Abstract
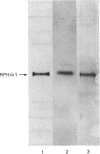
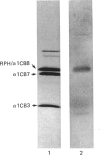
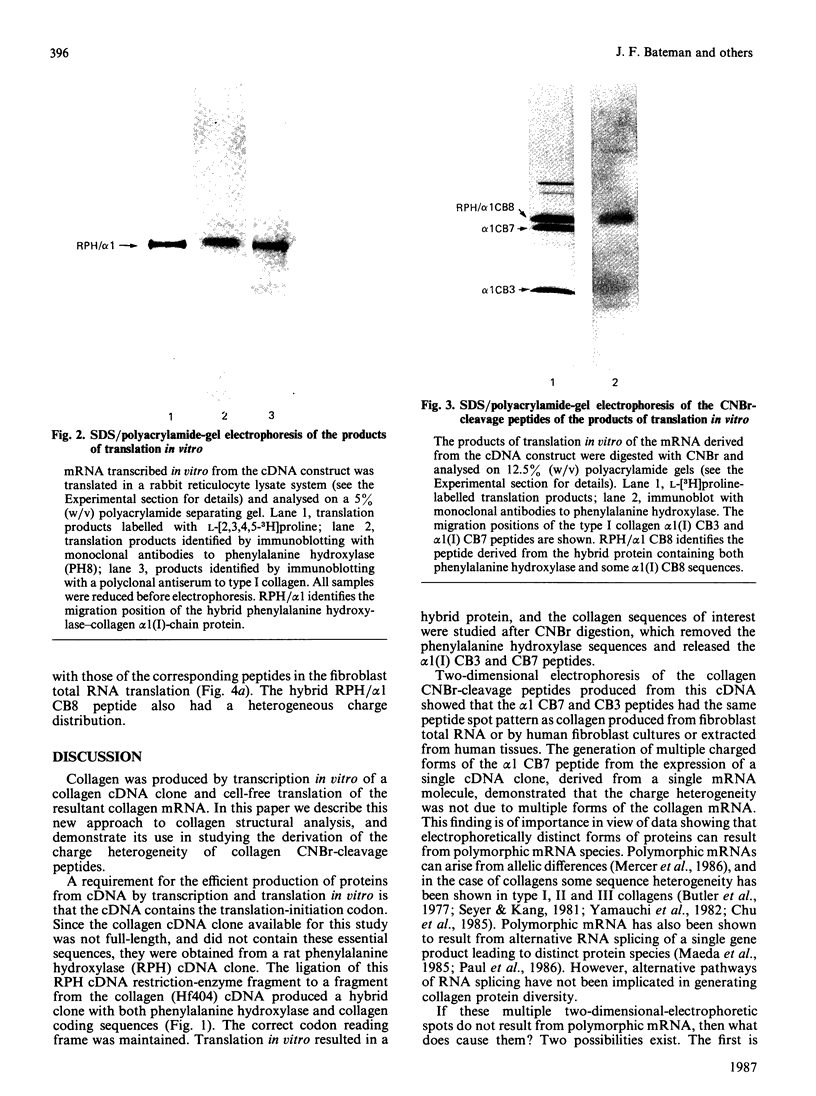
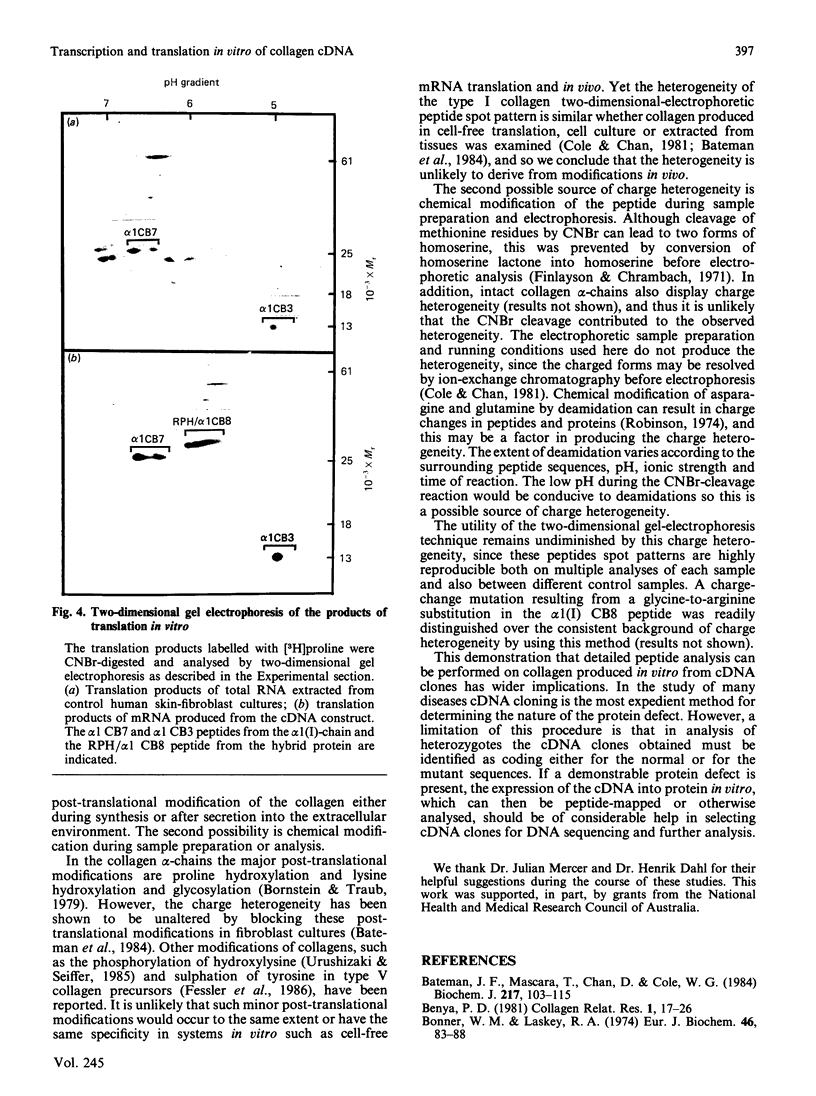
When collagen CNBr-cleavage peptides are analysed by two-dimensional gel electrophoresis each peptide is resolved into a reproducible set of charged forms. To test whether this peptide heterogeneity resulted from polymorphic mRNA, collagen was produced by transcription and translation in vitro of a collagen cDNA clone, and the peptides were mapped by two-dimensional gel electrophoresis. A cDNA construct was produced by ligation of the 5' end of the rat phenylalanine hydroxylase cDNA [Dahl & Mercer (1986) J. Biol. Chem. 261, 4148-4153], containing the translation-initiation codon, to a human alpha 1(I) cDNA [Chu, Myers, Bernard, Ding & Ramirez (1982) Nucleic Acids Res. 10, 5925-5934] coding for a large portion of helical region including the complete CB7 and CB3 CNBr-cleavage peptides. This cDNA construct was ligated into the transcription vector pSP65, and cell-free translation of the mRNA transcribed from the pSP65 plasmid was performed with a rabbit reticulocyte lysate system. After CNBr cleavage of the hybrid protein translation products, the collagen CB7 and CB3 peptides were resolved by two-dimensional electrophoresis into the same multiple charged forms whether the mRNA was produced from the cDNA construct or was extracted from normal fibroblast cultures. This result demonstrated that the multiple peptide spots were not due to polymorphic mRNA species. The heterogeneity must result from some uncharacterized specific post-translational modification or chemical alterations during sample preparation. This method of expression and analysis of proteins from cDNA clones should be of considerable use in the identification and characterization of clones that code for mutant proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bateman J. F., Mascara T., Chan D., Cole W. G. Abnormal type I collagen metabolism by cultured fibroblasts in lethal perinatal osteogenesis imperfecta. Biochem J. 1984 Jan 1;217(1):103–115. doi: 10.1042/bj2170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D. Two-dimensional CNBr peptide patterns of collagen types I, II and III. Coll Relat Res. 1981;1(1):17–26. doi: 10.1016/s0174-173x(80)80004-5. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Finch J. E., Jr, Miller E. J. The covalent structure of cartilage collagen. Evidence for sequence heterogeneity of bovine alpha1(II) chains. J Biol Chem. 1977 Jan 25;252(2):639–643. [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M. L., Weil D., de Wet W., Bernard M., Sippola M., Ramirez F. Isolation of cDNA and genomic clones encoding human pro-alpha 1 (III) collagen. Partial characterization of the 3' end region of the gene. J Biol Chem. 1985 Apr 10;260(7):4357–4363. [PubMed] [Google Scholar]
