Abstract
Background
The triglyceride glucose (TyG) index, defined as Ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2], provides insights into overall metabolic status. However, the association between the TyG index and gout has not been investigated. Therefore, this study explored the correlation between the TyG index and gout.
Methods
Using data from the National Health and Nutrition Examination Survey, which was conducted from 2007 to 2018, this study investigated the relationship between the TyG index and gout. Demographic data and potential risk factors were analyzed and compared using t tests for continuous data and chi-square tests for categorical data. Logistic regression and subgroup analysis were performed to examine the association between the TyG index and gout.
Results
A total of 14,924 participants were enrolled, among whom 726 (4.86%) were diagnosed with gout. Without controlling for any covariates, a significant positive correlation was observed between an elevated TyG index and increased risk of gout, with an odds ratio (OR) of 2.07 and a 95% confidence interval (CI) ranging from 1.76 to 2.43. After full adjustment, this association remained statistically significant, with an adjusted OR of 1.43 and a 95% CI from 1.14 to 1.80. Subgroup analyses revealed significant interactions, particularly for females (OR = 2.55; 95% CI: 2.00-3.26), individuals with no military service history (OR = 2.15; 95% CI: 1.66–2.43), and those without diabetes (OR = 2.00; 95% CI: 1.64–2.43).
Conclusion
A positive correlation was observed between the TyG index and gout. Consequently, further large-scale prospective studies are warranted for a comprehensive analysis of the role of the TyG index in gout.
Keywords: Gout, Triglyceride glucose index, Hyperuricemia, National health and nutrition examination survey, Cross-sectional study, Insulin resistance
Background
Gout is a clinical condition characterized by recurrent episodes of acute arthritis caused by the deposition of monosodium urate crystals as a result of an excessive uric acid concentration in the blood [1, 2]. Epidemiological studies have reported that the prevalence of gout ranges from less than 1–4% globally. Recent trends have shown a significant increase in the prevalence of gout along with the increase in metabolic syndrome components [3].
The triglyceride glucose (TyG) index, first proposed in 2010, has emerged as a novel indicator of insulin resistance—a critical aspect of metabolic dysfunction [4]. In recent years, there has been a burgeoning interest in the predictive potential of the TyG index for various metabolic conditions. Previous studies have supported the use of the TyG index as a reliable surrogate for insulin resistance, which is correlated with an increased risk of cardiovascular diseases [5–7]. However, the specific relationship between the TyG index and gouty arthritis is unclear and remains to be further investigated.
Our study aimed to bridge this knowledge gap by analyzing data from the NHANES to explore the correlation of the TyG index with gout. This study offers innovative insights into the metabolic underpinnings of gout, provides practical guidance for risk prediction, and identifies a potential locus for intervention to alleviate the burden of this metabolic disorder.
Methods
Data sources
The National Health and Nutrition Examination Survey (NHANES) is a cross-sectional survey designed to evaluate the health and nutritional conditions of both adult and pediatric populations in the U.S., especially due to its interviews and physical examinations. It is a nationally representative survey that scrutinizes a sample of the U.S. population, involving approximately 5,000 individuals annually [8]. The National Health and Nutrition Examination Survey (NHANES) was approved by the National Center for Health Statistics Ethics Review Board, and all the participants provided written informed consent. The NHANES datasets can be freely obtained from the website.
Assessment of the TyG index
The formula for calculating the TyG index is Ln [fasting triglyceride (mg/dL) × fasting glucose (mg/dL)/2] [4]. Fasting triglyceride and fasting glucose levels were extracted from the NHANES database. Subsequently, the TyG index was calculated by the formula above.
Assessment of gout diagnosis
Gout diagnosis was ascertained through a self-report questionnaire (MCQ160n), which included the following question: “Has a doctor or other health professional ever told you that you had gout?”. Participants were asked to choose between two response options: “Yes” or “No.”
Covariates
Demographic data, including age, sex (female/male), poverty status, race and ethnicity (Mexican American, non-Hispanic Black, non-Hispanic White, other Hispanic, and other races including multiple racial), marital status (married or with a partner and single), military service history (yes/no), and educational level (less than high school, high school or GED, and above high school), were collected.
