Abstract
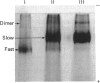
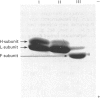
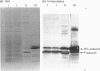
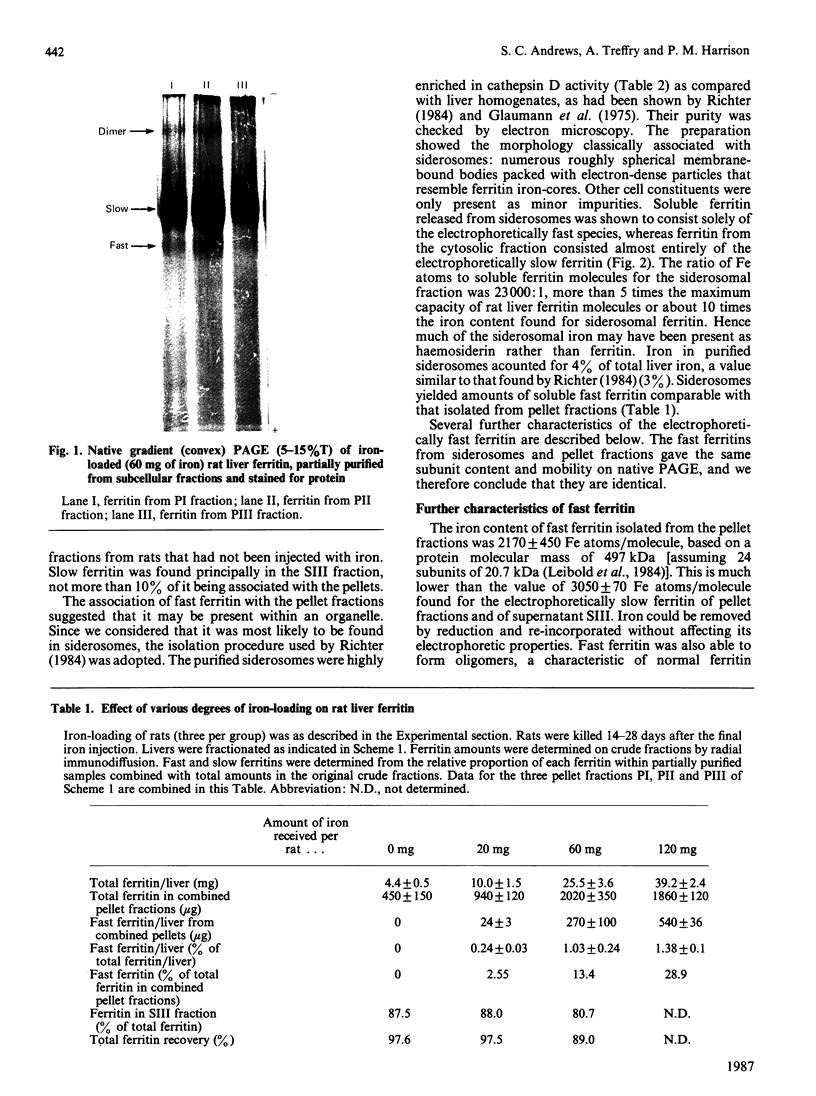
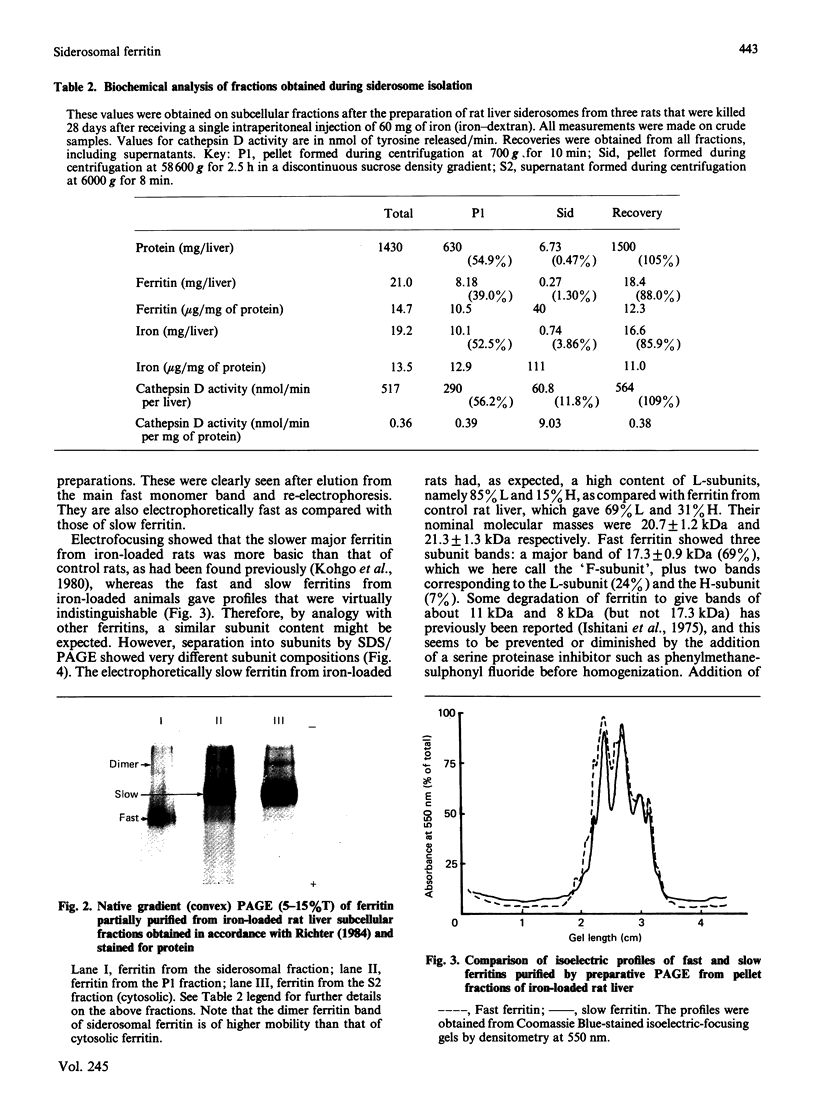
A minor electrophoretically fast component was found in ferritin from iron-loaded rat liver in addition to a major electrophoretically slow ferritin similar to that observed in control rats. The electrophoretically fast ferritin showed immunological identity with the slow component, but on electrophoresis in SDS it gave a peptide of 17.3 kDa, in contrast with the electrophoretically slow ferritin, which gave a major band corresponding to the L-subunit (20.7 kDa). Thus the electrophoretically fast ferritin resembles that reported by Massover [(1985) Biochim. Biophys. Acta 829, 377-386] in livers of mice with short-term parenteral iron overload. The electrophoretically fast ferritin had a lower iron content (2000 Fe atoms/molecule) than the electrophoretically slow ferritin (3000 Fe atoms/molecule). Removal and re-incorporation of iron was possible without effect on the electrophoretic mobility of either ferritin species. On subcellular fractionation the electrophoretically fast ferritin was enriched in pellet fractions and was the sole soluble ferritin isolated from iron-laden secondary lysosomes (siderosomes). The amount and relative proportion of the electrophoretically fast species increased with iron loading. Haemosiderin isolated from siderosomes was found to contain a peptide reactive to anti-ferritin serum and corresponding to the 17.3 kDa peptide of the electrophoretically fast ferritin species. Unlike the electrophoretically slow ferritin, the electrophoretically fast ferritin did not become significantly radioactive in a 1 h biosynthetic labelling experiment. We conclude that the minor ferritin is not, as has been suggested for mouse liver ferritin, 'a completely new species of smaller holoferritin that represents a shift in the ferritin phenotype' in response to siderosis, but a precursor of haemosiderin, in agreement with the proposal by Richter [(1984) Lab. Invest. 50, 26-35] concerning siderosomal ferritin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. C., Treffry A., Harrison P. M. A new form of ferritin heterogeneity explained. Isolation and identification of a nineteen-amino-acid-residue fragment from siderosomal ferritin of rat liver. Biochem J. 1987 Jul 15;245(2):447–453. doi: 10.1042/bj2450447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Bell S. H., Weir M. P., Dickson D. P., Gibson J. F., Sharp G. A., Peters T. J. Mössbauer spectroscopic studies of human haemosiderin and ferritin. Biochim Biophys Acta. 1984 Jun 28;787(3):227–236. doi: 10.1016/0167-4838(84)90313-3. