Abstract
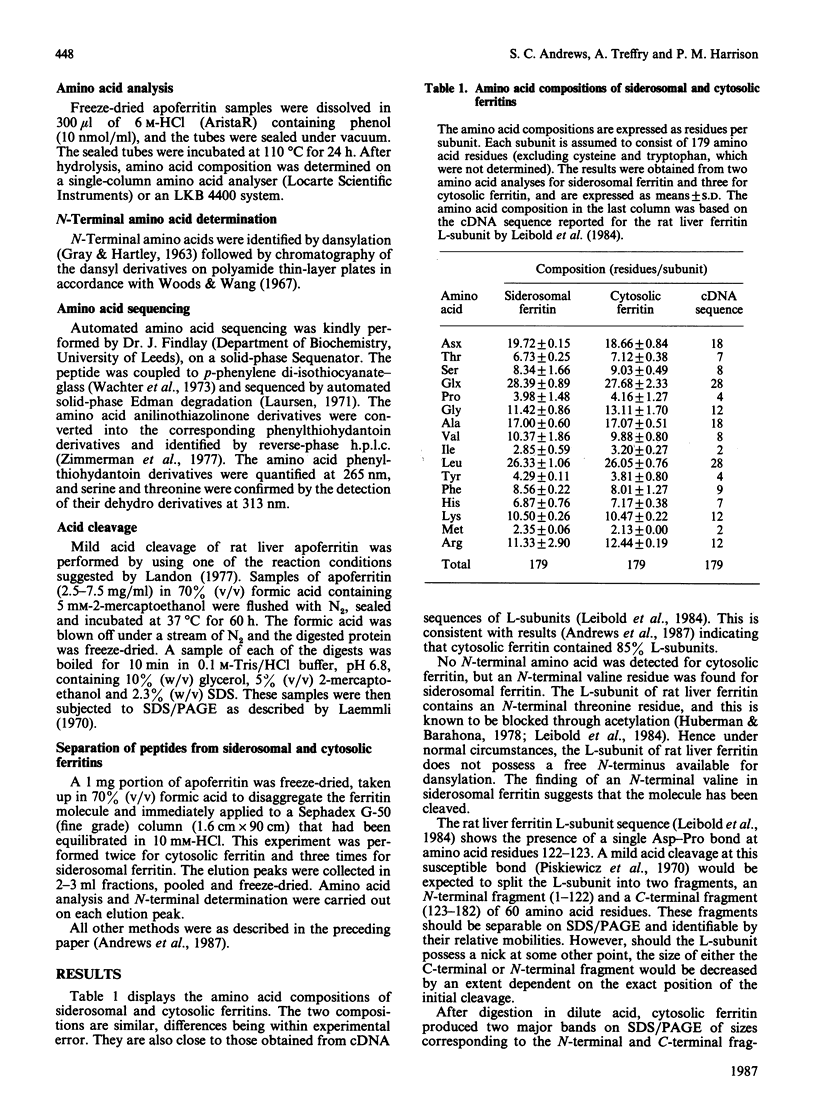
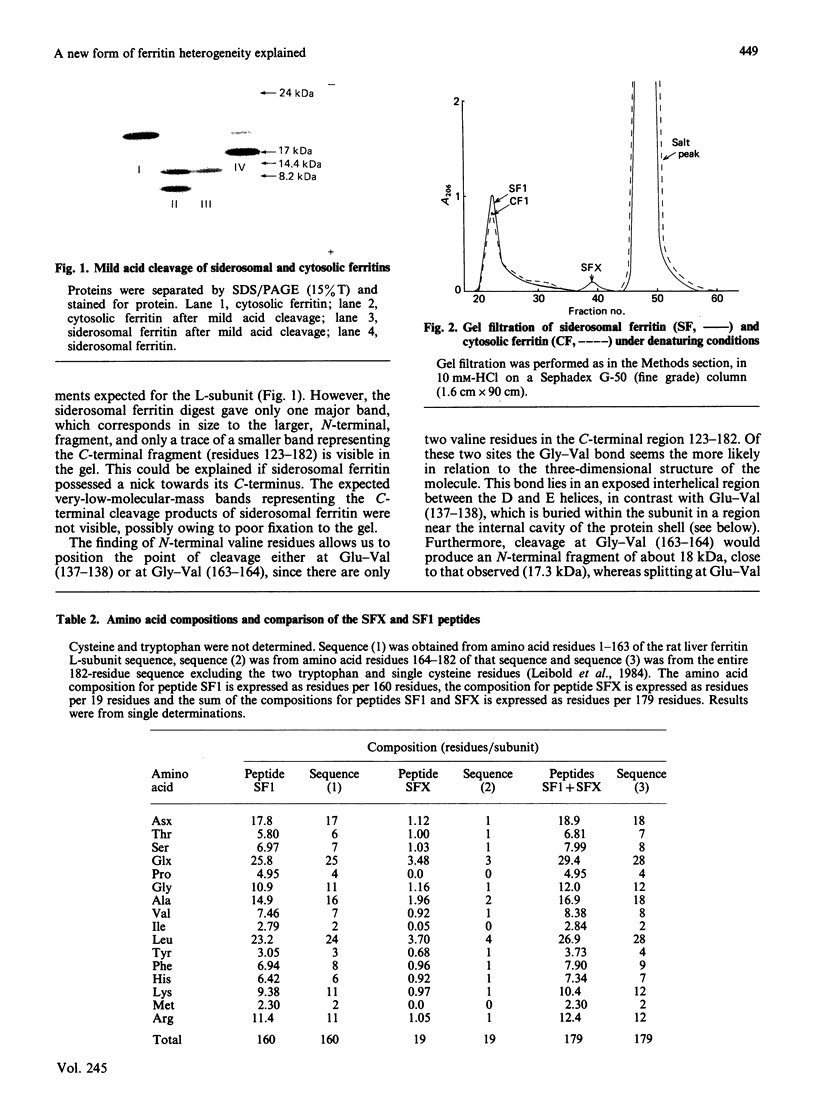
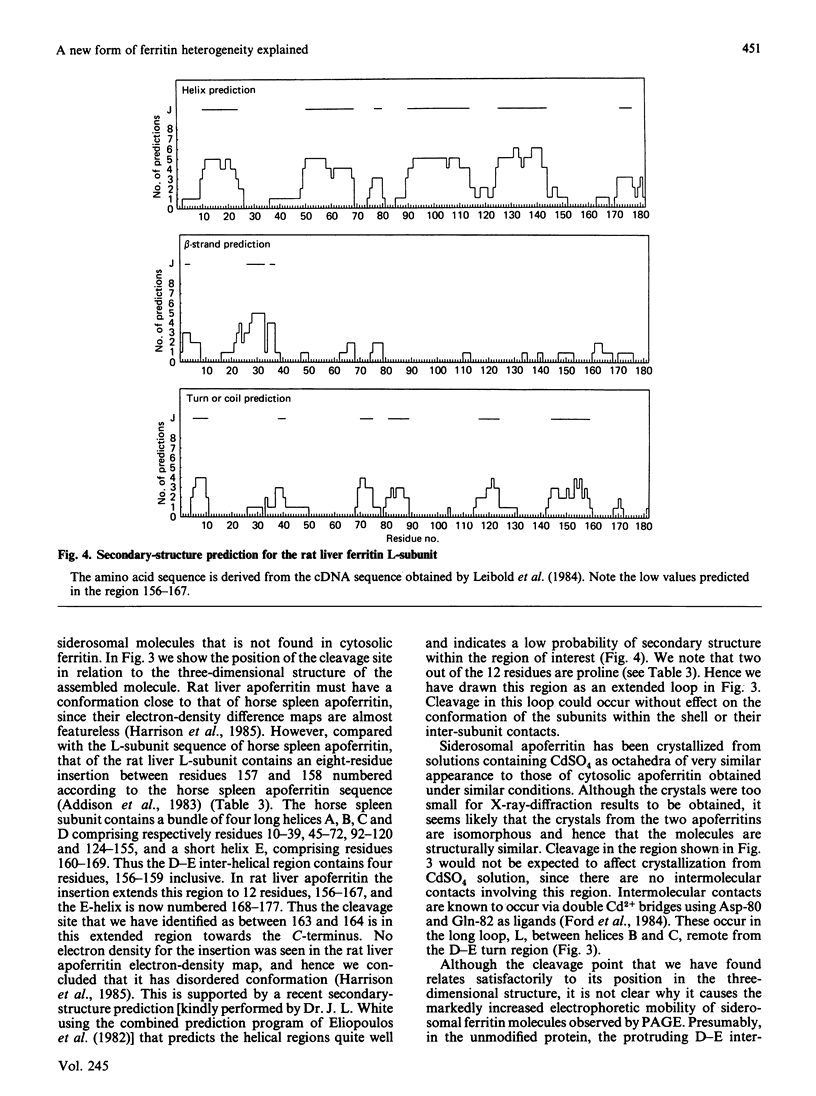
Ferritin present within siderosomes of iron-loaded rats has a faster anodal mobility than that of cytosolic ferritin from the same rats. A 19-amino-acid-residue peptide was isolated from this fast ferritin and shown to be derived from the C-terminal end of its L-subunit. A 17.3 kDa peptide seen on electrophoresis in denaturing gels of this ferritin accounts for the major portion of the original 182-residue subunit. The two peptides arise from cleavage within the 'insertion region' of the L-subunit sequence that occurs between the D and E helices and lies on the outside of the assembled molecule. This cleavage is present in about 80% of the L-subunits of siderosomal ferritin but nevertheless leaves the molecular structure otherwise intact. It gives rise to an apparent decrease in molecular size, accounting for the faster anodal mobility on native gels. Hence a new form of heterogeneity in ferritin preparations has been explained.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Addison J. M., Fitton J. E., Lewis W. G., May K., Harrison P. M. The amino acid sequence of human liver apoferritin. FEBS Lett. 1983 Nov 28;164(1):139–144. doi: 10.1016/0014-5793(83)80037-4. [DOI] [PubMed] [Google Scholar]