- Cole W. G., Chan D. Analysis of the heterogeneity of human collagens by two-dimensional polyacrylamide-gel electrophoresis. Biochem J. 1981 Aug 1;197(2):377–383. doi: 10.1042/bj1970377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole W. G., Chan D., Hickey A. J., Wilcken D. E. Collagen composition of normal and myxomatous human mitral heart valves. Biochem J. 1984 Apr 15;219(2):451–460. doi: 10.1042/bj2190451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl H. H., Mercer J. F. Isolation and sequence of a cDNA clone which contains the complete coding region of rat phenylalanine hydroxylase. Structural homology with tyrosine hydroxylase, glucocorticoid regulation, and use of alternate polyadenylation sites. J Biol Chem. 1986 Mar 25;261(9):4148–4153. [PubMed] [Google Scholar]
- Epstein E. H., Jr (Alpha1(3))3 human skin collagen. Release by pepsin digestion and preponderance in fetal life. J Biol Chem. 1974 May 25;249(10):3225–3231. [PubMed] [Google Scholar]
- Fessler L. I., Brosh S., Chapin S., Fessler J. H. Tyrosine sulfation in precursors of collagen V. J Biol Chem. 1986 Apr 15;261(11):5034–5040. [PubMed] [Google Scholar]
- Finlayson G. R. Isoelectric focusing in polyacrylamide gel and its preparative application. Anal Biochem. 1971 Apr;40(2):292–311. doi: 10.1016/0003-2697(71)90388-5. [DOI] [PubMed] [Google Scholar]
- Gay S., Miller E. J. Characterization of lens capsule collagen: evidence for the presence of two unique chains in molecules derived from major basement membrane structures. Arch Biochem Biophys. 1979 Dec;198(2):370–378. doi: 10.1016/0003-9861(79)90509-5. [DOI] [PubMed] [Google Scholar]
- Jennings I. G., Russell R. G., Armarego W. L., Cotton R. G. Functional analysis of the effect of monoclonal antibodies on monkey liver phenylalanine hydroxylase. Biochem J. 1986 Apr 1;235(1):133–138. doi: 10.1042/bj2350133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C. P., Slate D. L., Gravel R., Ruddle F. H. Biological detection of specific mRNA molecules by microinjection. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4503–4506. doi: 10.1073/pnas.76.9.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Kim H. S., Azen E. A., Smithies O. Differential RNA splicing and post-translational cleavages in the human salivary proline-rich protein gene system. J Biol Chem. 1985 Sep 15;260(20):11123–11130. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J. F., McAdam W., Chambers G. W., Walker I. D. The W and L allelic forms of phenylalanine hydroxylase in the rat differ by a threonine to isoleucine substitution. Biochem J. 1986 Jun 15;236(3):679–683. doi: 10.1042/bj2360679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of a collagen from chick cartilage containing three identical alpha chains. Biochemistry. 1971 Apr 27;10(9):1652–1659. doi: 10.1021/bi00785a024. [DOI] [PubMed] [Google Scholar]
- Paul J. I., Schwarzbauer J. E., Tamkun J. W., Hynes R. O. Cell-type-specific fibronectin subunits generated by alternative splicing. J Biol Chem. 1986 Sep 15;261(26):12258–12265. [PubMed] [Google Scholar]
- Robinson A. B. Evolution and the distribution of glutaminyl and asparaginyl residues in proteins. Proc Natl Acad Sci U S A. 1974 Mar;71(3):885–888. doi: 10.1073/pnas.71.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P. G., Veis A. The cyanogen bromide peptides of bovine soluble and insoluble collagens. I. Characterization of peptides from soluble type I collagen by sodium dodecylsulphate polyacrylamide gel electrophoresis. Connect Tissue Res. 1976;4(2):107–116. doi: 10.3109/03008207609152206. [DOI] [PubMed] [Google Scholar]
- Seyer J. M., Kang A. H. Covalent structure of collagen: amino acid sequence of alpha 1(III)-CB9 from type III collagen of human liver. Biochemistry. 1981 Apr 28;20(9):2621–2627. doi: 10.1021/bi00512a040. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szewczyk B., Kozloff L. M. A method for the efficient blotting of strongly basic proteins from sodium dodecyl sulfate-polyacrylamide gels to nitrocellulose. Anal Biochem. 1985 Nov 1;150(2):403–407. doi: 10.1016/0003-2697(85)90528-7. [DOI] [PubMed] [Google Scholar]
- Urushizaki Y., Seifter S. Phosphorylation of hydroxylysine residues in collagen synthesized by cultured aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3091–3095. doi: 10.1073/pnas.82.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake S. A., Mercer J. F. Induction of metallothionein mRNA in rat liver and kidney after copper chloride injection. Biochem J. 1985 Jun 1;228(2):425–432. doi: 10.1042/bj2280425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M., Noyes C., Kuboki Y., Mechanic G. L. Collagen structural microheterogeneity and a possible role for glycosylated hydroxylysine in type I collagen. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7684–7688. doi: 10.1073/pnas.79.24.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]