Abstract
Background
Osimertinib is an irreversible third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). It is the preferred first-line treatment for EGFR-mutated non-small cell lung cancer (NSCLC) compared to first-generation EGFR-TKIs. However, limited research has compared its clinical effectiveness with second-generation (2nd G) EGFR-TKIs.
Materials and methods
This study recruited patients diagnosed with stage IIIb-IV EGFR-mutated NSCLC who received first-line treatment with either 2nd G EGFR-TKIs (afatinib and dacomitinib) or osimertinib between April 2020 and April 2023.
Results
The final analysis included 168 patients, of whom 113 received 2nd G EGFR-TKIs (afatinib or dacomitinib) and 55 received osimertinib. The median progression-free survival (PFS) did not differ significantly between 2nd G EGFR-TKIs and osimertinib (del 19: 17.6 months; L858R: 20.0 months vs. 28.3 months, p = 0.081). In patients with the EGFR exon 19 deletion, osimertinib conferred a longer median PFS (28.3 vs. 17.6 months, p = 0.118) and time to treatment failure (30.2 vs. 22.7 months, p = 0.722) than 2nd G EGFR-TKIs. However, the differences were not statistically significant. In patients with with the EGFR exon 19 deletion and central nervous system metastasis, the median PFS did not differ significantly between those treated with osimertinib (14.3 months) and those treated with 2nd G EGFR-TKIs (17.6 months; p = 0.881). Multivariate regression analysis revealed that the NSCLC stage was the only independent negative predictor of PFS. The treatment patterns in the second line also differed significantly between groups (p = 0.008).
Conclusions
This study found comparable effectiveness between osimertinib and 2nd G EGFR-TKIs as first-line treatment for advanced EGFR-mutated NSCLC, with only the NSCLC stage identified as a negative predictor of PFS. However, whether the different second-line treatments affect overall survival should be examined.
Keywords: NSCLC, EGFR, Osimertinib, Afatinib, Dacomitinib
Introduction
Lung cancer is a common malignancy worldwide and is now a major cause of cancer-related deaths. Non-small cell lung cancer (NSCLC) accounts for 80%–85% of all lung cancer cases [1]. With the advent of genomic medicine, precision oncology has proven effective in improving treatment outcomes compared to conventional chemotherapy [2]. In Asia, 50%–60% of patients with lung adenocarcinoma have epidermal growth factor receptor (EGFR) mutations [3]. Most EGFRmutations occur within the tyrosine kinase domain (exons 18–21). Notably, in-frame deletions in exon 19 (19Del) and the L858R point mutation (c.2573T>G, p.Leu858Arg) in exon 21 are among the most common mutations observed, driving tumorigenesis and tumor growth by activating the EGFR pathway [4]. EGFR-tyrosine kinase inhibitors (TKIs) have become the established first-line treatment for advanced EGFR-mutated NSCLC. Three generations of EGFR-TKIs are currently available for use as first-line treatment in clinical practice.
First-generation EGFR-TKIs (erlotinib and gefitinib) reversibly inhibit the EGFR’s ATP binding sites, blocking downstream signaling. In the IPASS trial [5], gefitinib outperformed traditional chemotherapy in patients with EGFR-positive metastatic NSCLC, who showed improved progression-free survival (PFS), response rates, and quality of life. Similarly, the EURTAC trial [6] demonstrated erlotinib’s superiority in PFS and response rates over chemotherapy in patients with advanced EGFR-mutated NSCLC.
Second-generation EGFR-TKIs (afatinib and dacomitinib) form irreversible bonds with ERBB receptors, providing an alternative treatment for patients who develop resistance to first-generation EGFR-TKIs. In the LUX-Lung 7 study [7], afatinib demonstrated significant improvements compared to gefitinib in patients with EGFR-mutated NSCLC. Additionally, dacomitinib notably enhanced PFS compared to gefitinib as a first-line treatment for patients with EGFR-mutated NSCLC [8].
Third-generation EGFR-TKIs (osimertinib) target both sensitizing and T790M EGFRmutations in tumors, the cause of resistance in about 50% of patients treated with first- and second-generation EGFR-TKIs. In the FLAURA trial, first-line osimertinib significantly improved PFS and overall survival (OS) compared to first-generation EGFR TKIs [9, 10]. The second- and third-generation EGFR-TKIs appear to confer superior clinical efficacy to first-generation EGFR-TKIs.
While osimertinib is the favored EGFR-TKI for first-line treatment, the ambiguity regarding subsequent therapeutic strategies after osimertinib failure requires thoughtful consideration. In this context, the sequential administration of afatinib and osimertinib shows promise for Asian patients with EGFR-mutated NSCLC, especially those with T790M-mediated resistance and del19-positive disease [11–13]. In many countries, osimertinib is not fully covered by insurance for first-line treatment. In Taiwan, osimertinib is only reimbursed for patients with a del19 mutation. Therefore, the use of second-generation EGFR-TKIs as a subsequent treatment option following osimertinib failure remains a favorable strategy. Whether to choose second-generation EGFR-TKIs or osimertinib for initial treatment is still being debated. However, no randomized controlled trials have compared second-generation EGFR-TKIs with osimertinib. Nonetheless, most real-world studies did not strongly favor osimertinib over afatinib in achieving longer median PFS and OS during first-line treatment [14–17].
