, Chile.
Joshua L Warren, Department of Epidemiology, Microbial Diseases and Public Health Modeling Unit, Yale School of Public Health, Yale University, New Haven, Connecticut, USA; Department of Biostatistics, Yale School of Public Health, Yale University, New Haven, Connecticut, USA.
Daniel M Weinberger, Department of Epidemiology, Microbial Diseases and Public Health Modeling Unit, Yale School of Public Health, Yale University, New Haven, Connecticut, USA.
Lucia H de Oliveira, Comprehensive Immunization Program, Pan-American Health Organization, Washington, District of Columbia, USA.
Notes
Acknowledgments . The authors thank the country teams and Expanded Program on Immunization (EPI) programs for data collection and cleaning. The authors also thank Keith Klugman and Gail Rodgers for their critical feedback; and Rodrigo Puentes for their support with the invasive pneumococcal disease data from Chile.
Disclaimer . The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the decisions or policies of the Pan American Health Organization.
Financial support . This work was supported by the Bill and Melinda Gates Foundation (grant number 365011 to the Pan-American Health Organization who was responsible for convening and leading the study implementation).
References
- 1. de Oliveira LH, Shioda K, Valenzuela MT, et al. Declines in pneumonia mortality following the introduction of pneumococcal conjugate vaccines in Latin American and Caribbean countries. Clin Infect Dis 2021; 73:306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. Lancet 2016; 388:3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanquet G, Krizova P, Valentiner-Branth P, et al. Effect of childhood pneumococcal conjugate vaccination on invasive disease in older adults of 10 European countries: implications for adult vaccination. Thorax 2019; 74:473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wahl B, O'Brien KL, Greenbaum A, et al. Burden of Streptococcus pneumoniae and Haemophilus influenzae type B disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health 2018; 6:e744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whitney CG, Toscano CM. Direct effects of pneumococcal conjugate vaccines among children in Latin America and the Caribbean. Lancet Infect Dis 2021; 21:306–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Oliveira LH, Trumbo SP, Ruiz Matus C, Sanwogou NJ, Toscano CM. Pneumococcal conjugate vaccine introduction in Latin America and the Caribbean: progress and lessons learned. Expert Rev Vaccines 2016; 15:1295–304. [DOI] [PubMed] [Google Scholar]
- 7. Bruhn CA, Hetterich S, Schuck-Paim C, et al. Estimating the population-level impact of vaccines using synthetic controls. Proc Natl Acad Sci U S A 2017; 114:1524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldblatt D. The indirect effect of pneumococcal conjugate vaccine. Lancet Glob Health 2017; 5:e6–7. [DOI] [PubMed] [Google Scholar]
- 9. Ciruela P, Broner S, Izquierdo C, et al. Indirect effects of paediatric conjugate vaccines on invasive pneumococcal disease in older adults. Int J Infect Dis 2019; 86:122–30. [DOI] [PubMed] [Google Scholar]
- 10. Pelton SI, Bornheimer R, Doroff R, Shea KM, Sato R, Weycker D. Decline in pneumococcal disease attenuated in older adults and those with comorbidities following universal childhood PCV13 immunization. Clin Infect Dis 2019; 68:1831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simonsen L, Taylor RJ, Young-Xu Y, Haber M, May L, Klugman KP. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. mBio 2011; 2:e00309–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kleynhans J, Tempia S, Shioda K, Von Gottberg A, Weinberger DM, Cohen C. Estimated impact of the pneumococcal conjugate vaccine on pneumonia mortality in South Africa, 1999 through 2016: an ecological modelling study. PLoS Med 2021; 18:e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewnard JA, Hanage WP. Making sense of differences in pneumococcal serotype replacement. Lancet Infect Dis 2019; 19:e213–20. [DOI] [PubMed] [Google Scholar]
- 14. Flasche S, Lipsitch M, Ojal J, Pinsent A. Estimating the contribution of different age strata to vaccine serotype pneumococcal transmission in the pre vaccine era: a modelling study. BMC Med 2020; 18:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shioda K, Schuck-Paim C, Taylor RJ, et al. Challenges in estimating the impact of vaccination with sparse data. Epidemiology 2019; 30:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shioda K, Cai J, Warren JL, Weinberger DM. Incorporating information on control diseases across space and time to improve estimation of the population-level impact of vaccines. Epidemiology 2021; 32:360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. International Vaccine Access Center . Pneumococcal conjugate vaccine (PVC). VIEW-hub. https://view-hub.org/vaccine/pcv. Accessed 8 February 2024.
- 18. Pan American Health Organization/World Health Organization . Lineamientos básicos para el análisis de la mortalidad [in Spanish]. Washington, DC: WHO, 2017. [Google Scholar]
- 19. Instituto de Salud Pública de Chile . Vigilancia de Laboratorio de Streptococcus pneumoniae procedente de enfermedad invasora. Chile, 2012–2021. https://www.ispch.cl/wp-content/uploads/2022/12/BoletinNeumococo-26122022A-1.pdf. Accessed 8 February 2024.
- 20. Plummer M, Stukalov A, Denwood M. rjags: Bayesian graphical models using MCMC. https://rdrr.io/cran/rjags/. Accessed 30 January 2023.
- 21. Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017; 46:348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Von Gottberg A, De Gouveia L, Tempia S, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. New Engl J Med 2014; 371:1889–99. [DOI] [PubMed] [Google Scholar]
- 23. Sotomayor RJ, Toscano CM, Choez XS, et al. Impact of pneumococcal conjugate vaccine on pneumonia hospitalization and mortality in children and elderly in Ecuador: time series analyses. Vaccine 2020; 38:7033–9. [DOI] [PubMed] [Google Scholar]
- 24. Pan American Health Organization . Basic guidelines for the analysis of mortality. Washington, DC: PAHO, 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.