Abstract
A simple method is described for the separation and quantification of the subunits of GSH transferases present in rat tissue extracts. This method, involving GSH-agarose affinity chromatography followed by reverse-phase h.p.l.c., is rapid and sufficiently sensitive to measure 5 micrograms of each subunit in a mixture. Examples are given of its application to extracts of rat kidney, adrenal, testicular interstitial cells and seminiferous tubules. The analysis of seminiferous tubules indicates that the technique may be of value for the identification of novel subunits. Preliminary separations of subunits from human GSH transferases are also described.
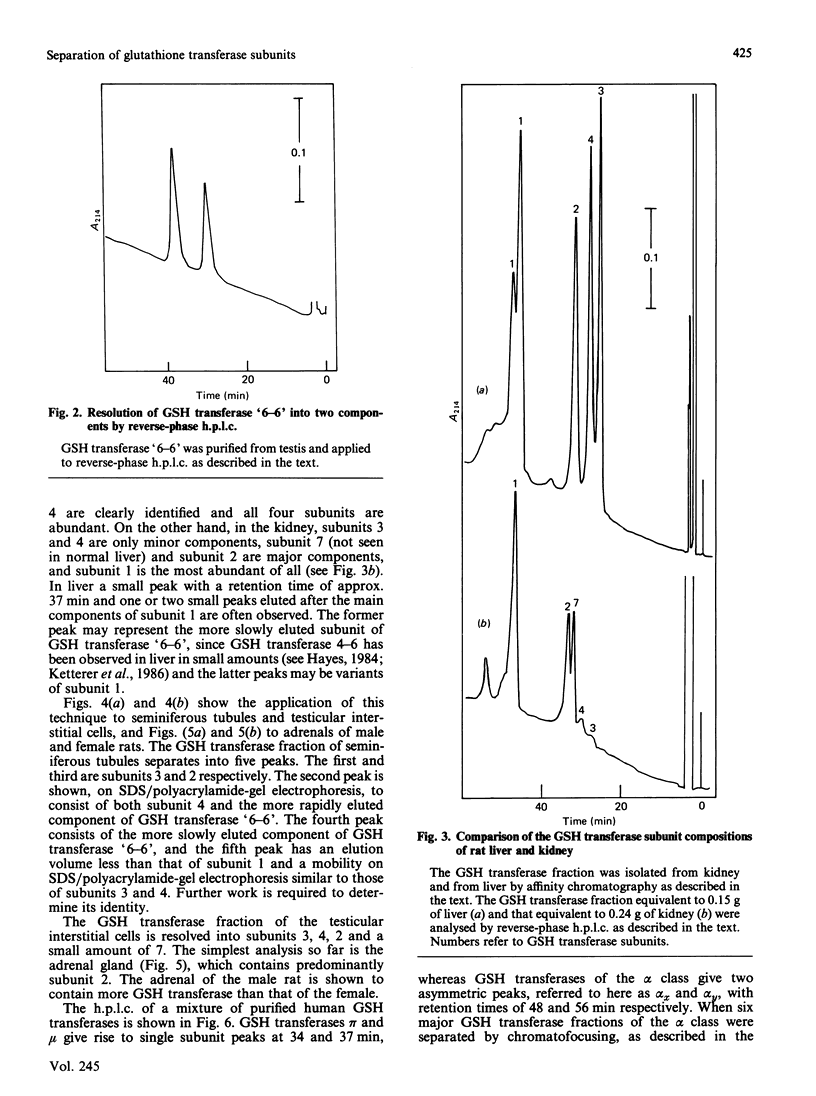
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass N. M., Kirsch R. E., Tuff S. A., Saunders S. J. Radioimmunoassay of ligandin. Biochim Biophys Acta. 1977 Sep 27;494(1):131–143. doi: 10.1016/0005-2795(77)90141-6. [DOI] [PubMed] [Google Scholar]
- Beale D., Ketterer B., Carne T., Meyer D., Taylor J. B. Evidence that the Ya and Yc subunits of glutathione transferase B (ligandin) are the products of separate genes. Eur J Biochem. 1982 Sep 1;126(3):459–463. doi: 10.1111/j.1432-1033.1982.tb06802.x. [DOI] [PubMed] [Google Scholar]
- Beale D., Meyer D. J., Taylor J. B., Ketterer B. Evidence that the Yb subunits of hepatic glutathione transferases represent two different but related families of polypeptides. Eur J Biochem. 1983 Dec 1;137(1-2):125–129. doi: 10.1111/j.1432-1033.1983.tb07805.x. [DOI] [PubMed] [Google Scholar]
- Christ-Hazelhof E., Nugteren D. H. Purification and characterisation of prostaglandin endoperoxide D-isomerase, a cytoplasmic, glutathione-requiring enzyme. Biochim Biophys Acta. 1979 Jan 29;572(1):43–51. doi: 10.1016/0005-2760(79)90198-x. [DOI] [PubMed] [Google Scholar]
- Danielson U. H., Mannervik B. Kinetic independence of the subunits of cytosolic glutathione transferase from the rat. Biochem J. 1985 Oct 15;231(2):263–267. doi: 10.1042/bj2310263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G. J., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Nucleotide sequence analysis of a Yb1 cDNA clone and prediction of the complete amino acid sequence of the Yb1 subunit. J Biol Chem. 1985 Oct 25;260(24):13268–13271. [PubMed] [Google Scholar]
- Drabkin D. L. The environment of function of liver and red blood cells. Ann N Y Acad Sci. 1975 Apr 15;244:603–623. doi: 10.1111/j.1749-6632.1975.tb41557.x. [DOI] [PubMed] [Google Scholar]
- Guthenberg C., Alin P., Mannervik B. Glutathione transferase from rat testis. Methods Enzymol. 1985;113:507–510. doi: 10.1016/s0076-6879(85)13067-3. [DOI] [PubMed] [Google Scholar]
- Hall P. F., Irby D. C., De Kretser D. M. Conversion of cholesterol to androgens by rat testes: comparison of interstitial cells and seminiferous tubules. Endocrinology. 1969 Mar;84(3):488–496. doi: 10.1210/endo-84-3-488. [DOI] [PubMed] [Google Scholar]