Based on previous studies [9, 10], several other covariates were included in this study: serum uric acid, body mass index (BMI), coffee, drinking, smoking, hypertension, diabetes, and osteoporosis. Hyperuricemia was defined as a serum uric acid level ≥ 7 mg/dL (420 µmol/L) in men and ≥ 6 mg/dL (360 µmol/L) in women [11].
Statistical analysis
To ensure the national representativeness of the data, we used a complex sampling method provided by the NHANES for our analysis [12]. We computed both unweighted sample sizes and weighted percentages of individuals from 2007 to 2018. To assess whether there were significant differences between different groups, demographic data and potential risk factors were compared using t tests for continuous data and chi-square tests for categorical data. Logistic regression was used to examine the correlation between gout incidence and the TyG index, and odds ratios (ORs) were calculated to identify risk factors for gout. All analyses were carried out using R version 4.3.1, and P < 0.05 indicated statistical significance.
Results
Characteristics of participants
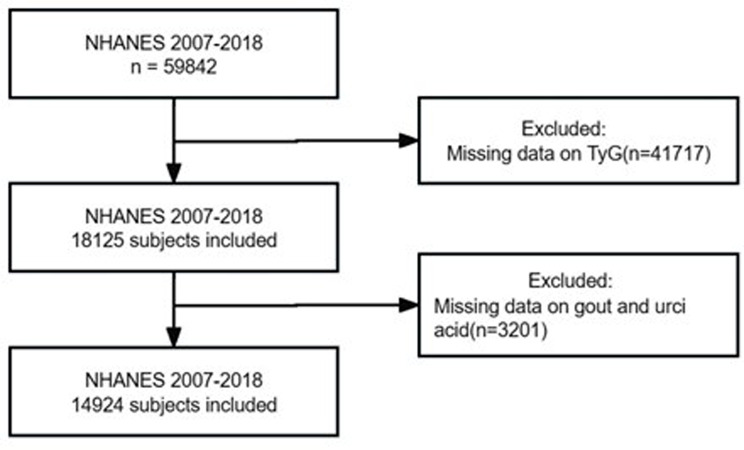
Initially, a total of 59,842 participants were enrolled from six cycles of the NHANES from 2007 to 2018 to analyze the association between triglyceride glucose index and gout. After excluding the patients whose TyG index, gout, and uric acid data were missing, the final sample size was 14,924, representing the noninstitutionalized U.S. population, which comprises 102.23 million adults (Fig. 1). In the sample, the incidence of gout was 4.86%. All procedures in the NHANES were approved by the National Center for Health Statistics Ethics Review Board, and written informed consent was obtained from participants at the time of enrollment. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for cross-sectional studies.
Fig. 1.
Flowchart of participant selection
The characteristics of the participants with gout or not were illustrated in Table 1. For the descriptive statistics, we used the mean (standard error, SE) for continuous variables, and categorical variables are expressed as the number of cases and percentage. As shown in Table 1, there were significant differences in age, sex, race, marital status, military service history, BMI, smoking, alcohol use, hypertension, diabetes, uric acid, hyperuricemia and the TyG index (all p < 0.05). No significant differences in poverty, education, coffee, or osteoporosis were found (all p > 0.05). Compared with individuals without gout, participants with gout were more likely to be older, male, black and other races, have a history of military service, have a higher BMI, have a smoking history, have a history of alcohol use, have hypertension, have diabetes, have a higher uric acid level, and have a higher TyG index.
Table 1.