- Andrews S. C., Treffry A., Harrison P. M. Siderosomal ferritin. The missing link between ferritin and haemosiderin? Biochem J. 1987 Jul 15;245(2):439–446. doi: 10.1042/bj2450439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomford A., Conlon-Hollingshead C., Munro H. N. Adaptive responses of rat tissue isoferritins to iron administration. Changes in subunit synthesis, isoferritin abundance, and capacity for iron storage. J Biol Chem. 1981 Jan 25;256(2):948–955. [PubMed] [Google Scholar]
- Costanzo F., Colombo M., Staempfli S., Santoro C., Marone M., Frank R., Delius H., Cortese R. Structure of gene and pseudogenes of human apoferritin H. Nucleic Acids Res. 1986 Jan 24;14(2):721–736. doi: 10.1093/nar/14.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Santoro C., Colantuoni V., Bensi G., Raugei G., Romano V., Cortese R. Cloning and sequencing of a full length cDNA coding for a human apoferritin H chain: evidence for a multigene family. EMBO J. 1984 Jan;3(1):23–27. doi: 10.1002/j.1460-2075.1984.tb01756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg S. J., Wagstaff M., Worwood M. Detection of a glycosylated subunit in human serum ferritin. Biochem J. 1981 Dec 1;199(3):565–571. doi: 10.1042/bj1990565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W. Ferritin phenotypes: structure and metabolism. Ciba Found Symp. 1976 Dec 7;(51):41–67. doi: 10.1002/9780470720325.ch3. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Harrison P. M., Rice D. W., Smith J. M., Treffry A., White J. L., Yariv J. Ferritin: design and formation of an iron-storage molecule. Philos Trans R Soc Lond B Biol Sci. 1984 Feb 13;304(1121):551–565. doi: 10.1098/rstb.1984.0046. [DOI] [PubMed] [Google Scholar]
