Abstract
Background
Postoperative pancreatic fistula (POPF) continues to be the most common complication after distal pancreatectomy (DP). Recent advancements in surgical techniques have established minimally invasive distal pancreatectomy (MIDP) as the standard treatment for various conditions, including pancreatic cancer. However, MIDP has not demonstrated a clear advantage over open DP in terms of POPF rates, indicating the need for additional strategies to prevent POPF in MIDP. This trial (WRAP study) aims to evaluate the efficacy of wrapping the pancreatic stump with polyglycolic acid (PGA) mesh and fibrin glue in preventing clinically relevant (CR-) POPF following MIDP.
Methods
This multicenter, randomized controlled trial will include patients scheduled for laparoscopic or robotic DP for tumors in the pancreatic body and/or tail. Eligible participants will be centrally randomized into either the control group (Group A) or the intervention group (Group B), where the pancreatic stump will be reinforced by PGA mesh and fibrin glue. In both groups, pancreatic transection will be performed using a bioabsorbable reinforcement-attached stapler. A total of 172 patients will be enrolled across 14 high-volume centers in Japan. The primary endpoint is the incidence of CR-POPF (International Study Group of Pancreatic Surgery grade B/C).
Discussion
The WRAP study will determine whether the reinforcement of the pancreatic stump with PGA mesh and fibrin glue, a technique whose utility has been previously debated, could become the best practice in the era of MIDP, thereby enhancing its safety.
Trial registration
This trial was registered with the Japan Registry of Clinical Trials on June 15, 2024 (jRCTs032240120).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12893-024-02610-0.
Keywords: Distal pancreatectomy, Fibrin glue, Minimally invasive surgery, Polyglycolic acid mesh, Pancreatic fistula
Background
Postoperative pancreatic fistula (POPF) is a leading complication after distal pancreatectomy (DP), with incidence rates reported to range between 13 to 64% [1–3]. POPF often contributes to additional complications, including intra-abdominal abscess, hemorrhage, wound infections, and sepsis, which in turn lead to extended hospitalizations and higher healthcare costs [4, 5].
With advancements in technology and surgical techniques, laparoscopic and robotic surgeries have progressed in the field of pancreatic surgery, leading to the increased use of minimally invasive distal pancreatectomy (MIDP) not only for benign pancreatic tumors but also for pancreatic cancer [6, 7]. While MIDP has proven effective in addressing cosmetic concerns and promoting early postoperative recovery, it has unfortunately not shown significant improvement in POPF rates compared to open surgery [8].
In order to reduce the incidence of POPF following DP, various methods have been attempted, including hand-sewn closure, the use of stapling devices, fibrin sealants, and the application of patches and sheets [9–15]. A randomized controlled trial conducted in Korean centers introduced a straightforward approach to minimize the risk of POPF by applying polyglycolic acid (PGA) mesh to the pancreatic stump after transection with a stapler [16]. Although this trial involved patients undergoing open-DP, this technique could potentially be advantageous in MIDP, where stapling devices are frequently used for pancreatic transection [6, 7]. However, the effectiveness of PGA mesh reinforcement in the context of MIDP has not been extensively studied.
This multicenter randomized controlled trial (WRAP study) is designed to evaluate the efficacy of wrapping the pancreatic stump with polyglycolic acid (PGA) mesh and fibrin glue in preventing clinically relevant (CR-) POPF after MIDP. The trial will include 172 patients across 14 high-volume centers in Japan.
Methods/Design
Aim
The WRAP study aims to evaluate the efficacy of wrapping the pancreatic stump with PGA mesh and fibrin glue in preventing CR-POPF after laparoscopic or robotic DP.
Study design
This is a multicenter, prospective, randomized controlled trial with 1:1 allocation (Fig. 1). The study is registered with the Japan Registry of Clinical Trials (jRCTs032240120). Ethical approval was granted by the Certified Review Board of Cancer Institute Hospital of Japanese Foundation for Cancer Research. Written informed consent will be obtained from patients by the designated attending physicians after explaining the trial. A total of 172 patients will be enrolled across 14 high-volume centers in Japan. The registration period is scheduled for 3 years, with a 3-month follow-up period. The study design follows Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 guidelines (Supplementary Fig. 1) [17].
Fig. 1.