To our knowledge, no studies have compared the efficacy of osimertinib and second-generation EGFR TKIs (afatinib or dacomitinib) as first-line treatments for EGFR-mutated NSCLC. Our single-center retrospective study aimed to assess and compare the efficacy of second-generation EGFR-TKIs and osimertinib as first-line treatment for Taiwanese patients with advanced EGFR-mutated NSCLC.
Material and methods
Eligible patients
This retrospective study was conducted at China Medical University Hospital, a tertiary medical center in central Taiwan. It aimed to analyze patients diagnosed with advanced-stage EGFR-mutated NSCLC between April 2020 and April 2023. An advanced stage was defined as stages IIIB-IV according to the American Joint Committee on Cancer, Eighth Edition [18]. Patient data were extracted and reviewed from electronic medical records, including sex, age, smoking history, Eastern Cooperative Oncology Group Performance Status (ECOG PS), TNM stage at initial diagnosis, distant metastasis patterns, EGFR mutation subtype, first-line and subsequent treatments, T790M status after disease progression, and treatment duration.
Assessment of treatment and safety
All enrolled patients underwent computed tomography (CT) examinations, brain imaging (e.g., brain CT or magnetic resonance imaging [MRI]) in response to changes in neurological symptoms, and positron emission tomography (PET) at the initial diagnosis to establish baseline staging. During EGFR-TKI therapy, chest CT scans were conducted every 12–16 weeks to evaluate the tumor response, which is required for National Health Insurance reimbursement. Additional imaging modalities, including brain CT or MRI, were obtained and interpreted by a radiologist. PFS was defined as the duration from initiating EGFR-TKI therapy to radiological progression (according to the Response Evaluation Criteria in Solid Tumors v1.1) [19], death, or censorship at the last follow-up date (October 1, 2023). OS was defined as the time between initiating EGFR-TKI therapy and death or censorship at the last follow-up date (October 1, 2023). Time to treatment failure (TTF) was defined as the period from initiating EGFR-TKI therapy to its premature discontinuation. The EGFR mutation status in tumor tissue was assessed using version 2 of the cobas® EGFR Mutation Test (Roche Molecular Systems, Pleasanton, CA, USA).
Statistical analyses
Statistical analyses were conducted using MedCalc for Windows (version 18.10; MedCalc Software, Ostend, Belgium). Data are presented using descriptive statistics, using means (standard deviations) or medians (interquartile ranges) for normally and non-normally distributed variables, respectively. Data were compared between groups using a t-test for normally distributed continuous variables and a Kruskal–Wallis test for ordinal and non-normally distributed variables. Categorical variables are presented as numbers (percentages) and compared between groups using a chi-square test. Survival analyses, encompassing PFS and OS, were conducted using the Kaplan–Meier method. Prognostic factors were identified through Cox proportional hazards regression analysis. The hazard ratio (HR) for mortality was calculated for each variable in univariate analyses, and significant variables and clinically important factors were incorporated into the multivariate regression model. The strength of association is presented as the HR with its associated 95% confidence interval (CI). A p-value of <0.05 was considered statistically significant in all analyses.
Results
Patients’ characteristics
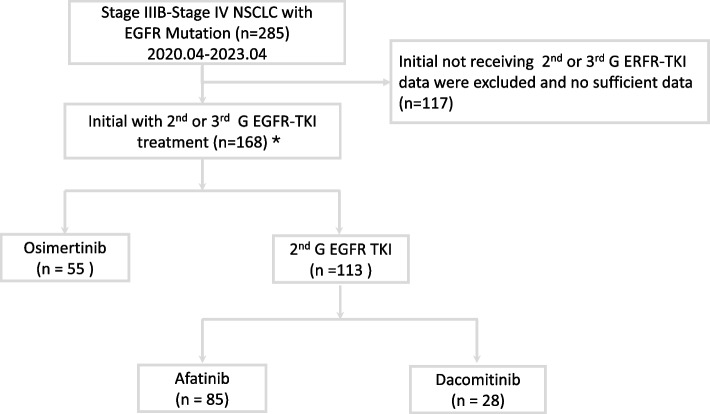
As shown in Fig. 1, 285 patients were initially screened between April 2020 and April 2023, of whom 168 received either second-generation EGFR-TKIs or osimertinib. The 168 enrolled patients were divided into two groups based on their first-line treatment: 55 received osimertinib, and 113 received second-generation EGFR-TKIs (85 received afatinib, and 28 received dacomitinib). Notably, all patients with the EGFR p.L858R variant were treated with second-generation TKIs due to health insurance coverage constraints.
Fig. 1.
Flowchart of patient enrollment. Key: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; NSCLC, non-small cell lung cancer; 2nd G, second generation; 3rd G, third generation (osimertinib). *First-line treatment with osimertinib or second-generation EGFR-TKIs is influenced by the reimbursement policies of Taiwan's health insurance system
Table 1 shows the enrolled patients’ baseline characteristics. Among the 168 patients, 89 (53.0%) were aged ≥65 years, 64 (38.1%) were male, and 51 (30.4%) had a history of smoking. Only 12 individuals (7.2%) had an ECOG PS of ≥2. Sex, age, and distant metastasis patterns did not differ significantly between patients who received osimertinib and second-generation EGFR TKIs. However, significantly fewer patients with a smoking history received osimertinib. Regarding EGFR mutation subtypes, 96 cases (57.1%) had a del19 mutation, 68 cases (40.5%) had the L858R point mutation in exon 21, and four cases (2.4%) had other mutations: One patient in the 2nd G EGFR-TKIs group had an L861Q/G719X mutation, another had an L861Q mutation, and two patients in the osimertinib group had a del19 mutation plus a de novo T790M mutation. Notably, patients who received osimertinib mostly had a del19 mutation, emphasizing the influence of coverage on treatment choices. Regarding tumor treatment response in the first three months, second-generation EGFR-TKIs conferred a higher partial response rate than osimertinib (88 cases [77.9%] vs. 30 cases [54.5%]; Table 1).