- GRAY W. R., HARTLEY B. S. THE STRUCTURE OF A CHYMOTRYPTIC PEPTIDE FROM PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:379–380. doi: 10.1042/bj0890379. [DOI] [PubMed] [Google Scholar]
- Huberman A., Barahona E. Primary structure of rat liver apoferritin. The amino end. Biochim Biophys Acta. 1978 Mar 28;533(1):51–56. doi: 10.1016/0005-2795(78)90546-9. [DOI] [PubMed] [Google Scholar]
- Ihara K., Maeguchi K., Young C. T., Theil E. C. Cell-specific properties of red cell and liver ferritin from bullfrog tadpoles probed by phosphorylation in vitro. J Biol Chem. 1984 Jan 10;259(1):278–283. [PubMed] [Google Scholar]
- Jain S. K., Crampton J., Gonzalez I. L., Schmickel R. D., Drysdale J. W. Complementarity between ferritin H mRNA and 28 S ribosomal RNA. Biochem Biophys Res Commun. 1985 Sep 16;131(2):863–867. doi: 10.1016/0006-291x(85)91319-1. [DOI] [PubMed] [Google Scholar]
- Kohgo Y., Yokota M., Drysdale J. W. Differential turnover of rat liver isoferritins. J Biol Chem. 1980 Jun 10;255(11):5195–5200. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landon Cleavage at aspartyl-prolyl bonds. Methods Enzymol. 1977;47:145–149. doi: 10.1016/0076-6879(77)47017-4. [DOI] [PubMed] [Google Scholar]
- Laursen R. A. Solid-phase Edman degradation. An automatic peptide sequencer. Eur J Biochem. 1971 May 11;20(1):89–102. doi: 10.1111/j.1432-1033.1971.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Leibold E. A., Aziz N., Brown A. J., Munro H. N. Conservation in rat liver of light and heavy subunit sequences of mammalian ferritin. Presence of unique octopeptide in the light subunit. J Biol Chem. 1984 Apr 10;259(7):4327–4334. [PubMed] [Google Scholar]