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Bomford A., Conlon-Hollingshead C., Munro H. N. Adaptive responses of rat tissue isoferritins to iron administration. Changes in subunit synthesis, isoferritin abundance, and capacity for iron storage. J Biol Chem. 1981 Jan 25;256(2):948–955. [PubMed] [Google Scholar]
- Brooks K. P., Sander E. G. Preparative polyacrylamide gel electrophoresis: removal of polyacrylate from proteins. Anal Biochem. 1980 Sep 1;107(1):182–186. doi: 10.1016/0003-2697(80)90509-6. [DOI] [PubMed] [Google Scholar]
- DRYSDALE J. W., MUNRO H. N. SMALL-SCALE ISOLATION OF FERRITIN FOR THE ASSAY OF THE INCORPORATION OF 14C-LABELLED AMINO ACIDS. Biochem J. 1965 Jun;95:851–858. doi: 10.1042/bj0950851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach F. A., Gregory D. W., Harrison P. M., Hoy T. G., Williams J. M. On the structure of hemosiderin and its relationship to ferritin. J Ultrastruct Res. 1971 Dec;37(5):495–503. doi: 10.1016/s0022-5320(71)80020-5. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- Glaumann H., Jansson H., Arborgh B., Ericsson J. L. Isolation of liver lysosomes by iron loading. Ultrastructural characterization. J Cell Biol. 1975 Dec;67(3):887–894. doi: 10.1083/jcb.67.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. M., Gregory D. W. Evidence for the existence of stable "aggregates" in horse ferritin and apoferritin. J Mol Biol. 1965 Dec;14(2):626–629. doi: 10.1016/s0022-2836(65)80217-0. [DOI] [PubMed] [Google Scholar]
- Hoy T. G., Jacobs A. Changes in the characteristics and distribution of ferritin in iron-loaded cell cultures. Biochem J. 1981 Jan 1;193(1):87–92. doi: 10.1042/bj1930087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu T. C. Iron overload. Mol Aspects Med. 1983;6(1):1–100. doi: 10.1016/0098-2997(83)90004-3. [DOI] [PubMed] [Google Scholar]
- Kim K., Rhee S. G., Stadtman E. R. Nonenzymatic cleavage of proteins by reactive oxygen species generated by dithiothreitol and iron. J Biol Chem. 1985 Dec 15;260(29):15394–15397. [PubMed] [Google Scholar]