Table 1.
Patients’ baseline characteristics
| All (n = 168) | Osimertinib (n = 55) | 2nd-G EGFR-TKI (n = 113) | p-value | |
|---|---|---|---|---|
| Age ≥ 65 years | 89 (53.0) | 50 (54.5) | 59 (52.2) | 0.776 |
| Male | 64 (38.1) | 18 (32.7) | 46 (40.7) | 0.319 |
| Smoking | 51 (30.4) | 9 (16.4) | 42 (37.2) | 0.006 |
| ECOG PS ≥ 2 | 12 (7.2) | 6 (10.9) | 6 (5.4) | 0.198 |
| EGFR mutation | < 0.001 | |||
| Del 19 | 96 (57.1) | 53 (96.4) | 43 (38.1) | |
| L858R | 68 (40.5) | 0 (0) | 68 (60.2) | |
| Othersa | 4 (2.4) | 2 (3.2) | 2 (1.8) | |
| Metastasis organ | ||||
| Lung metastasis | 64 (38.1) | 25 (45.5) | 39 (34.5) | 0.172 |
| LN metastasis | 122 (72.6) | 36 (65.5) | 86 (76.1) | 0.147 |
| Pleural metastasis | 69 (41.1) | 19 (34.5) | 50 (44.2) | 0.232 |
| Liver metastasis | 19 (11.3) | 5 (9.1) | 14 (12.4) | 0.527 |
| Bone Metastasis | 65 (38.7) | 22 (40.0) | 43 (38.7) | 0.808 |
| CNS metastasis | 34 (20.2) | 15 (27.3) | 19 (16.8) | 0.114 |
| Adrenal metastasis | 7 (4.2) | 3 (5.5) | 4 (3.5) | 0.561 |
| Stage | 0.479 | |||
| IIIb/IIIc | 10 (6.0) | 4 (7.3) | 6 (5.3) | |
| IVa | 72 (42.9) | 20 (36.4) | 52 (46.0) | |
| IVb | 86 (51.2) | 31 (56.4) | 55 (48.7) | |
| Combination Treatment | ||||
| Anti-VEGF | 18 (10.7) | 4 (7.3) | 14 (12.4) | 0.315 |
| RT | 50 (29.8) | 14 (25.5) | 36 (31.9) | 0.395 |
| Response | 0.004 | |||
| Partial response | 118 (70.2) | 30 (54.5) | 88 (77.9) | |
| Stable disease | 46 (27.4) | 24 (43.6) | 22 (19.5) | |
| Progressive disease | 4 (2.4) | 1 (1.8) | 3 (2.7) | |
CNS Central nervous system, ECOG PS Eastern Cooperative Oncology Group performance status, EGFR Epidermal growth factor receptor, LN Lymph node, RT Radiotherapy, VEGF Vascular endothelial growth factor
Categorical variables are presented as numbers (percentages)
aOne patient in the 2nd G EGFR-TKIs group had an L861Q/G719X mutation, another had an L861Q mutation, and two patients in the osimertinib group had a del19 mutation plus a de novo T790M mutation.
Survival outcome
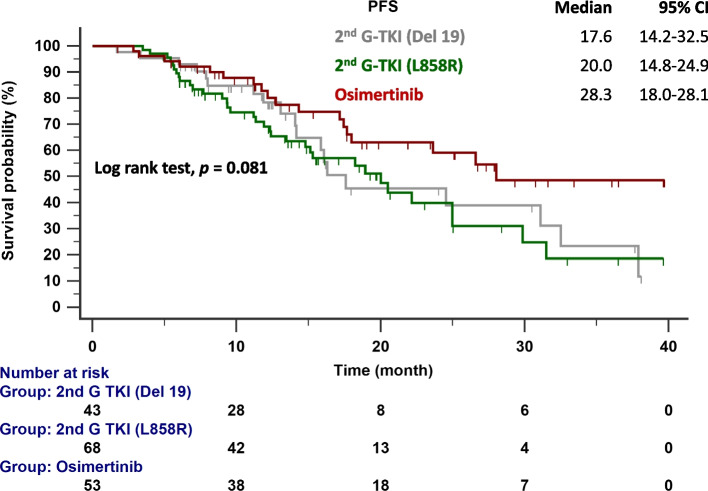
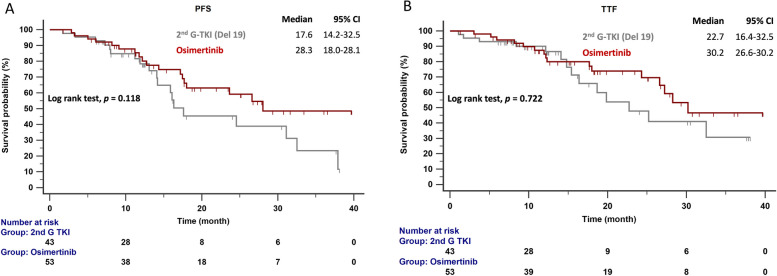
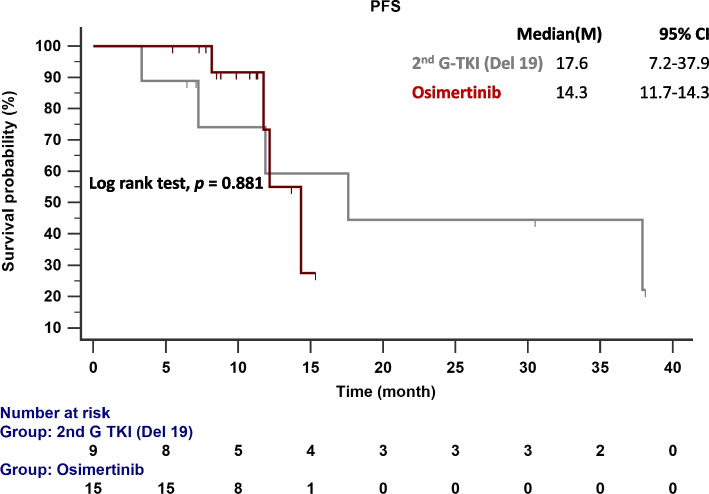
The follow-up ended on October 1, 2023. The median follow-up time was 19.3 months (range = 19.2–23.3 months). Figure 2 compares the median PFS between patients given second-generation EGFR-TKIs and osimertinib. The median PFS for patients with the del19 and L858R mutations given second-generation EGFR-TKIs was 17.6 and 20.0 months, respectively. In contrast, patients given osimertinib had a median PFS of 28.3 months (p = 0.081). In patients with EGFR del19 mutations (Fig. 3A), the median PFS was 28.3 months with osimertinib and 17.6 months with second-generation EGFR-TKIs (p = 0.118). Figure 3B compares the TTF in patients with del19 mutations who received osimertinib (30.2 months) and second-generation EGFR-TKIs (22.7 months; p = 0.722). In patients with del19 mutations and central nervous system (CNS) metastasis, the median PFS was 14.3 months with osimertinib and 17.6 months with second-generation EGFR-TKIs (p =0.881; Fig. 4).
Fig. 2.
PFS in patients with EGFR-mutated NSCLC treated with osimertinib and second-generation EGFR-TKIs. Key: EGFR, epidermal growth factor receptor; 2nd G, second generation; PFS, progression-free survival; TKI, tyrosine kinase inhibitor
Fig. 3.
A PFS and (B) TTF in patients with EGFR-mutated (del19) NSCLC treated with osimertinib and second-generation EGFR-TKIs. Key: EGFR, epidermal growth factor receptor; 2nd G, second generation; PFS, progression-free survival; TTF, time to treatment failure; TKI, tyrosine kinase inhibitor