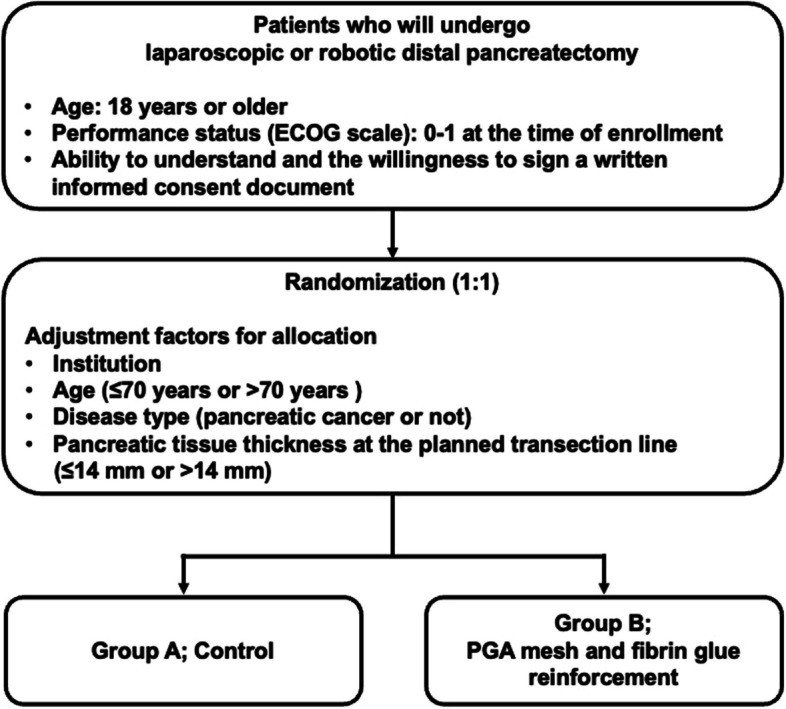
Flow diagram of the WRAP study
Study population
Patients undergoing laparoscopic or robotic DP are eligible, regardless of disease type. Both spleen-preserving DP and conventional DP are included in the study. Detailed eligibility criteria are presented in Table 1.
Table 1.
Eligibility criteria of the WRAP study
| Inclusion criteria |
| (1) Scheduled laparoscopic or robotic distal pancreatectomy |
| (2) Planned pancreatic transection using a bioabsorbable reinforcement-attached stapler |
| (3) Eastern Cooperative Oncology Group (ECOG) Performance status: 0–1 |
| (4) Age: 18 years or older |
| (5) Sufficient organ function, defined as: |
| (a) Leukocyte count ≥ 2500 mm3, ≤ 14000 mm3 |
| (b) Hemoglobin ≥ 9.0 g/dl |
| (c) Platelet count ≥ 100,000 mm3 |
| (d) Total bilirubin ≤ 2.0 mg/dl |
| (e) Creatinine ≤ 2.0 mg/dl |
| (6) Capability to comprehend and willingness to provide written informed consent |
| Exclusion criteria |
| (1) Scheduled open distal pancreatectomy |
| (2) Pancreatic transection without the use of a stapler |
| (3) History of upper abdominal surgery except for cholecystectomy |
| (4) Requirement for emergency surgery |
| (5) Necessity for arterial reconstruction, such as the superior mesenteric artery, common hepatic artery, or celiac artery |
| (6) Severe ischaemic heart disease |
| (7) Significant liver dysfunction due to cirrhosis or active hepatitis |
| (8) Severe respiratory disorder requiring oxygen therapy |
| (9) Chronic renal failure requiring dialysis |
| (10) Requirement for resection of organs other than the left adrenal gland or gallbladder during distal pancreatectomy |
| (11) Current immunosuppressive treatment |
| (12) History of severe hypersensitivity to polyglycolic acid felt or fibrin glue |
| (13) History of other severe drug allergies |
| (14) Iodine-based contrast media allergy |
| (15) Active secondary malignancy that may influence adverse events |
| (16) Planned use of octreotide |
| (17) Severe psychological or neurological disorders |
Informed consent
Patients scheduled for MIDP will be screened for eligibility. When deemed eligible, written informed consent will be obtained from the patients by the attending surgeons at the participating centers. Upon completion of the informed consent process, eligible patients will be formally enrolled and randomized.
Randomization and blinding
After confirming eligibility and obtaining written informed consent, patients will be randomized in a 1:1 ratio to either the control group (Group A) or the intervention group (Group B). Central randomization and registration will be conducted using the Electronic Data Capture (EDC) system. To minimize background bias between the groups, stratification will be based on institution, age (≤ 70 years or > 70 years), disease type (pancreatic cancer or not), and pancreatic tissue thickness at the planned transection line (≤ 14 mm or > 14 mm) [18]. The transection line is determined by the surgeon based on preoperative computerized tomography (CT) planning, and the thickness of the pancreas at the transection line is measured on CT. Pocock and Simon’s minimization method will be used for random assignment. A flow diagram of the WRAP study is shown in Fig. 1.
