ABSTRACT
The objective of this study was to determine risk factors and sources attributed to yersiniosis in Aotearoa New Zealand (NZ). A risk factor questionnaire was administered to 247 notified yersiniosis cases and 258 control participants from the Canterbury and/or Wellington regions of NZ. Yersinia sp. isolates from clinical cases and a range of food sources were whole-genome sequenced and genetically compared. Yersinia enterocolitica (YE) bioserotype 2/3, O:9 [McNally multi-locus sequence type (ST) 12] and YE Biotype (BT) 1A (46 different STs) predominated within the consented cases (45 and 27%, respectively). Exposure to pork was identified as a significant risk factor for cases associated with YE ST12. The presence of YE ST12 was confirmed in retail raw meat, primarily raw pork. Single-nucleotide polymorphism (SNP) analysis identified multiple genomically very closely related clusters (0–5 SNPs) of YE ST12, predominately from raw pork with clinical cases from one or both regions. Risk factors associated with YE BT 1A included the consumption of cooked seafood, sushi, tofu, and some vegetable types. Analysis of specific risk factors and SNP analysis, combined, indicate that raw pork is a significant risk factor for exposure and infection to pathogenic YE cases, but not BT 1A cases.
KEYWORDS: Yersinia enterocolitica, case-control study, whole-genome sequencing
INTRODUCTION
Yersiniosis is a gastrointestinal infectious disease caused by the bacteria Yersinia enterocolitica (YE) and less frequently Y. pseudotuberculosis (YP). Symptoms in humans includes diarrhea, vomiting, fever, and abdominal pain. Yersiniosis is a notifiable disease under the Health Act 1956 in Aotearoa, New Zealand (NZ). Since 2015, the rates of yersiniosis in NZ have significantly increased, with the 2022 rate of 25.3 cases per 100,000 population being higher compared with other industrialized countries that ranged from 0.2 to 1.9 cases per 100,000 (1–5).
Up until 2012, the most common NZ strain was YE biotype (BT) 4 but the emergent bioserotype since this time is YE BT 2/3, serotype O:9 (5), and the reasons why this change has occurred are unclear. Yersiniosis is not a notifiable disease in many countries, but the predominance of YE BT 4 continues to be reported in countries where surveillance and typing are available (1, 6).
In NZ, YE BT 1A is notifiable, yet many other countries consider this BT to be non-pathogenic due to this BT lacking the classical YE virulence determinants (7, 8). The pathogenicity of YE BT 1A is controversial, but there is growing epidemiological and experimental evidence to suggest that YE BT 1A can cause human disease and may cause illness through an alternative mechanism (8, 9).
Previous epidemiological studies have shown that the majority of human yersiniosis cases in NZ are sporadic with no identifiable source (10). In addition, there is a paucity of local information on source attribution, meaning that there is currently no evidential base for interventions to reduce disease incidence. Baseline data on Yersinia from foods and the environment are lacking or historic as food surveillance is not routinely performed, and thus, the etiology of yersiniosis in NZ remains unclear (5, 11).
We report a case-control study involving notified human yersiniosis cases within two NZ regions, as well as whole-genome sequence (WGS) analysis of YE isolates obtained from clinical cases and from source testing. The objective of the study was to generate up-to-date evidence on potential sources of YE in NZ in order to devise control strategies to improve the yersiniosis public health outcomes.
MATERIALS AND METHODS
Case-control study
A confirmed case of yersiniosis in NZ is defined as a clinically compatible illness accompanied by laboratory definitive evidence of either (i) isolation of YE or YP from blood or feces or (ii) detection of Yersinia spp. nucleic acid from feces (12). Yersiniosis cases for the study were recruited following notification between 25 June 2021 and 30 November 2022 from the Canterbury region (comprising the Canterbury, West Coast, and South Canterbury districts), and between 1 August and 28 November 2022 from the Wellington region (comprising the Capital and Coast, Hutt, and Wairarapa districts).