Fig. 4.
PFS in patients with EGFR-mutated NSCLC and initial brain metastasis treated with osimertinib and second-generation EGFR-TKIs. Key: EGFR, epidermal growth factor receptor; 2nd G, second generation; PFS, progression-free survival; TKI, tyrosine kinase inhibitor
Independent predictor of PFS in patients with EGFR mutated NSCLC
Univariate regression analyses identified potential predictors of PFS, including non-smoker status (HR = 0.783, 95% CI = 0.62–0.99), lung cancer stage (HR = 1.726, 95% CI = 1.17–2.54), and osimertinib use (HR = 0.727, 95% CI = 0.55–0.94). However, multivariate regression analysis revealed that the NSCLC stage was the only independent negative predictor of PFS (Table 2).
Table 2.
Univariate and multivariate analyses of progression-free survival
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age ≥ 65 | 0.833 | 0.53–1.32 | 0.441 | 0.992 | 0.61–1.61 | 0.975 |
| Sex, male | 1.456 | 0.92-2.31 | 0.113 | 1.311 | 0.75-2.27 | 0.334 |
| Non-Smoking | 0.783 | 0.62-0.99 | 0.047 | 0.897 | 0.67-1.19 | 0.459 |
| Stage | 1.726 | 1.17-2.54 | 0.005 | 1.617 | 1.07-2.43 | 0.021 |
| ECOG ≥ 2 | 1.872 | 0.81–4.34 | 0.179 | 1.366 | 0.53-3.49 | 0.515 |
| L858R | 1.519 | 0.95-2.43 | 0.082 | 1.076 | 0.59-1.95 | 0.809 |
| Osimertinib | 0.727 | 0.55-0.95 | 0.024 | 0.813 | 0.58-1.14 | 0.241 |
Second-line treatment pattern
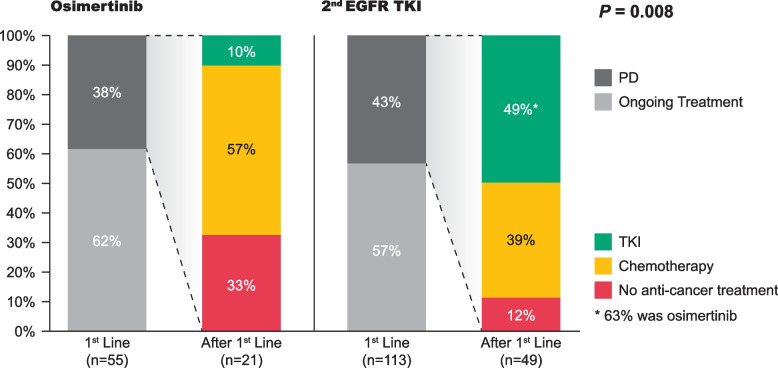
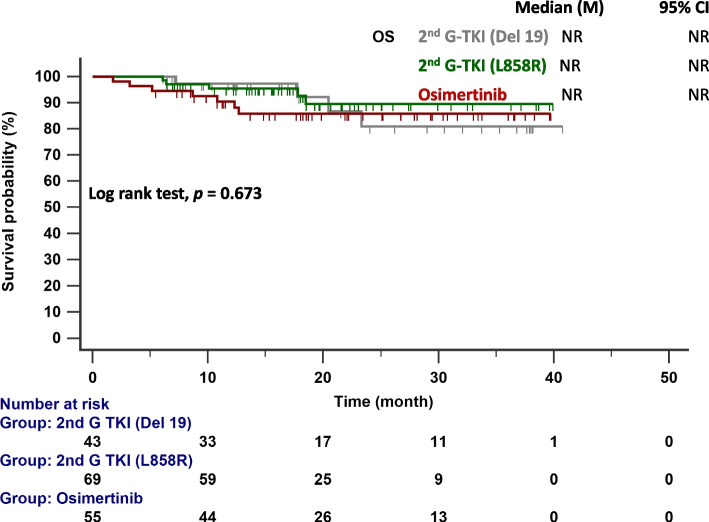
A notable shift in treatment patterns was observed within our study cohort. Figure 5 shows a significant difference in second-line treatment patterns between patients treated with osimertinib and second-generation EGFR-TKIs (p = 0.008). Disease progression occurred in 43% of patients given second-generation EGFR-TKIs and 38% of patients given osimertinib as first-line treatment. Among those given second-generation EGFR-TKIs as the first-line treatment, the predominant choices for second-line treatment were chemotherapy (39%) and and TKI (49%), with 63% of the latter choosing osimertinib (31%). In contrast, among those given osimertinib as the first-line treatment, chemotherapy (57%) was the most common choice for second-line treatment. However, approximately one-third of patients in this subgroup opted not to pursue additional anticancer treatment. The median OS was not reached in either group (p = 0.673; Fig. 6).
Fig. 5.
Subsequent treatment regimens for patients who experienced disease progression while on osimertinib and second-generation EGFR-TKIs. Key: EGFR, epidermal growth factor receptor; 2nd G, second generation; PD, progressive disease; TKI, tyrosine kinase inhibitor
Fig. 6.
OS in patients with EGFR-mutated NSCLC treated with osimertinib and second-generation EGFR-TKIs. Key: EGFR, epidermal growth factor receptor; 2nd G, second generation; OS, overall survival; TKI, tyrosine kinase inhibitor
Discussion
This study is the first to compare the clinical effectiveness of second-generation EGFR-TKIs (dacomitinib or afatinib) and osimertinib in Taiwanese patients with NSCLC with common EGFR mutations. Our findings indicate that, in real-world scenarios, both second-generation EGFR-TKIs and osimertinib confer comparable median PFS and TTF. Notably, median PFS did not differ significantly between these two treatment strategies in patients with initial brain metastasis. The initial disease stage independently predicts PFS. Moreover, second-line treatment patterns differed significantly between second-generation EGFR-TKIs (dacomitinib or afatinib) and osimertinib.