Patients will be blinded to the surgical approach they receive. The attending surgeons will not be blinded to the assigned treatment; however, outcome assessors and statisticians will be blinded to group assignments.
Endpoints
The primary endpoint is the incidence of CR-POPF. CR-POPF is defined as International Study Group of Pancreatic Surgery (ISGPS) grade B or C [19]. Secondary endpoints include drain fluid amylase levels on postoperative days (POD) 1–3, duration of drain placement, length of hospital stay, incidence of overall biochemical leak and POPF, incidence of delayed gastric emptying (DGE), intra-abdominal abscess, post-pancreatectomy hemorrhage (PPH), need for interventional drainage, overall postoperative complications, POPF-related complications (POPF + DGE + abscess + PPH), 3-month mortality, re-operation, re-hospitalization, and fluid collection around the pancreatic stump on contrast-enhanced CT 3 months post-surgery.
Biochemical leak, POPF [19], DGE [20], and PPH [21] will be defined and graded according to ISGPS criteria and Clavien–Dindo classification [22]. Other postoperative complications will be graded according to Clavien–Dindo classification [22].
Intervention
Surgical procedure
In both groups, pancreatic transection will be performed with a bioabsorbable reinforcement-attached stapler (Tri-StapleTM 2.0 Reinforced; Covidien, Japan, or ECHELON ENDOPARH® Staple Line Reinforcement; ETHICON, Japan). If pancreatic transection is performed without a bioabsorbable reinforcement-attached stapler, the protocol treatment will be terminated.
In Group A, no additional reinforcement of the pancreatic stump is permitted. In Group B, the pancreatic stump will be covered with a 0.15-mm PGA mesh (Neoveil®; Gunze Medical, Japan) and then fibrin glue (BOLHEAL®; KM Biologics, Japan) will be spread there. It will then be covered with another 0.15-mm PGA mesh and spread with fibrin glue (Fig. 2).
Fig. 2.
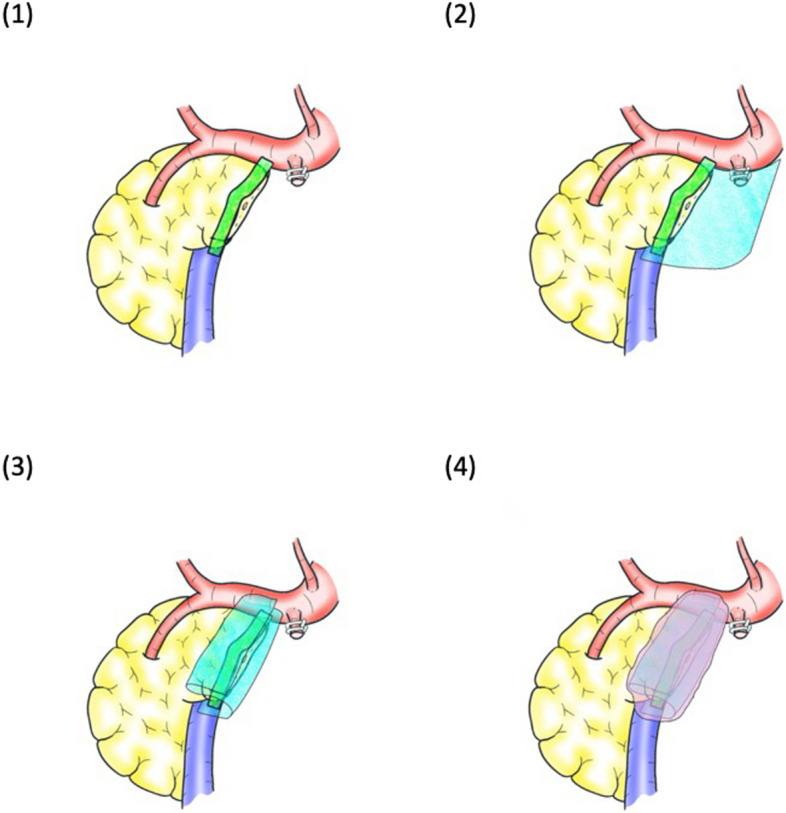
The surgical procedure of PGA mesh and fibrin glue reinforcement of the pancreatic stump
The number and type of drains will be at the surgeon's discretion, but at least one closed drain must be placed at the pancreatic stump. When converting to open surgery, the protocol treatment will be terminated, and those cases will be excluded from the study at that point.
Intraoperative photography
To ensure the correct surgical procedure, intraoperative photographs of the surgical fields are required in both groups. The pancreatic stump will be photographed immediately after pancreatic transection (both groups) and after spraying with fibrin glue (Group B only). Central assessment will be conducted every 6 months for all registered patients by the central review committee. Inappropriate cases will be excluded from the per-protocol analysis set and reviewed in the final report/publication.