The characteristics of participants with gout
| Variable | Total | Gout | Without gout | P value |
|---|---|---|---|---|
| age | 48.00(0.25) | 61.01(0.58) | 47.44(0.26) | < 0.0001 |
| gender | < 0.0001 | |||
| female | 7704(51.57) | 222( 2.75) | 7482(97.25) | |
| male | 7220(48.43) | 504( 5.54) | 6716(94.46) | |
| poverty | 2.96(0.04) | 2.97(0.10) | 2.96(0.04) | 0.93 |
| race | < 0.001 | |||
| mexican american | 2285( 8.68) | 60( 2.08) | 2225(97.92) | |
| non-hispanic black | 2983(10.43) | 175( 4.45) | 2808(95.55) | |
| non-hispanic white | 6186(66.84) | 353( 4.42) | 5833(95.58) | |
| other hispanic | 1635( 6.05) | 49( 2.39) | 1586(97.61) | |
| other race - including multi-racial | 1835( 7.99) | 89( 4.49) | 1746(95.51) | |
| marital | 0.01 | |||
| Married or with partner | 8926(63.83) | 471( 4.51) | 8455(95.49) | |
| Single | 5993(36.14) | 255( 3.39) | 5738(96.61) | |
| military service history | < 0.0001 | |||
| yes | 1594(10.23) | 183( 8.89) | 1411(91.11) | |
| no | 13,327(89.75) | 543( 3.56) | 12,784(96.44) | |
| education | 0.94 | |||
| Above high school | 7793(60.59) | 355( 4.05) | 7438(95.95) | |
| High school or GED | 3362(22.86) | 180( 4.17) | 3182(95.83) | |
| Less than high school | 3752(16.50) | 191( 4.19) | 3561(95.81) | |
| BMI(kg/m2) | 29.04(0.10) | 32.06(0.41) | 28.91(0.10) | < 0.0001 |
| coffee(g) | 325.88(7.60) | 378.33(28.80) | 323.66( 7.72) | 0.07 |
| smoke | < 0.0001 | |||
| never | 8294(55.24) | 307( 3.28) | 7987(96.72) | |
| former | 3636(25.38) | 299( 6.72) | 3337(93.28) | |
| now | 2982(19.34) | 120( 3.01) | 2862(96.99) | |
| alcohol user | < 0.001 | |||
| heavy | 2674(18.95) | 101( 3.35) | 2573(96.65) | |
| mild | 4521(33.95) | 228( 4.32) | 4293(95.68) | |
| moderate | 2001(15.52) | 76( 3.14) | 1925(96.86) | |
| never | 1875( 9.83) | 69( 3.15) | 1806(96.85) | |
| former | 2064(11.58) | 148( 6.61) | 1916(93.39) | |
| osteoporosis | 0.59 | |||
| normal | 4455(28.01) | 234( 4.57) | 4221(95.43) | |
| osteopenia | 2251(14.14) | 121( 4.93) | 2130(95.07) | |
| osteoporosis | 355( 1.95) | 16( 3.49) | 339(96.51) | |
| hypertension | < 0.0001 | |||
| yes | 5527(33.26) | 530( 8.62) | 4997(91.38) | |
| no | 9376(66.63) | 196( 1.85) | 9180(98.15) | |
| diabetes | < 0.0001 | |||
| yes | 2011(10.05) | 236(10.41) | 1775(89.59) | |
| no | 12,904(89.89) | 490( 3.40) | 12,414(96.60) | |
| uric acid(mg.dl) | 5.48(0.02) | 6.53(0.08) | 5.43(0.02) | < 0.0001 |
| hyperuricemia | < 0.0001 | |||
| yes | 2918(18.78) | 349( 9.99) | 2569(90.01) | |
| no | 12,006(81.22) | 377( 2.74) | 11,629(97.26) | |
| TyG | 8.59(0.01) | 8.95(0.05) | 8.57(0.01) | < 0.0001 |
| TyG_group | < 0.0001 | |||
| [5.64721212316781,8.151] | 3729(25.94) | 96( 2.24) | 3633(97.76) | |
| (8.151,8.572] | 3736(25.75) | 136( 2.96) | 3600(97.04) | |
| (8.572,9.02] | 3720(24.70) | 189( 4.26) | 3531(95.74) | |
| (9.02,12.8449048658017] | 3739(23.61) | 305( 7.22) | 3434(92.78) |
Logistic regression analyses
Four logistic regression models were used to investigate the correlation between gout incidence and the TyG index (Table 2). According to the crude model (Model 1), the unadjusted analysis demonstrated a significant positive correlation between gout incidence and the TyG index (OR = 2.07, 95% CI: 1.76–2.43, P < 0.0001). This implies that without adjusting for any potential confounders, for each unit increase in the TyG index, the risk of developing gout increased by 107% (95% CI: 1.76–2.43). After adjusting for age and sex in Model 2, this correlation still existed (OR = 1.80, 95% CI: 1.48–2.18, P < 0.0001). When we adjusted for race, poverty, education, marital status, military service history, coffe, smoking, and alcohol use, the TyG index still showed a significant increase in the risk of gout in Model 3. (OR = 1.95, 95% CI: 1.58–2.40, P < 0.0001). In addition, in Model 4 adjusted for all covariables, including BMI, uric acid, hypertension, and diabetes, the corrected association between gout and the TyG index was still significant (OR = 1.43, 95% CI: 1.14–1.80, P < 0.003). These results suggest that a high TyG index may be a potential risk factor for gout.