In treating advanced EGFR-mutated NSCLC, the FLAURA trial and its subsequent studies [10, 20, 21] have demonstrated the survival benefit of osimertinib as first-line therapy compared to first-generation EGFR-TKIs. Its safety profile and efficacy have led to its designation as a first-line treatment. However, inconsistent results have been reported in the Asian population and among EGFR mutation subtypes (the L858R mutation in exon 21) [21]. There is also limited research on whether osimertinib is superior to second-generation EGFR-TKIs as first-line treatment for EGFR-mutated NSCLC. Few real-world studies have investigated using osimertinib and afatinib as first-line treatment for EGFR-mutant NSCLC (Table 3) [14–17].
Table 3.
Comparative studies on the first-line treatment of EGFR-mutated NSCLC with second-generation EGFR-TKIs and osimertinib
| Huang et al [13] | CJLSG1903 [14] | Mitsuya et al [15] | Sophia et al [16] | In current study* | |
|---|---|---|---|---|---|
| Country | Taiwanese | Japanese | Japanese | USA | Taiwanese |
| Patients number | 128 | 550 | 49 | 86 | 168 |
| 3rd/2nd G EGFR-TKI | Osi 47/ Afa 81 | Osi 326/ Afa 224 | Osi 34/ Afa 15 | Osi 71/ Afa 15 | Osi 55/ 2nd G TKI 113 |
| Median follow up (m) | Osi 20.1/ Afa 22.7 | Osi 9.4 / Afa 26.2 | - | Osi 22/ Afa 56 | Osi 21.9 / 2nd G TKI 17.6 |
| Del 19/L858R |
Osi (26/21) Afa (35/46) |
Osi (163/155) Afa (114/74) |
- |
Osi (52/19) Afa (14/1) |
Osi (53/0) 2nd G TKI (43/68) |
|
PFS (m) (Osi vs Afa/Daco*) |
18.8 vs 13.1; p = 0.208 |
20.5 vs 16.5; p = 0.443 |
NR vs. 23 ; p = 0.877 |
29 vs. 27.9 ; p = 0.75 |
28.3 vs 17.6 vs 20.0;a p = 0.081 |
|
OS (m) (Osi vs Afa/Daco*) |
NR vs 41.7; p = 0.553 |
25.1 vs 36.2 ; p = 0.018 |
33 vs. 36 ; p = 0.112 |
NR vs. NR ; p = 0.18 |
NR vs NR vs NR; p = 0.673 |
|
CNS PFS (m) (Osi vs Afa/Daco*) |
22.1 m vs 10.9 m; p = 0.045 |
HR = 0.60; p = 0.062 |
- | - |
14.3 m vs 17.6 m; p = 0.881 |
Afa Afatinib, Daco Dacomitinib, CNS Central nervous system, EGFR Epidermal growth factor receptor, 2nd G Second generation, HR Hazard ratio, Osi Osimertinib, OS Overall survival, PFS Progression-free survival, TKI Tyrosine kinase inhibitor, m Months, NR Not reached, USA The United States of America, vs Versus;
aosimertinib versus 2nd G-EGFR-TKIs (del 19) versus 2nd G-EGFR-TKIs (L858R)
*Only the current study includes dacomitinib
The retrospective, multicenter CJLSG1903 study [15] in Japan compared afatinib (n = 224) and osimertinib (n = 326) as first-line treatments, showing that osimertinib did not significantly extend the time to discontinuation of EGFR-TKIs (20.5 vs. 18.6 months, p = 0.204), time to treatment failure (20.5 vs. 16.0 months, p = 0.443), or PFS (20.5 vs. 16.5 months, p = 0.864) compared to afatinib. Propensity-score-adjusted OS favored afatinib over osimertinib (25.1 vs. 36.2 months, p = 0.018). However, a considerable disparity in median follow-up periods between afatinib (26.2 months) and osimertinib (9.4 months) raises concerns about potential bias, indicating that the results should be interpreted cautiously. Another real-world study conducted in Japan by Mitsuya et al. [16] reported no significant difference in median OS between osimertinib and afatinib in patients with advanced EGFR-mutated NSCLC (36 vs. 33 months, p = 0.112). However, their results should also be interpreted cautiously due to their limited sample size