Postoperative management
Blood and biochemical examinations, along with amylase level measurements of drains, will be performed on POD 1 to POD 3. If the amylase level of anastomotic drains on POD 1 is 5000 IU/L or less, drain removal is recommended on POD 3–6 [23]. For patients with POPF or infectious signs requiring therapeutic drainage, drainage should continue. Prior to drain removal, a CT scan is recommended to assess the intra-abdominal situation. After POD 4, blood/biochemical examinations or drain amylase-level measurements before drain removal are performed at the physician’s discretion. The use of prophylactic antimicrobial agents after surgery or routine exchange of anastomotic drains does not affect the definition of POPF. For continued drainage after POD 4–6, drain removal is recommended when either or both of the following conditions persist for 2 consecutive days: the amylase level in the drainage fluid is less than three times the upper limit of normal serum amylase levels, or the drainage fluid volume is 20 mL/day or less.
Postoperative follow-up
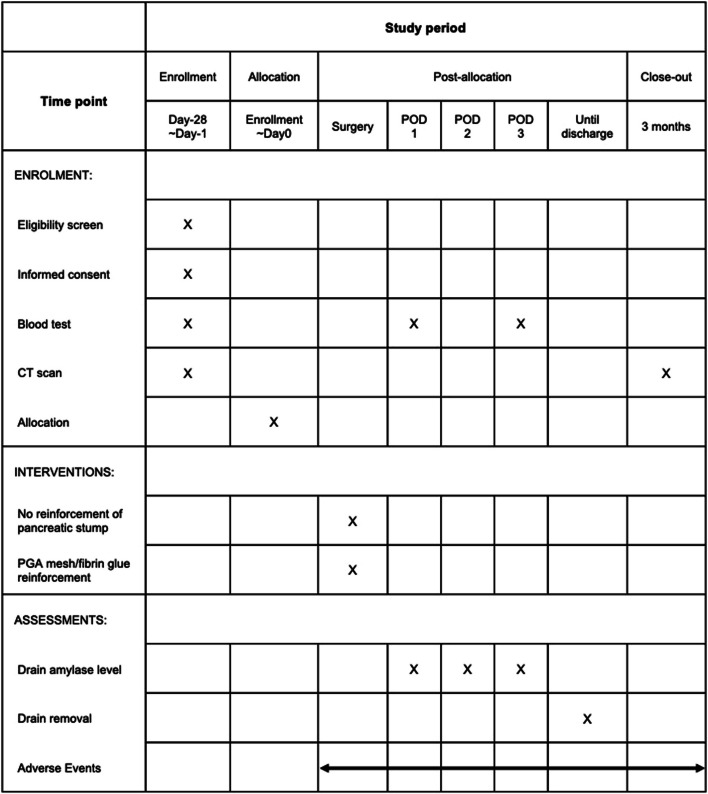
Patients will be followed for 3 months post-surgery to monitor for postoperative complications, re-drainage, re-hospitalization, and re-operation. Three months post-surgery, contrast-enhanced CT will evaluate fluid collection around the pancreatic stump. The study calendar is shown in Fig. 3.
Fig. 3.
Study calendar of the WRAP study
Post‒trial care
Participants will continue to receive follow-up care beyond the initial 3-month follow-up period to ensure that any ongoing health needs related to the trial are adequately addressed. The medical devices and pharmaceuticals used in the protocol treatment for this study are approved for use in the study population. As the treatment does not exceed the scope of standard medical practice, it will be conducted using the participants’ health insurance, in the same manner as regular medical care. Consequently, the portion of the medical expenses typically borne by the participant will remain their responsibility. However, in the event of any health damage, such as previously unreported side effects resulting from this study, compensation will be provided in accordance with legal liability.
Sample size estimation
This trial aims to evaluate the efficacy of Group B compared to Group A in reducing the incidence of CR-POPF. In our pilot study, the incidence of CR-POPF was 20% with PGA mesh reinforcement and 44% without (unpublished data). For statistical analysis with a significance level of α = 0.05 (two-sided) in a superiority design, 82 patients per arm are required, with a power of over 80%. Considering approximately 5% of patients may be ineligible for surgery, the sample size was increased to 172 patients (86 per arm).
Statistical analysis plan
Primary and secondary endpoints will be analyzed using full analysis set (FAS), which will include all subjects in each group without erroneous or duplicate registrations, subjects who did not receive protocol treatments, subjects who withdrew consent and declined all data usage, or subjects with no any efficacy data. Analysis using per protocol set is to be conducted, as reference, but will exclude subjects with a protocol violation from FAS. A safety analysis will be conducted on the safety analysis population, which will include all subjects in each group who received at least protocol treatments.