All cases were contacted by telephone and administered a questionnaire, which included routine public health investigation information and more detailed risk factor and exposure data (Supplementary Data 1). All yersiniosis cases were initially eligible for inclusion in the study, regardless of age, gender, and ethnicity. The routine protocol for public health investigation is that a parent or guardian is interviewed on behalf of a notified case that is under 16 years of age.
The parent or caregiver must reside in the same household as the case. For the case-control study, cases aged 7–15 years were provided the opportunity to participate in the interview or they could assent for their parent or caregiver to be interviewed on their behalf.
A case was excluded from the study if, during the interview, it was determined that the case was either asymptomatic, was not able to communicate (personally or through a proxy) in English, was overseas within 10 days prior to illness onset, or was a resident in an institution or equivalent (i.e., did not have access to a personal telephone) at the date of notification.
Following completion of the interview, all eligible cases (or their assented proxy reporters) were provided information regarding the study (via email/postal mail and telephone) and were subsequently contacted to seek consent to use their anonymized responses to the questionnaire for the purpose of the case-control study.
Controls were obtained from participants in the NZ Health Survey (NZHS) cohort, who had given consent to be approached to participate in further studies. The NZHS is an annual survey of individuals from approximately 13,000 adults and the parents or primary caregivers of approximately 4,000 children (13). Controls from the Canterbury region were matched by gender, age group, and urban/rural classification (14). Control individuals were randomly selected in batches throughout the study and notified of the study by email or postal mail. Individuals were subsequently contacted via telephone. Control inclusion criteria include those who resided in the Canterbury region during the study period and those who
had not been notified with yersiniosis in the last 10 days,
had not had a recent (within the last 10 days) acute illness with symptoms of diarrhea, abdominal pain, and/or cramps, which may be accompanied by fever and blood in the stool,
could communicate (personally or through a proxy) in English,
were not residents in an institution or equivalent (i.e., did not have access to a personal telephone) at the date of selection,
are not part of an enteric disease outbreak already identified at the date of selection,
had not been overseas in the last 10 days, and
consented to participation in the study.
Individuals that met the criteria were administered the same questionnaire as cases, excluding yersiniosis illness-specific questions. Control participants were requested to recall their activities and food consumed or prepared in their households in the 7 days prior to the interview.
All questionnaires were administered by the same interviewer and piloted prior to beginning the study with a range of individuals. Internal review also included an assessment of the questionnaire for Māori cultural appropriateness and language provided by a Māori public health advisor.
Source testing
Concurrently with the case-control study, a sampling programme was conducted to isolate YE from food samples from various retailers within the Canterbury region. Samples of food, water from bores or potentially untreated sources, and/or feces from animals that consenting cases had indicated exposure 7 days prior to their illness, were also tested when available.
Testing and isolation of YE from all source samples were performed using International Organization for Standardization (ISO) methods (15, 16). Either 25 g or 25 mL of samples was diluted in 225 mL peptone, sorbitol, and bile salts (PSB) broth (Fort Richard, Auckland, NZ) and homogenized for 2 min using a stomacher. A 10-mL aliquot of this homogenate was inoculated into 90 mL Irgasan, ticarcillin, and potassium chlorate (ITC) broth and incubated at 25 ± 1°C, 44 h ± 3 h. The PSB enrichment was incubated at 25 ± 1°C for 24 h ± 3 h, and a DNA extraction was prepared using 1 mL of the enrichment and the Qiagen DNeasy bloody and tissue kit (Qiagen, Hilden, Germany). The DNA was screened using the real-time polymerase chain reaction (RT-PCR) outlined in ISO/TS18867:2015 (16).