Table 2.
The correlation between the TyG index and gout incidence
| OR(95% CI) | P Value | |
|---|---|---|
| Model 1 | 2.07(1.76,2.43) | < 0.0001 |
| Model 2 | 1.80(1.48,2.18) | < 0.0001 |
| Model 3 | 1.95(1.58,2.40) | < 0.0001 |
| Model 4 | 1.43(1.14,1.80) | 0.003 |
Model 1: Without adjustment
Model 2: Adjusted for age and sex
Model 3: Model 2 + race, poverty status, education status, marital status, military service history, coffee, smoking, and alcohol use
Model 4: Model 3 + BMI, uric acid, hypertension, and diabetes
Subgroup analysis
Subgroup analyses were performed to examine the potential modification effects of various covariates on the relationship between the TyG index and gout incidence, as detailed in Table 3. The correlation between gout and the TyG index exhibited consistency across several subgroups, including race, marital status, education, smoking, alcohol use, osteoporosis, and hypertension. However, notable significant interactions were observed between sex, military status, diabetes, and correction according to the TyG index (all p < 0.05). The positive association between the TyG index and gout was particularly strong among females (OR = 2.55; 95% CI: 2.00-3.26), individuals without a history of military service (OR = 2.15; 95% CI: 1.66,2.43), and those without diabetes (OR = 2.00; 95% CI: 1.64–2.43).
Table 3.
Subgroup analysis for the correlation between gout incidence and the TyG index
| Subgroup | OR(95% CI) | P value | P for interaction |
|---|---|---|---|
| Gender | 0.001 | ||
| male | 1.72(1.43,2.05) | < 0.0001 | |
| female | 2.55(2.00,3.26) | < 0.0001 | |
| Race | 0.17 | ||
| mexican american | 1.38(1.02,1.87) | 0.04 | |
| other hispanic | 2.35(1.66,3.33) | < 0.0001 | |
| other race - including multi-racial | 2.69(1.72,4.22) | < 0.0001 | |
| non-hispanic white | 2.21(1.73,2.80) | < 0.0001 | |
| non-hispanic black | 1.84(1.50,2.25) | < 0.0001 | |
| marital | 0.54 | ||
| Married or with partner | 2.01(1.66,2.43) | < 0.0001 | |
| Single | 2.18(1.72,2.77) | < 0.0001 | |
| military service history | 0.05 | ||
| no | 2.15(1.79,2.57) | < 0.0001 | |
| yes | 1.55(1.13,2.13) | 0.01 | |
| Education | 0.15 | ||
| Less than high school | 1.86(1.52,2.28) | < 0.0001 | |
| Above high school | 2.30(1.84,2.87) | < 0.0001 | |
| High school or GED | 1.76(1.30,2.39) | < 0.001 | |
| smoke | 0.63 | ||
| never | 2.13(1.66,2.74) | < 0.0001 | |
| now | 1.75(1.25,2.44) | 0.001 | |
| former | 2.02(1.59,2.55) | < 0.0001 | |
| alcohol.user | 0.17 | ||
| heavy | 2.34(1.67,3.28) | < 0.0001 | |
| never | 1.36(0.97,1.91) | 0.07 | |
| mild | 2.27(1.71,3.01) | < 0.0001 | |
| former | 1.71(1.19,2.46) | 0.004 | |
| moderate | 2.19(1.56,3.08) | < 0.0001 | |
| osteoporosis | 0.29 | ||
| normal | 1.94(1.47,2.57) | < 0.0001 | |
| osteopenia | 1.46(1.03,2.07) | 0.04 | |
| osteoporosis | 1.53(0.74,3.17) | 0.24 | |
| Hypertension | 0.79 | ||
| no | 1.74(1.34,2.25) | < 0.0001 | |
| yes | 1.68(1.42,2.00) | < 0.0001 | |
| Diabetes | 0.01 | ||
| no | 2.00(1.64,2.43) | < 0.0001 | |
| DM | 1.36(1.07,1.74) | 0.01 | |
| Hyperuricemia | 0.31 | ||
| 0 | 1.96(1.60,2.41) | < 0.0001 | |
| 1 | 1.64(1.22,2.21) | 0.001 | |
| TyG_group | 0.05 | ||
| [5.64721212316781,8.151] | 9.62(3.33,27.77) | < 0.0001 | |
| (8.151,8.572] | 5.77(0.96,34.60) | 0.05 | |
| (8.572,9.02] | 3.52(0.50,24.91) | 0.20 | |