The primary endpoint is intergroup difference of the incidence of CR-POPF. Intergroup comparison will be performed by analysis of the Cochran-Mantel–Haenszel test and the Mantel–Haenszel risk difference with its confidence interval (CI) using age (≤ 70 years or > 70 years), disease type (pancreatic cancer or not), and pancreatic tissue thickness at the planned transection line (≤ 14 mm or > 14 mm). The secondary endpoints, drain fluid amylase levels on PODs 1–3, duration of drain placement, and length of hospital stay, will be analyzed using median, interquartile range, and the Wilcoxon rank-sum test for reference. The remaining secondary endpoints will be analyzed using proportion with its CI, intergroup differences of proportion with its CI, and the chi-square test for reference. The fluid collection around the pancreatic stump on contrast-enhanced CT 3 months post-surgery will also have its volume analyzed using mean with its CI, intergroup difference of mean with its CI, and the Welch t test for reference. Subject characteristics will be presented as frequencies and proportions for categorical data, and summary statistics (number of subjects, mean, standard deviation, minimum, median, interquartile range, and maximum) for continuous data. The significance level is 5% (two-sided) with a confidence coefficient of 0.95 (two-sided). Details and other analyses will be performed according to the statistical analysis plan, which will be finalized before database lock.
Coordinating center
The coordinating center comprises the principal investigator, project manager, data manager, and biostatistician. The principal investigator provides overall leadership for the study and ensures adherence to the protocol. The project manager is responsible for managing the daily operations of the trial, coordinating with study sites, and overseeing the study timeline. The data manager handles data collection, entry, and ensures data integrity. The biostatistician develops the statistical analysis plan, performs data analyses, and interprets the results.
Data and safety monitoring committee
A Data and Safety Monitoring Committee (DSMC) will be established for this trial. The DSMC will be composed of three independent experts, and review reported adverse events (AEs) and provide recommendations regarding the continuation of the trial.
Data management
The data collection/management system of this project is the EDC system. The EDC system that has been systematically verified and designed with trace management and user permission management functions will be selected. To protect confidentiality, only authorized and trained investigators will have access to the data of enrolled patients.
Central monitoring will be conducted by the data manager to ensure that the trial is conducted safely, adheres to the protocol, and that data is collected accurately. On-site monitoring will be implemented as necessary for facilities where potential non-compliance is identified through central monitoring.
Confidentiality
Unique participant codes will be assigned to all study participants, and names as well as other direct identifiers will be removed from the dataset to protect confidentiality. Electronic data stored in the EDC system will be maintained on a securely managed cloud server. Access to this data will be limited to individuals authorized by the principal investigator, and a record of those granted access rights will be maintained. Electronic data stored outside of the EDC system will be secured on an external storage device with appropriate security measures, such as avoiding the use of computers connected to external networks, employing antivirus protection, and implementing password access restrictions. Furthermore, this storage device will be kept in a locked cabinet.
Adverse event reporting and harms
AE refers to any adverse medical events occurring to the subjects regardless of their relevance to the intervention. All AEs occurring during the study will be carefully recorded via the EDC system. DSMC will assess the causal relationship between the AEs and the intervention. AEs that result in death, life-threatening situations, prolonged hospital stay, or disability/incapacity will be reported as severe AEs to the Certified Review Board of Cancer Institute Hospital of Japanese Foundation for Cancer Research within 15 days.
Audit
Audits are planned for this study. An initial audit will be conducted to assess the study implementation system at the start of the trial. Subsequent audits will be conducted as deemed necessary, based on the study results.
Protocol amendments
When any amendments to the study protocol occur during this trial, the principal investigator will submit the changes to the Certified Review Board of Cancer Institute Hospital of Japanese Foundation for Cancer Research for approval. Once approval is obtained, the principal investigator will promptly report the changes and the reasons for them to the lead investigators at each participating institution. The lead investigators at each institution will then submit the amendments to the administrators of their respective institutions and obtain approval.
Dissemination
The results of this study will be published in peer-reviewed journals, and be presented at academic conferences. We will follow the International Committee of Medical Journal Editors rules for authorship.
Trial status
This study was approved by the Certified Review Board of Cancer Institute Hospital of Japanese Foundation for Cancer Research on May 16, 2024 (GKC-2402) and registered with the Japan Registry of Clinical Trials on June 15, 2024 (jRCTs032240120). The current protocol version is Version 3.0 (approved on August 15, 2024). The recruitment was started on June 16, 2024, and the current status is still recruiting.