Enrichments that resulted in a cycle threshold of ≤38 were selected for further testing, which involved alkaline treatment of 0.5-mL aliquot of the PSB enrichment into 4.5 mL 0.5% (wt/vol) potassium hydroxide solution prepared the day prior, mixing the suspension gently for 20 sec before inoculating 10 µL and 100 µL onto separate Cefsulodin-Irgasan-Novobiocin (CIN) agar plates (Fort Richard) and CHROMagar Yersinia (CH-Yersinia, Fort Richard, NZ) and streaking each aliquot on each plate using a sterile loop to obtain single colonies. Following incubation at 30°C for 24 h ± 2 h, plates were assessed for typical YE colonies on CIN previously described (15, 17). Five colonies were selected, and each streaked onto Columbia blood agar (CBA, Fort Richard) using a sterile loop. For CH-Yersinia, five colonies presenting a mauve or white phenotype and, if not present, then the blue phenotype were selected and streaked onto CBA and incubated at 30°C for 24 h ± 2 h. The plating and colony selection process was repeated on the PSB enrichment following 48 h incubation and the ITC enrichment. Presumptive colonies were prepared using Chelex 100 (Bio-Rad, Hercules, CA. US) preparation screened for YE using the RT-PCR. The Chelex preparations for all YE RT-PCR-positive isolates for each sample were screened using a PCR to provide a presumptive serotype (O:9, O:5, O:3, or O:8) for each isolate (18). For each sample, one isolate for each presumptive serotype was selected for further biochemical confirmation (Microgen GN-ID, Microgen Bioproducts, Camberley, UK) for YE and whole-genome sequencing.
The same DNA extraction for the PSB enrichment used for screening of pathogenic YE was screened by conventional PCR for YE BT 1A using gene targets foxA and ystB (19, 20). For those enrichments that resulted in the detection of both foxA and ystB, the colonies selected for CIN pathogenic YE above were screened using PCR. For CH-Yersinia, five blue colonies were selected and streaked onto CBA and incubated at 30°C for 24 h ± 2 h. The colony selection process was repeated using plates from PSB following 48 h incubation and for the ITC enrichment. Presumptive colonies were prepared using Chelex were screened for YE BT 1A using PCR. For each sample, one presumptive YE BT 1A was selected for further biochemical confirmation (Microgen, Supplier) for YE and whole-genome sequencing.
Bacterial strains and whole-genome sequencing
All YE and YP isolates from notified yersiniosis cases associated with the case-control study underwent whole-genome sequencing. All clinical isolates were grown on Tryptone Soya Agar (Fort Richard) at 28°C for 24 h ± 2 h. This was then used to inoculate 1 mL TE buffer to a density of 2–4 McFarland Standard, which was then heat inactivated at 65°C for 1 h. These suspensions were stored at 4°C until genomic DNA extraction using a Chemagic 360 extraction platform (PerkinElmer, Waltham, MA, US). Selected YE isolates from source sampling were grown on CBA and incubated at 30°C for 24 h ± 2 h prior to DNA extraction using the Qiagen DNeasy Kit. DNA quality and concentration were performed using PicoGreen (Quant-iT; Thermo Fisher Scientific). Sequencing libraries containing 1 ng of DNA were prepared using Nextera XT chemistry (Illumina, San Diego, CA. USA) for 150 bp pair-end sequencing on an Illumina NextSeq sequencer, according to the manufacture’s recommendations (Illumina).
Quality reference genomes were produced for YE ST12 (16ER3959), ST14 (20ER2233), and ST18 (20ER2231), using reads from Illumina and Oxford nanopore sequencing [Oxford Nanopore Technologies (ONT); Oxford, UK]. DNA extraction for both sequencing platforms was performed using the Qiagen DNeasy Kit. Illumina sequencing was performed as per methods above. Nanopore sequencing was performed using the native barcoding kit (SQK-RAD004, ONT), run on a MinION flow cell (FLO-MIN106, ONT). Reads were base called on the MinION instrument using the high-accuracy base-calling model. Quality trimming was performed using Filtlong v. 0.2.0 (https://github.com/rrwick/Filtlong), removing reads less than 1,000 bp and retaining 90% of the best reads. A hybrid assembly was performed using Unicycler v.0.4.8 (https://github.com/rrwick/Unicycler), with nanopore and Illumina reads, using the conservative mode.
Raw sequence reads for all isolates analyzed in this study are available on the National Center for Biotechnology Information short read archive with BioProject number PRJNA 1142067.
Initial sequence quality and species identification using the Illumina data were determined using the Nullarbor version 2 pipeline (21). The multi-locus ST was inferred using WGS data and the McNally scheme for YE and Achtman scheme for YP (22, 23).