- Kohgo Y., Yokota M., Drysdale J. W. Differential turnover of rat liver isoferritins. J Biol Chem. 1980 Jun 10;255(11):5195–5200. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lahav M., Schoenfeld N., Epstein O., Atsmon A. A method for obtaining high recovery of purified subcellular fractions of rat liver homogenate. Anal Biochem. 1982 Mar 15;121(1):114–122. doi: 10.1016/0003-2697(82)90563-2. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Aziz N., Brown A. J., Munro H. N. Conservation in rat liver of light and heavy subunit sequences of mammalian ferritin. Presence of unique octopeptide in the light subunit. J Biol Chem. 1984 Apr 10;259(7):4327–4334. [PubMed] [Google Scholar]
- MCKAY R. H., FINEBERG R. A. HORSE SPLEEN HEMOSIDERIN. I. ISOLATION. Arch Biochem Biophys. 1964 Mar;104:487–495. doi: 10.1016/0003-9861(64)90493-x. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Massover W. H. Molecular size heterogeneity of ferritin in mouse liver. Biochim Biophys Acta. 1985 Jul 1;829(3):377–386. doi: 10.1016/0167-4838(85)90248-1. [DOI] [PubMed] [Google Scholar]
- Richter G. W. Studies of iron overload. Lysosomal proteolysis of rat liver ferritin. Pathol Res Pract. 1986 May;181(2):159–167. doi: 10.1016/S0344-0338(86)80005-X. [DOI] [PubMed] [Google Scholar]
- Richter G. W. Studies of iron overload. Rat liver siderosome ferritin. Lab Invest. 1984 Jan;50(1):26–35. [PubMed] [Google Scholar]
- Samarel A., Bern M. M. Distribution of iron in splenic ferritin. Lab Invest. 1978 Jul;39(1):10–12. [PubMed] [Google Scholar]
- Shinjo S., Harrison P. M. Artifacts in ferritin isoelectric focussing profiles. FEBS Lett. 1979 Sep 15;105(2):353–356. doi: 10.1016/0014-5793(79)80647-x. [DOI] [PubMed] [Google Scholar]
- Treffry A., Lee P. J., Harrison P. M. Iron-induced changes in rat liver isoferritins. Biochem J. 1984 Jun 15;220(3):717–722. doi: 10.1042/bj2200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treffry A., Lee P. J., Harrison P. M. The effect of iron on ferritin turnover in rat liver. FEBS Lett. 1984 Jan 9;165(2):243–246. doi: 10.1016/0014-5793(84)80177-5. [DOI] [PubMed] [Google Scholar]
- Trefry A., Harrison P. M. Incorporation and release of inorganic phosphate in horse spleen ferritin. Biochem J. 1978 May 1;171(2):313–320. doi: 10.1042/bj1710313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Weir M. P., Gibson J. F., Peters T. J. Haemosiderin and tissue damage. Cell Biochem Funct. 1984 Oct;2(4):186–194. doi: 10.1002/cbf.290020402. [DOI] [PubMed] [Google Scholar]
- Williams M. A., Harrison P. M. Electron-microscopic and chemical studies of oligomers in horse ferritin. Biochem J. 1968 Nov;110(2):265–280. doi: 10.1042/bj1100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixom R. L., Prutkin L., Munro H. N. Hemosiderin: nature, formation, and significance. Int Rev Exp Pathol. 1980;22:193–225. [PubMed] [Google Scholar]