| (9.02,12.8449048658017] | 1.81(1.42,2.29) | < 0.0001 |
Discussion
This investigation explored the relationship between the TyG index and gout, revealing a robust significant positive correlation that persisted even after adjusting for all covariates.
Gout is the most common form of inflammatory arthritis and is caused by uric acid metabolism disorders and hyperuricemia [2]. In our study, the incidence rate of gout among patients with hyperuricemia was found to be 9.9%, which is in line with previous literature reports (8.3–18.2) [13–16]. A recent study, leveraging the NHANES database, demonstrated the predictive capacity of the TyG index in gout, reaching conclusions consistent with our findings [17]. The association between the TyG index and hyperuricemia has been a subject of interest, with several studies delving into their positive correlation [18–22]. For each unit increase in the TyG index, serum uric acid levels increased by 0.32 (95% CI: 0.31–0.33). Furthermore, individuals in the fourth quartile of the TyG index exhibited a 44.2% prevalence of hyperuricemia [19].
The triglyceride glucose (TyG) index, as an accessible measure of insulin resistance, has demonstrated significant potential in recent research for predicting a range of metabolic abnormalities, including the development of diabetes, nonalcoholic fatty liver disease, and cardiovascular disease [5–7, 18, 23]. A high TyG index is also associated with an increased incidence of diseases such as arthritis and osteoporosis [9, 24]. Our study contributes to this burgeoning field by assessing the capacity of the TyG index to predict gout.
Insulin resistance plays a key role in the occurrence and progression of hyperuricemia. There is a bidirectional relationship between insulin resistance and hyperuricemia [21]. Insulin resistance impedes uric acid clearance by enhancing sodium reabsorption in the renal tubules, while elevated uric acid levels contribute to insulin resistance by diminishing the availability of nitric oxide, exacerbating mitochondrial oxidative stress, and promoting inflammation through various pathways [25, 26]. Kottgen et al. [27] suggested that insulin resistance leads to increased serum uric acid levels by facilitating the production of ribose pyrophosphate and 5-ribose phosphate from glycolysis and pentose phosphate pathway intermediates. Insulin resistance is often accompanied by metabolic disorders such as obesity and metabolic syndrome, both of which are closely related to gout [28].
The influence of lifestyle on metabolic health cannot be overlooked. Intake of sugars, especially fructose, has the potential to simultaneously increase levels of uric acid and triglycerides, which may precipitate conditions such as gout, diabetes and metabolic syndrome. Reducing fructose intake is particularly crucial for the treatment of hyperuricemia, gout, insulin resistance, and metabolic syndrome. Diets that emphasize particular fatty acids, such as the Mediterranean and DASH diets, have demonstrated a beneficial effect on insulin resistance [29–31]. Engaging in appropriate physical training significantly mitigates inflammation and the accumulation of triglycerides in tissues, with resistance training proving particularly efficacious in enhancing glycemic stability, insulin sensitivity, and reducing hepatic lipid content [32].