of 49 patients. Similarly, a real-world retrospective study conducted in the USA by Gilardone et al. [17] reported no differences in PFS or OS between patients treated with afatinib or osimertinib as first-line therapy. However, the median follow-up periods differed significantly between the afatinib (56 months) and osimertinib (22 months) groups. A Taiwanese study by Huang et al. [14] demonstrated no significant difference between osimertinib and afatinib in median PFS (18.8 vs. 13.1 months, p = 0.208) and OS (not reached vs. 41.7 months, p = 0.553).
In our observational study conducted in Taiwan, the median follow-up time was 17.6 months for patients given second-generation EGFR-TKIs and 21.9 months for patients given osimertinib. In addition, the median PFS was not significantly longer with osimertinib (28.3 months) than with second-generation EGFR-TKIs (17.6 months for NSCLC with del19 mutations and 20.0 months for NSCLC with the L858R mutation in exon 21). Notably, the second-generation EGFR-TKIs conferred comparable PFS for patients with del19 and L858R mutations. Kohsaka et al. found that 15.9% of 390 specimens from EGFR-mutated NSCLC had compound EGFR mutations. Notably, the L858R mutation (19.5%) was more common than the del19 mutation (4.7%) [22].
A recent next-generation sequencing study indicated that around 10% of patients with NSCLC have compound EGFR mutations involving multiple distinct genetic changes in the EGFRgene at initial diagnosis [23]. Patients with compound EGFR mutations typically show reduced responsiveness to EGFR-TKI therapies than those with a single EGFRmutation [24, 25]. Yang et al.validated the effectiveness of afatinib in treating NSCLC with very rare mutations (G719X, S768I, and L861Q) [26]. Li et al. demonstrated the effectiveness of dacomitinib in patients with NSCLC and rare EGFR mutations, both in first-line and subsequent treatments [27, 28]. Therefore, second-generation EGFR-TKIs appear to overcome reduced treatment responses with compound EGFR mutations. Wang et al. also reported that afatinib and osimertinib demonstrated efficacy against rare EGFR mutations, with afatinib conferring a longer PFS than osimertinib [29]. However, in our study, the osimertinib group primarily comprised patients with a del19 mutation (n=53, 96.4%) due to health insurance coverage constraints. When considering only patients with del19 mutations, osimertinib did not confer significantly longer PFS (28.3 vs. 17.6 months; p = 0.118) and TTF (30.2 vs. 22.7 months; p = 0.772) than second-generation EGFR-TKIs.
Several studies have suggested that osimertinib may reduce the risk of CNS progression compared to standard EGFR-TKIs [30, 31]. The CJLSG1903 study observed that osimertinib had a similar median PFS (HR = 0.60, p = 0.062) and time to EGFR-TKI discontinuation (HR = 0.66, p= 0.0103) to afatinib, indicating no clear advantage [15]. However, our study does not definitively establish osimertinib as superior to second-generation EGFR-TKIs in the first-line treatment of patients with NSCLC, the del19 mutation, and CNS metastasis. The observed difference in median PFS between osimertinib (14.3 months) and second-generation EGFR-TKIs (17.6 months) was not statistically significant. Huang et al. reported that patients with NSCLC and brain metastasis treated with osimertinib had significantly improved median PFS compared to those treated with afatinib. Larger-scale studies are needed to provide further clarity in this area.