Discussion
This clinical trial is designed to assess the effectiveness of wrapping the pancreatic stump with PGA mesh and fibrin glue in preventing CR-POPF after laparoscopic or robotic DP. The LEOPARD trial, a multicenter patient-blinded randomized controlled trial, reported that CR-POPF occurred in 39% of patients following MIDP, compared to 23% after open DP (p = 0.07) [24]. Similarly, the DIPLOMA trial, an international randomized trial, demonstrated that MIDP was non-inferior to open DP in terms of achieving radical resection in patients with resectable pancreatic cancer, with comparable short-term outcomes, including CR-POPF (p = 0.41) and severe complications (p = 0.78) [25]. These findings suggest the need for additional strategies to prevent POPF in MIDP. Considering that stapler use for pancreatic stump closure in MIDP is preferred worldwide [26, 27], the combination of PGA mesh wrapping with a stapler may be widely acceptable.
This trial mandates the use of bioabsorbable reinforcement-attached staplers for pancreatic transection. Some studies have reported that reinforced staplers decrease the risk of POPF [12, 28]. However, Kondo et al. found no reduction in CR-POPF incidence with reinforced staplers compared to standard staplers in their multicenter randomized controlled trial [18], indicating that the efficacy of reinforced staplers remains controversial. To avoid potential bias, only reinforced staplers will be used in this trial.
Randomization will stratify for age, disease type (pancreatic cancer or not), and pancreatic tissue thickness at the planned transection line. These factors have been associated with POPF incidence. Studies have shown that older age is protective against POPF, possibly due to a decline in pancreatic exocrine function with age [29, 30]. Pancreatic cancer has been associated with a lower incidence of POPF [31]. Our pilot study also indicated that pancreatic cancer reduced the risk of CR-POPF (odds ratio = 0.45, 95% confidence interval: 0.23–0.88) [unpublished data]. Pancreatic thickness is a well-established risk factor for POPF [28, 32–35]. The 14 mm cut-off for pancreatic thickness at the transection line was based on a multicenter randomized controlled trial by Kondo et al. in Japan [18]. While pancreatic texture is another known predictor for POPF, it is challenging to assess objectively, so it was not included in the stratification factors.
Few clinical trials focus solely on MIDP for methods to prevent POPF. If this trial demonstrates the efficacy of PGA mesh and fibrin glue in preventing CR-POPF after MIDP, it will significantly enhance the safety of MIDP. Additionally, reducing CR-POPF incidence could offer economic and oncological benefits, such as shorter hospital stays and timely initiation of postoperative adjuvant chemotherapy.
Supplementary Information
Acknowledgements
We would like to express our deep gratitude to Moe Kambe and Mayumi Miyamoto for their significant contributions to the Certified Review Board and to Yoshiko Matsui for her dedicated work in creating the EDC system. Their support and expertise have been invaluable to the success of the WRAP study.
Abbreviations
- POPF
Postoperative pancreatic fistula
- DP
Distal pancreatectomy
- MIDP
Minimally invasive distal pancreatectomy
- PGA
Polyglycolic acid
- CR-POPF
Clinically relevant postoperative pancreatic fistula
- EDC
Electronic data capture
- CT
Computerized tomography
- ISGPS
The International Study Group of Pancreatic Surgery
- POD
Postoperative days
- DGE
Delayed gastric emptying
- PPH
Post-pancreatectomy hemorrhage
- FAS
Full analysis set
- CI
Confidence interval
- DSMC
Data and Safety Monitoring Committee
- AE
Adverse event
Authors’ contributions
All authors participated in conceptualization of the study and preparing the study protocol, YT coordinated the process. NM devised the statistical analysis plan. HB and AO drafted the manuscript and KT, TMiu, DB, MEd, YIs, KOh, HTan, RS, NI, TI, YK, RM, SY, ST, SA, TMiz, MT, HU, HD, HTak, KOk, KN, YM, HO, KS, DH, YIn, SH, MU, MEs, MK, KA, TS, TE, KU, MN, TO, YN, TF, SS, and YT have revised and approved the final version.
Funding
Research funding from Gunze Medical, Ltd. and KM Biologics Co., Ltd.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval was granted by the Certified Review Board of Cancer Institute Hospital of Japanese Foundation for Cancer Research. The Patients provided their written informed consent to participate in this trial.
Consent for publication
Not applicable.