Subgroup analysis in our study revealed that the relationship between TyG index and gout was pervasive across the population, with particularly strong correlations observed in females, individuals with no history of military service, and those without a history of diabetes. Previous literature has also reported differences in the correlation between lipid metabolism and uric acid between genders. Zhang et al. [1] reported that the triglyceride-glucose index tended to be a better predictor of hyperuricemia in females than in males. Additionally, a study from Italy found that triglycerides were independently associated with serum uric acid levels in women, but not in men [2]. Regional variations in diet and lifestyle could be contributing factors leading to these divergent results. The association between TyG and gout is more pronounced in individuals without a history of military service, possibly because individuals with a military service background engage in more physical activity. Regular exercise can significantly reduce inflammation and the accumulation of triglycerides in tissues [32]. The TyG index is a marker of insulin resistance, and it tends to be in a high level in individuals with diabetes, which may be the reason why the association between TyG and gout is more significant in non-diabetic patients.
In summary, the correlation between the TyG index and gout may be primarily mediated through the central mechanism of insulin resistance. Insulin resistance can directly affect uric acid metabolism and may also exacerbate hyperuricemia and gout through indirect factors such as obesity and chronic inflammation. Further fundamental and clinical research is needed in the future to elucidate the causal relationship and molecular mechanisms between the TyG index and the pathogenesis of gout. The TyG index holds potential as a convenient tool for gauging gout risk, offering new perspectives for disease prevention and management.
Despite these promising findings, our study is subject to several limitations that warrant careful consideration. Firstly, there is an inherent potential for selection bias due to the nature of the population studied and the retrospective analysis design, which may affect the generalizability of our results. Secondly, while the TyG index shows promise as a predictor of gout, it may not fully encompass the complexity of metabolic interactions involved in the disease pathogenesis. Additionally, our study’s limitations are compounded by the methodological constraints of the NHANES dataset, including recall bias and the use of self-reported questionnaires for diagnosing gout, which contrasts with the standardized diagnostic criteria employed in clinical practice. Lastly, the TyG index in our study was obtained at random times and not necessarily during the acute flare-ups of gout patients, which may introduce a certain degree of statistical bias. There may be significant differences in the TyG index between the acute and intercritical periods for gout patients, which requires further research to confirm.
Conclusion
The TyG index was positively correlated with gout. A high TyG index might be a risk factor for gout. This correction remained consistent across different subgroups. Further large-scale prospective studies are warranted for a comprehensive analysis of the role of the TyG index in gout.
Acknowledgements
Thanks to Zhang Jing (Department of Infectious Disease, Shanghai Fifth People’s Hospital, Fudan University) for his work on the NHANES database.
Author contributions
Yahui Dai and Yushan Zhang contributed equally to this article. Yahui Dai conducted the database search, prepared the extracted data for the NHANES database, and had primary responsibility in writing this article; Yushan Zhang contributed to processing and analyzing the data. Bo Wang took responsibility for the writing and reversing of the article; Lei Cao was responsible for the interpretation and description of the statistical results. Zhiyuan Wang was responsible for the design of this study. All the authors have read and approved the final manuscript.
Funding
This work was supported by the Shanghai Municipal Health Commission’s Special Clinical Research Project for the Hygiene Industry under Grant 20234Y0044.
Data availability
The datasets analyzed in this study are available from the NHANES databases. (Available from https://www.cdc.gov/nchs/nhanes/participant.htm).
Declarations
Ethics approval and consent to participate
This study involved human participants and was approved by an ethics committee(s) or institutional board(s). All procedures in each NHANES were approved by the National Center for Health Statistics Ethics Review Board, and written informed consent was obtained from participants at the time of enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yahui Dai and Yushan Zhang contributed equally to this work.
Contributor Information
Lei Cao, Email: joney19802223@sina.com.cn.
Zhiyuan Wang, Email: wangzhiyuan@tongji.edu.cn.