Numerous small-scale studies have examined the independent predictive factors for shorter PFS in first-line EGFR-TKI treatment of EGFR-mutated NSCLC. These factors include poor ECOG PS [32], carrying the T790M mutation before treatment [33], concomitant mutations (e.g., in axin 2 [AXIN2], phospholipase C gamma 2 [PLCG2], and RAD51 paralog C [RAD51C]) and/or high marker of proliferation Ki-67 (MKI67) expression [34], tumor protein p53 (TP53) co-mutations [35], and dynamic plasma EGFR mutation status [36]. However, the results of these studies lack consistency and are not easily applied in clinical practice. Our multivariate regression analysis revealed that the NSCLC stage was the only independent negative predictor of PFS. There was no compelling evidence suggesting a significant impact on PFS between osimertinib and second-generation EGFR-TKIs, consistent with Huang et al. [14].
Our study observed significant differences in second-line treatment patterns after disease progression between osimertinib and second-generation EGFR-TKIs (p = 0.008). Upon failure of first-line afatinib treatment, chemotherapy (39%) and osimertinib (31%) are the most common second-line treatments. In contrast, upon failure of first-line osimertinib treatment, chemotherapy (57%) is the most common second-line treatments, followed by opting for no further treatment (33%).
In real-world clinical practice, such as in the GioTag [11], RESET [12], and UpSwinG [13] studies, sequential administration of afatinib and osimertinib has shown encouraging outcomes in patients with EGFR-mutated NSCLC, particularly those with del19 mutations and Asian ancestry. Interestingly, the RESET study [12] revealed that patients treated with pemetrexed-platinum doublet therapy in the second line had a comparable OS (50.0 months) to those treated with osimertinib in the second line (54.3 months) after afatinib failure (p= 0.6). In the FLAURA study [10], the OS with first-line osimertinib treatment was 38.6 months. These findings reemphasize the potential advantages of using second-generation EGFR-TKIs instead of osimertinib as the first-line treatment.
While contributing valuable insights, this study had several limitations. Firstly, its retrospective design introduces potential bias since there is an inherent risk of inaccuracies or incomplete documentation. Secondly, since it was conducted at a single center in Taiwan with a small sample size, the applicability of its findings to broader populations may be limited, suggesting the need for multicenter studies. Thirdly, given the relatively short follow-up period, the OS analysis is still in its early stages, limiting the comprehensive understanding of long-term outcomes. Fourthly, health insurance coverage constraints have impacted the data, with the osimertinib group predominantly composed of patients with del19 mutations, preventing a thorough examination of osimertinib’s effects on NSCLC with the L858R mutation in exon 21. Finally, it did not analyze adverse events associated with EGFR-TKIs, leaving an important aspect of treatment outcomes unexplored.
Conclusion
This study is the first to compare the effectiveness of second-generation EGFR-TKIs (afatinib or dacomitinib) and osimertinib in the first-line treatment of EGFR-mutated NSCLC. Its results indicate that while osimertinib prolongs PFS, it does not provide a statistically significant advantage over second-generation EGFR-TKIs. Second-line treatments differed significantly between osimertinib and second-generation EGFR-TKIs, which may impact OS in subsequent lines. Further research is needed to explore these differences and their implications for OS.
Acknowledgments
Not applicable.
Abbreviations
- CT
computed tomography
- EGFR
epidermal growth factor receptor
- EGFR-TKI
epidermal growth factor receptor tyrosine kinase inhibitor
- ECOG
Eastern Cooperative Oncology Group
- MRI
magnetic resonance imaging
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PET
positron emission tomography
- PFS
progression-free survival
- PS
performance status
- TTF
time to treatment failure
Authors’ contributions
WCC, HYC, and CYT participated in study conception and design. WCC, HYC, YCL, WCL, CHC, HJC, CYT, and TCH participated in data acquisition. WCC and HYC participated in data analysis and interpretation. WCC, CHC,and HYC drafted the manuscript, and all authors reviewed it critically for intellectual content. All authors have read and approved the final version of the manuscript.
Funding
Not applicable.
Data availability
The corresponding author is willing to provide the datasets used and/or analyzed in the current study upon reasonable request.
Declarations
Ethics approval and consent to participate
The retrospective study was approved by the Institutional Review Board of China Medical University Hospital (CMUH110-REC1-244). The need for individual patient consent was waived by the Institutional Review Board (IRB) of China Medical University Hospital (CMUH) due to the retrospective design. All methods in the study adhered to relevant guidelines and regulations, including the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hsu-Yuan Chen and Chia-Hung Chen contributed equally to this work.
Contributor Information
Wen-Chien Cheng, Email: wcchengdr@gmail.com.