- Massover W. H. Molecular size heterogeneity of ferritin in mouse liver. Biochim Biophys Acta. 1985 Jul 1;829(3):377–386. doi: 10.1016/0167-4838(85)90248-1. [DOI] [PubMed] [Google Scholar]
- Mertz J. R., Theil E. C. Subunit dimers in sheep spleen apoferritin. The effect on iron storage. J Biol Chem. 1983 Oct 10;258(19):11719–11726. [PubMed] [Google Scholar]
- Piszkiewicz D., Landon M., Smith E. L. Anomalous cleavage of aspartyl-proline peptide bonds during amino acid sequence determinations. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1173–1178. doi: 10.1016/0006-291x(70)90918-6. [DOI] [PubMed] [Google Scholar]
- Richter G. W. Studies of iron overload. Rat liver siderosome ferritin. Lab Invest. 1984 Jan;50(1):26–35. [PubMed] [Google Scholar]
- Santoro C., Marone M., Ferrone M., Costanzo F., Colombo M., Minganti C., Cortese R., Silengo L. Cloning of the gene coding for human L apoferritin. Nucleic Acids Res. 1986 Apr 11;14(7):2863–2876. doi: 10.1093/nar/14.7.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treffry A., Lee P. J., Harrison P. M. Functional studies on rat-liver isoferritins. Biochim Biophys Acta. 1984 Feb 28;785(1-2):22–29. doi: 10.1016/0167-4838(84)90229-2. [DOI] [PubMed] [Google Scholar]
- Wachter E., Machleidt W., Hofner H., Otto J. Aminopropyl glass and its p-phenylene diisothiocyanate derivative, a new support in solid-phase Edman degradation of peptides and proteins. FEBS Lett. 1973 Sep 1;35(1):97–102. doi: 10.1016/0014-5793(73)80585-x. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]