References
- 1.Neilson J, Bonnon A, Dickson A, Roddy E. Gout: diagnosis and management—summary of NICE guidance. BMJ-Brit Med J. 2022:o1754. [DOI] [PubMed]
- 2.FitzGerald JD, Dalbeth N, Mikuls T, Brignardello Petersen R, Guyatt G, Abeles AM, Gelber AC, Harrold LR, Khanna D, King C, et al. 2020 American College of Rheumatology Guideline for the management of gout. Arthritis Rheumatol. 2020;72(6):879–95. [DOI] [PubMed] [Google Scholar]
- 3.Dehlin M, Jacobsson L, Roddy E. Global epidemiology of gout: prevalence, incidence, treatment patterns and risk factors. Nat Rev Rheumatol. 2020;16(7):380–90. [DOI] [PubMed] [Google Scholar]
- 4.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, Jacques-Camarena O, Rodriguez-Moran M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocr Metab. 2010;95(7):3347–51. [DOI] [PubMed] [Google Scholar]
- 5.Muhammad IF, Bao X, Nilsson PM, Zaigham S. Triglyceride-glucose (TyG) index is a predictor of arterial stiffness, incidence of diabetes, cardiovascular disease, and all-cause and cardiovascular mortality: a longitudinal two-cohort analysis. Front Cardiovasc Med. 2023;9. [DOI] [PMC free article] [PubMed]
- 6.Yao Y, Wang B, Geng T, Chen J, Chen W, Li L. The association between TyG and all-cause/non-cardiovascular mortality in general patients with type 2 diabetes mellitus is modified by age: results from the cohort study of NHANES 1999–2018. Cardiovasc Diabetol. 2024;23(1). [DOI] [PMC free article] [PubMed]
- 7.Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, Ma J, Zhao Y, Zhu W, Wang J. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21(1). [DOI] [PMC free article] [PubMed]
- 8.Centers For Disease CAP: National Health and Nutrition Examination Survey. In. 2023;2023.
- 9.Zhan H, Liu X, Piao S, Rong X, Guo J. Association between triglyceride-glucose index and bone mineral density in US adults: a cross sectional study. J Orthop Surg Res. 2023;18(1). [DOI] [PMC free article] [PubMed]
- 10.Han Y, Cao Y, Han X, Di H, Yin Y, Wu J, Zhang Y, Zeng X. Hyperuricemia and gout increased the risk of long-term mortality in patients with heart failure: insights from the National Health and Nutrition Examination Survey. J Transl Med. 2023;21(1). [DOI] [PMC free article] [PubMed]
- 11.Ndrepepa G. Uric acid and cardiovascular disease. Clin Chim Acta. 2018;484:150–63. [DOI] [PubMed] [Google Scholar]
- 12.Chen TC, Clark J, Riddles MK, Mohadjer LK, Fakhouri T. National Health and Nutrition Examination Survey, 2015–2018: Sample Design and Estimation procedures. Vital Health Stat. 2020;2(184):1–35. [PubMed]
- 13.Li J, Lee J, Lu C, Su Y, Chiu C, Chen S, Geng J, Chen C. Hyperuricemia and Its Association with Osteoporosis in a Large Asian Cohort. Nutrients. 2022;14(11):2206. [DOI] [PMC free article] [PubMed]
- 14.Song J, Jin C, Shan Z, Teng W, Li J. Prevalence and risk factors of hyperuricemia and gout: a cross-sectional survey from 31 provinces in mainland China. J Transl Intern Med. 2022;10(2):134–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu R, Han C, Wu D, Xia X, Gu J, Guan H, Shan Z, Teng W. Prevalence of Hyperuricemia and Gout in Mainland China from 2000 to 2014: A Systematic Review and Meta-Analysis. Biomed Res Int. 2015;2015:1–12. [DOI] [PMC free article] [PubMed]
- 16.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–41. [DOI] [PubMed] [Google Scholar]
- 17.Cao S, Hu Y. Interpretable machine learning framework to predict gout associated with dietary fiber and triglyceride-glucose index. Nutr Metab. 2024;21(1). [DOI] [PMC free article] [PubMed]