Competing interests
Atsushi Oba has been awarded a grant (Bayer Yakuhin, Ltd.) to conduct an observational study to investigate the clinical impact of EOB-MRI. The other authors have no conflicts of interest to declare. Sohei Satoi declared research fundings from Nihon Servier, Amino-up co. and Boston Scientific.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Knaebel H, Diener M, Wente M, Büchler M, Seiler C. Systematic review and meta-analysis of techniques for closure of the pancreatic remnant after distal pancreatectomy. Br J Surg. 2005;92:539–46. [DOI] [PubMed] [Google Scholar]
- 2.Lillemoe KD, Kaushal S, Cameron JL, Sohn TA, Pitt HA, Yeo CJ. Distal pancreatectomy: indications and outcomes in 235 patients. Ann Surg. 1999;229:693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodríguez JR, Germes SS, Pandharipande PV, Gazelle GS, Thayer SP, Warshaw AL, Fernández-del CC. Implications and cost of pancreatic leak following distal pancreatic resection. Arch Surg. 2006;141:361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strasberg SM, Linehan DC, Clavien PA, Barkun JS. Proposal for definition and severity grading of pancreatic anastomosis failure and pancreatic occlusion failure. Surgery. 2007;141:420–6. [DOI] [PubMed] [Google Scholar]
- 5.Butturini G, Marcucci S, Molinari E, Mascetta G, Landoni L, Crippa S, Bassi C. Complications after pancreaticoduodenectomy: the problem of current definitions. J Hepatobiliary Pancreat Surg. 2006;13:207–11. [DOI] [PubMed] [Google Scholar]
- 6.Kato T, Inoue Y, Oba A, Ono Y, Sato T, Ito H, Takahashi Y. Laparoscopic Radical Antegrade Modular Pancreatosplenectomy with Anterocranial Splenic Artery-First Approach for Left-Sided Resectable Pancreatic Cancer (with Videos). Ann Surg Oncol. 2022;29:3505–14. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Oba A, Kato T, Kobayashi K, Wu YHA, Ono Y, et al. Feasibility of laparoscopic radical antegrade modular pancreatosplenectomy (RAMPS) as a standard treatment for distal resectable pancreatic cancer. Langenbecks Arch Surg. 2023;408:217. [DOI] [PubMed] [Google Scholar]
- 8.Adams AM, Russell DM, Carpenter EL, Nelson DW, Yheulon CG, Vreeland TJ. Minimally invasive versus open distal pancreatectomy: a matched analysis using ACS-NSQIP. Surg Endosc. 2023;37:617–23. [DOI] [PubMed] [Google Scholar]
- 9.Miyasaka Y, Mori Y, Nakata K, Ohtsuka T, Nakamura M. Attempts to prevent postoperative pancreatic fistula after distal pancreatectomy. Surg Today. 2017;47:416–42. [DOI] [PubMed] [Google Scholar]
- 10.Bilimoria MM, Cormier JN, Mun Y, Lee JE, Evans DB, Pisters PW. Pancreatic leak after left pancreatectomy is reduced following main pancreatic duct ligation. Br J Surg. 2003;90:190–6. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto M, Hayashi MS, Nguyen NT, Nguyen TD, McCloud S, Imagawa DK. Use of Seamguard to prevent pancreatic leak following distal pancreatectomy. Arch Surg. 2009;144:894–9. [DOI] [PubMed] [Google Scholar]
- 12.Pulvirenti A, Landoni L, Borin A, De Pastena M, Fontana M, Pea A, et al. Reinforced stapler versus ultrasonic dissector for pancreatic transection and stump closure for distal pancreatectomy: a propensity matched analysis. Surgery. 2019;166:271–6. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Kuroda Y, Morita A, Fujino Y, Tanioka Y, Kawamura T, et al. Fibrin glue sealing for the prevention of pancreatic fistulas following distal pancreatectomy. Arch Surg. 1995;130:952–5. [DOI] [PubMed] [Google Scholar]
- 14.Park JS, Lee DH, Jang JY, Han Y, Yoon DS, Kim JK, et al. Use of TachoSil(®) patches to prevent pancreatic leaks after distal pancreatectomy: a prospective, multicenter, randomized controlled study. J Hepatobiliary Pancreat Sci. 2016;23:110–7. [DOI] [PubMed] [Google Scholar]
- 15.Ochiai T, Sonoyama T, Soga K, Inoue K, Ikoma H, Shiozaki A, et al. Application of polyethylene glycolic acid felt with fibrin sealant to prevent postoperative pancreatic fistula in pancreatic surgery. J Gastrointest Surg. 2010;14:884–90. [DOI] [PubMed] [Google Scholar]
- 16.Jang JY, Shin YC, Han Y, Park JS, Han HS, Hwang HK, et al. Effect of polyglycolic acid mesh for prevention of pancreatic fistula following distal pancreatectomy: a randomized clinical trial. JAMA Surg. 2017;152:150–5. [DOI] [PubMed] [Google Scholar]