- Hayes J. D., Mantle T. J. Use of immuno-blot techniques to discriminate between the glutathione S-transferase Yf, Yk, Ya, Yn/Yb and Yc subunits and to study their distribution in extrahepatic tissues. Evidence for three immunochemically distinct groups of transferase in the rat. Biochem J. 1986 Feb 1;233(3):779–788. doi: 10.1042/bj2330779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. D. Purification and characterization of glutathione S-transferases P, S and N. Isolation from rat liver of Yb1 Yn protein, the existence of which was predicted by subunit hybridization in vitro. Biochem J. 1984 Dec 15;224(3):839–852. doi: 10.1042/bj2240839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey A. J., Stockman P. K., Beckett G. J., Hayes J. D. Variations in the glutathione S-transferase subunits expressed in human livers. Biochim Biophys Acta. 1986 Nov 7;874(1):1–12. doi: 10.1016/0167-4838(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Li N., Weiss M. J., Reddy C. C., Tu C. P. The nucleotide sequence of a rat liver glutathione S-transferase subunit cDNA clone. J Biol Chem. 1984 May 10;259(9):5536–5542. [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Meyer D. J., Beale D., Tan K. H., Coles B., Ketterer B. Glutathione transferases in primary rat hepatomas: the isolation of a form with GSH peroxidase activity. FEBS Lett. 1985 May 6;184(1):139–143. doi: 10.1016/0014-5793(85)80670-0. [DOI] [PubMed] [Google Scholar]
- Pemble S. E., Taylor J. B., Craig R. K., Ketterer B. Differential tissue expression of the glutathione transferase multigene family. Biochem J. 1986 Sep 1;238(2):373–378. doi: 10.1042/bj2380373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickett C. B., Telakowski-Hopkins C. A., Ding G. J., Argenbright L., Lu A. Y. Rat liver glutathione S-transferases. Complete nucleotide sequence of a glutathione S-transferase mRNA and the regulation of the Ya, Yb, and Yc mRNAs by 3-methylcholanthrene and phenobarbital. J Biol Chem. 1984 Apr 25;259(8):5182–5188. [PubMed] [Google Scholar]
- Rothkopf G. S., Telakowski-Hopkins C. A., Stotish R. L., Pickett C. B. Multiplicity of glutathione S-transferase genes in the rat and association with a type 2 Alu repetitive element. Biochemistry. 1986 Mar 11;25(5):993–1002. doi: 10.1021/bi00353a007. [DOI] [PubMed] [Google Scholar]
- Suguoka Y., Kano T., Okuda A., Sakai M., Kitagawa T., Muramatsu M. Cloning and the nucleotide sequence of rat glutathione S-transferase P cDNA. Nucleic Acids Res. 1985 Sep 11;13(17):6049–6057. doi: 10.1093/nar/13.17.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. H., Meyer D. J., Coles B., Ketterer B. Thymine hydroperoxide, a substrate for rat Se-dependent glutathione peroxidase and glutathione transferase isoenzymes. FEBS Lett. 1986 Oct 27;207(2):231–233. doi: 10.1016/0014-5793(86)81494-6. [DOI] [PubMed] [Google Scholar]
- Telakowski-Hopkins C. A., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. Construction of a cDNA clone complementary to a Yc mRNA and prediction of the complete amino acid sequence of a Yc subunit. J Biol Chem. 1985 May 10;260(9):5820–5825. [PubMed] [Google Scholar]
- Tipping E., Ketterer B. The influence of soluble binding proteins on lipophile transport and metabolism in hepatocytes. Biochem J. 1981 May 1;195(2):441–452. doi: 10.1042/bj1950441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Garcia K. B., Royer R. E. Isolation and characterization of the multiple glutathione S-transferases from human liver. Evidence for unique heme-binding sites. J Biol Chem. 1985 Sep 25;260(21):11603–11610. [PubMed] [Google Scholar]