- 18.Liu S, Zhou Z, Wu M, Zhang H, Xiao Y. Association between the Triglyceride Glucose Index and Hyperuricemia in Patients with Primary Hypertension: A Cross-Sectional Study. Int J Endocrinol. 2023;2023:1–11. [DOI] [PMC free article] [PubMed]
- 19.Lertsakulbunlue S, Sangkool T, Bhuriveth V, Mungthin M, Rangsin R, Kantiwong A, Sakboonyarat B. Associations of triglyceride-glucose index with hyperuricemia among Royal Thai Army personnel. BMC Endocr Disord. 2024;24(1). [DOI] [PMC free article] [PubMed]
- 20.Zhao Q, Zhang M, Chu Y, Ban B. Association between serum uric acid and triglyceride-glucose index in children and adolescents with short stature. Sci Rep-UK. 2023;13(1). [DOI] [PMC free article] [PubMed]
- 21.Sun J, Sun M, Su Y, Li M, Ma S, Zhang Y, Zhang A, Cai S, Cheng B, Bao Q, et al. Mediation effect of obesity on the association between triglyceride-glucose index and hyperuricemia in Chinese hypertension adults. J Clin Hypertens. 2022;24(1):47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seifi N, Nosrati M, Koochackpoor G, Aghasizadeh M, Bahari H, Namdar HB, Afkhami N, Darban RA, Azarian F, Ferns GA, et al. The association between hyperuricemia and insulin resistance surrogates, dietary- and lifestyle insulin resistance indices in an Iranian population: MASHAD cohort study. Nutr J. 2024;23(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Du T, Zhang J, Lu H, Lin X, Xie J, Yang Y, Yu X. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Y, Zhou L, La R, Jiang M, Jiang D, Huang L, Xu W, Wu Q. The association between triglyceride glucose index and arthritis: a population-based study. Lipids Health Dis. 2023;22(1). [DOI] [PMC free article] [PubMed]
- 25.Muscelli E, Natali A, Bianchi S, Bigazzi R, Galvan AQ, Sironi AM, Frascerra S, Ciociaro D, Ferrannini E. Effect of insulin on renal sodium and uric acid handling in essential hypertension. Am J Hypertens. 1996;9(8):746–52. [DOI] [PubMed] [Google Scholar]
- 26.Ter Maaten JC, Voorburg A, Heine RJ, Ter Wee PM, Donker AJ, Gans RO. Renal handling of urate and sodium during acute physiological hyperinsulinaemia in healthy subjects. Clin Sci. 1997;92(1):51–8. [DOI] [PubMed] [Google Scholar]
- 27.Kottgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, Pistis G, Ruggiero D, O’Seaghdha CM, Haller T, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thottam GE, Krasnokutsky S, Pillinger MH. Gout and metabolic syndrome: a tangled web. Curr Rheumatol Rep. 2017;19(10):60. [DOI] [PubMed] [Google Scholar]
- 29.Lubawy M, Formanowicz D. High-fructose Diet-Induced Hyperuricemia accompanying metabolic syndrome-mechanisms and dietary therapy proposals. Int J Env Res Pub He. 2023;20(4). [DOI] [PMC free article] [PubMed]
- 30.Gołąbek K, Regulska-Ilow B. Dietary support in insulin resistance: an overview of current scientific reports. Adv Clin Exp Med. 2019;28(11):1577–85. [DOI] [PubMed] [Google Scholar]
- 31.Macdonald IA. A review of recent evidence relating to sugars, insulin resistance and diabetes. Eur J Nutr. 2016;55(Suppl 2):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Botezelli JD, Coope A, Ghezzi AC, Cambri LT, Moura LP, Scariot PPM, Gaspar RS, Mekary RA, Ropelle ER, Pauli JR. Strength training prevents hyperinsulinemia, insulin resistance, and inflammation Independent of Weight loss in Fructose-Fed animals. Sci Rep-UK. 2016;6(1). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in this study are available from the NHANES databases. (Available from https://www.cdc.gov/nchs/nhanes/participant.htm).