Chih-Yen Tu, Email: chesttu@gmail.com.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-386. [DOI] [PubMed] [Google Scholar]
- 2.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284(1):31–53. [DOI] [PubMed] [Google Scholar]
- 5.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–74. [DOI] [PubMed] [Google Scholar]
- 6.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. [DOI] [PubMed] [Google Scholar]
- 7.Park K. Afatinib for patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer: clinical implications of the LUX-Lung 7 study. Ann Transl Med. 2016;4(23):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–66. [DOI] [PubMed] [Google Scholar]
- 9.Cho BC, Chewaskulyong B, Lee KH, et al. Osimertinib versus Standard of Care EGFR TKI as First-Line Treatment in Patients with EGFRm Advanced NSCLC: FLAURA Asian Subset. J Thorac Oncol. 2019;14(1):99–106. [DOI] [PubMed] [Google Scholar]
- 10.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382(1):41–50. [DOI] [PubMed] [Google Scholar]
- 11.Hochmair MJ, Morabito A, Hao D, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol. 2020;16(34):2799–808. [DOI] [PubMed] [Google Scholar]
- 12.Kim T, Jang TW, Choi CM, et al. Final report on real-world effectiveness of sequential afatinib and osimertinib in EGFR-positive advanced non-small cell lung cancer: updated analysis of the RESET study. Cancer Res Treat. 2023;55(4):1152–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popat S, Jung HA, Lee SY, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive NSCLC and acquired T790M: A global non-interventional study (UpSwinG). Lung Cancer. 2021;162:9–15. [DOI] [PubMed] [Google Scholar]
- 14.Huang YH, Hsu KH, Tseng JS, et al. The difference in clinical outcomes between osimertinib and afatinib for first-line treatment in patients with advanced and recurrent EGFR-mutant non-small cell lung cancer in Taiwan. Target Oncol. 2022;17(3):295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito K, Morise M, Wakuda K, et al. A multicenter cohort study of osimertinib compared with afatinib as first-line treatment for EGFR-mutated non-small-cell lung cancer from practical dataset: CJLSG1903. ESMO Open. 2021;6(3):100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitsuya S, Tsuruoka K, Kanaoka K, et al. Comparison between second- and third-generation epidermal growth factor receptor tyrosine kinase inhibitors as first-line treatment in patients with non-small-cell lung cancer: a retrospective analysis. Anticancer Res. 2021;41(10):5137–45. [DOI] [PubMed] [Google Scholar]
- 17.Gilardone S, Thapa R, Laborde J, et al. Osimertinib vs. afatinib as first-line treatment for patients with metastatic non-small cell lung cancer with an EGFR exon 19 deletion or exon 21 L858R mutation. J Thorac Dis. 2023;15(11):6115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin MB, Greene FL, Edge SB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Okada M, Kaji Y, et al. New response evaluation criteria in solid tumours-revised RECIST guideline (version 1.1). Gan To Kagaku Ryoho. 2009;36(13):2495–501. [PubMed] [Google Scholar]
- 20.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25. [DOI] [PubMed] [Google Scholar]
- 21.Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. New England J Med. 2019;382(1):41–50. [DOI] [PubMed] [Google Scholar]
- 22.Kohsaka S, Nagano M, Ueno T, et al. A method of high-throughput functional evaluation of EGFR gene variants of unknown significance in cancer. Sci Transl Med. 2017;9(416):eaan6566. [DOI] [PubMed]
- 23.Hata A, Yoshioka H, Fujita S, et al. Complex mutations in the epidermal growth factor receptor gene in non-small cell lung cancer. J Thorac Oncol. 2010;5(10):1524–8. [DOI] [PubMed] [Google Scholar]
- 24.Kauffmann-Guerrero D, Huber RM, Reu S, et al. NSCLC patients harbouring rare or complex EGFR mutations are more often smokers and might not benefit from first-line tyrosine kinase inhibitor therapy. Respiration. 2018;95(3):169–76. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi S, Canepa HM, Bailey AS, et al. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol. 2013;8(1):45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JC, Schuler M, Popat S, et al. Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: a database of 693 cases. J Thorac Oncol. 2020;15(5):803–15. [DOI] [PubMed] [Google Scholar]
- 27.Li HS, Wang SZ, Xu HY, et al. Afatinib and dacomitinib efficacy, safety, progression patterns, and resistance mechanisms in patients with non-small cell lung cancer carrying uncommon EGFR mutations: a comparative cohort study in China (AFANDA Study). Cancers (Basel). 2022;14(21):5307. [DOI] [PMC free article] [PubMed]
- 28.Li HS, Yang GJ, Cai Y, et al. Dacomitinib for advanced non-small cell lung cancer patients harboring major uncommon EGFR alterations: a dual-center, single-arm, ambispective cohort study in China. Front Pharmacol. 2022;13:919652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C, Zhao K, Hu S, Dong W, Gong Y, Xie C. Clinical outcomes of afatinib versus osimertinib in patients with non-small cell lung cancer with uncommon EGFR mutations: a pooled analysis. Oncologist. 2023;28(6):e397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res. 2016;22(20):5130–40. [DOI] [PubMed] [Google Scholar]
- 31.Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018:JCO2018783118. [DOI] [PubMed]
- 32.Teranishi S, Sugimoto C, Nagaoka S, et al. Retrospective analysis of independent predictors of progression-free survival in patients with EGFR mutation-positive advanced non-small cell lung cancer receiving first-line osimertinib. Thorac Cancer. 2022;13(19):2741–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding D, Yu Y, Li Z, Niu X, Lu S. The predictive role of pretreatment epidermal growth factor receptor T790M mutation on the progression-free survival of tyrosine-kinase inhibitor-treated non-small cell lung cancer patients: a meta-analysis. Onco Targets Ther. 2014;7:387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen R, Chen Y, Long J, et al. Aggressive progression to EGFR tyrosine kinase inhibitors in advanced NSCLC patients: concomitant mutations, prognostic indicator and subsequent management. J Cancer Res Clin Oncol. 2023;149(11):8307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roeper J, Falk M, Chalaris-Rißmann A, et al. TP53 co-mutations in EGFR mutated patients in NSCLC stage IV: a strong predictive factor of ORR, PFS and OS in EGFR mt+ NSCLC. Oncotarget. 2020;11(3):250–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tseng JS, Yang TY, Tsai CR, et al. Dynamic plasma EGFR mutation status as a predictor of EGFR-TKI efficacy in patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol. 2015;10(4):603–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author is willing to provide the datasets used and/or analyzed in the current study upon reasonable request.