- 17.Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krle AJK, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Rev Panam Salud Publica. 2015;38(6):506–14. [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo N, Uemura K, Nakagawa N, Okada K, Kuroda S, Sudo T, et al. Hiroshima Surgical Study Group of Clinical Oncology. A Multicenter, Randomized, Controlled Trial Comparing Reinforced Staplers with Bare Staplers During Distal Pancreatectomy (HiSCO-07 Trial). Ann Surg Oncol. 2019;26(5):1519–27. [DOI] [PubMed] [Google Scholar]
- 19.Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–91. [DOI] [PubMed] [Google Scholar]
- 20.Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761–8. [DOI] [PubMed] [Google Scholar]
- 21.Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–5. [DOI] [PubMed] [Google Scholar]
- 22.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kosaka H, Satoi S, Yamamoto T, Hirooka S, Yamaki S, Kotsuka M, et al. Clinical impact of the sequentially-checked drain removal criteria on postoperative outcomes after pancreatectomy: a retrospective study. J Hepatobiliary Pancreat Sci. 2019;26:426–34. [DOI] [PubMed] [Google Scholar]
- 24.de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F, et al. Dutch Pancreatic Cancer Group. Minimally Invasive Versus Open Distal Pancreatectomy (LEOPARD): A Multicenter Patient-blinded Randomized Controlled Trial. Ann Surg. 2019;269:2–9. [DOI] [PubMed] [Google Scholar]
- 25.Allen PJ, Gönen M, Brennan MF, Bucknor AA, Robinson LM, Pappas MM, et al. Pasireotide for postoperative pancreatic fistula. N Engl J Med. 2014;370:2014–22. [DOI] [PubMed] [Google Scholar]
- 26.Maggino L, Malleo G, Salvia R, Bassi C, Vollmer CM Jr. Defining the practice of distal pancreatectomy around the world. HPB (Oxford). 2019;21:1277–87. [DOI] [PubMed] [Google Scholar]
- 27.Elkomos BE, Elkomos PE, Salem AA, Adly PB. The outcome of bioabsorbable staple line reinforcement versus standard stapler for distal pancreatectomy: A systematic review and meta-analysis. J Minim Access Surg. 2022;18:338–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaida H, Kono H, Amemiya H, Hosomura N, Saito R, Takahashi K, et al. Use of a reinforced triple-row stapler following distal pancreatectomy reduces the incidence of postoperative pancreatic fistula in patients with a high BMI. Anticancer Res. 2019;39:1013–8. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka R, Saiura A, Koga R, Seki M, Kishi Y, Morimura R, et al. Risk factors for clinical pancreatic fistula after distal pancreatectomy: analysis of consecutive 100 patients. World J Surg. 2010;34:121–5. [DOI] [PubMed] [Google Scholar]
- 30.Löhr JM, Panic N, Vujasinovic M, Verbeke CS. The ageing pancreas: a systematic review of the evidence and analysis of the consequences. J Intern Med. 2018;283:446–60. [DOI] [PubMed] [Google Scholar]
- 31.Halle-Smith JM, Vinuela E, Brown RM, Hodson J, Zia Z, Bramhall SR, et al. A comparative study of risk factors for pancreatic fistula after pancreatoduodenectomy or distal pancreatectomy. HPB (Oxford). 2017;19(8):727–34. [DOI] [PubMed] [Google Scholar]
- 32.Eguchi H, Nagano H, Tanemura M, Takeda Y, Marubashi S, Kobayashi S, et al. A thick pancreas is a risk factor for pancreatic fistula after a distal pancreatectomy: selection of the closure technique according to the thickness. Dig Surg. 2011;28:50–6. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura M, Shindo K, Ideno N, Ueda J, Takahata S, Nakashima H, Ohtsuka T, Shimizu S, Oda Y, Tanaka M. Prediction of pancreatic fistula by preoperatively assessable factors; retrospective review of unified operations by a single surgeon. Hepatogastroenterology. 2014;61(131):834–7. [PubMed] [Google Scholar]
- 34.De Pastena M, van Bodegraven EA, Mungroop TH, Vissers FL, Jones LR, Marchegiani G, et al. Distal Pancreatectomy Fistula Risk Score (D-FRS): Development and International Validation. Ann Surg. 2023;277(5). 10.1097/SLA.0000000000005497. [DOI] [PubMed]
- 35.Peng YP, Zhu XL, Yin LD, Zhu Y, Wei JS, Wu JL, Miao Y. Risk factors of postoperative pancreatic fistula in patients after distal pancreatectomy: a systematic review and meta-analysis. Sci Rep. 2017;7(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.