The WGS for each isolate was assembled within BioNumerics (BN) version 7.6.3 using Skesa v.2.3 (Applied Maths NV, Belgium), and the following analyses were performed within BN:
Whole-genome single-nucleotide polymorphism (SNP) analysis using strict SNP filtering options. Separate SNP comparisons were analyzed for isolates within YE ST12, ST14, ST18, and YP using the NZ reference genomes 16ER3959, 20ER2233, 20ER2231, and YP4713 (ST42) (24), respectively.
For YE BT 1A, assembly-based calls using the core-genome multi-locus sequence typing (cgMLST) scheme within BN with allelic profiles synchronized with public nomenclature hosted by the Pasteur Institute for YE (1,727 loci) (25) were performed on all isolates. Subsequent SNP analysis using the reference genome 16ER3959 (ST12), as well as whole-genome MLST (wgMLST; BN scheme; 20,638 loci), was performed on cgMLST clusters that contained clinical isolates only.
Additional analyses for YP included cgMLST analysis within BN as outlined above but using the YP scheme (1,921 loci) (26) and wgMLST scheme within BN.
Cluster analysis (categorical values) using the single-linkage algorithm was used for each analysis above, and a unique cluster identification was assigned to isolates that shared 0–5 SNP or allele differences between them. This threshold was based on a previous outbreak investigation that used SNP analysis (27), as well as ongoing surveillance observations in NZ. Similar threshold values were also reported in other studies that used cgMLST (28, 29). Whole-genome MLST demonstrated similar clustering of YE and YP isolates as observed for SNP analysis when using the 0–5 allele threshold (Supplementary data 2.3).
Statistical analysis
For the case-control study, logistic regression analysis was conducted for each risk factor adjusting for both gender and age and odds ratios (OR) with 95% confidence intervals were calculated in R version (4.2.2) with Tidyverse (30, 31). Variables analyzed are outlined in Table S1. Participant responses that were either “unknown” or “unsure” were excluded from calculations. The Pearson’s chi-squared test using R software was used to assess statistical significance within case-control and food testing data.
RESULTS
Case-control study
Of the 291 yersiniosis cases notified collectively within the study regions and periods, 93% (n = 272) was able to be contacted and 85% (n = 247) was eligible and completed the risk factor questionnaire and subsequently consented to the study (Table S2). Seventy-five percent of consenting cases was interviewed within 5 days from notification.
Of the consenting cases, 54% (n = 134) was female, 81% (n = 199) resided in urban areas, and they ranged in age from 4 months to 95 years (Table S2). The number of consenting cases did not significantly differ for gender (P = 0.84) or age group (P = 0.39) to those notified during the study period.
Forty-one of the consenting cases (17%), predominately from the Wellington region, were notified following the culture-independent diagnostic test detection of Yersinia spp., from their fecal sample, but a corresponding bacterial isolate was not recovered, and therefore, no further typing was possible for those cases.
Four epidemiological clusters of consenting cases (same household or preschool; total of 10 cases) were identified (Supplementary data 2.1). The delayed onset of symptoms, as well as the close-contact association of cases within two of these clusters, indicates possible person-to-person transmission. Thirteen consenting cases (five YE BT 2/3 and eight YE BT 1A) were notified with dual enteric infections (Supplementary data 2.2).
A total of 258 eligible and consenting control individuals completed a risk factor questionnaire. The number of control individuals did not significantly (P > 0.1) differ to those of consenting cases according to gender, age group, or urban/rural classification.
Table 1 outlines the statistically significant risk factors identified for each case definition. The complete data set of adjusted OR (aOR) calculations is available in Table S3. The aORs were comparable for each case definition with respect to different onset dates and exclusion/inclusion of dual diagnoses (Table S4).
TABLE 1.
Significant risk factors for yersiniosis based on different case definitionsa
| Did you eat or did anyone in the household prepare…? | Controls | All yersinosis | Pathogenic YE | YE ST12 | YE BT 1A | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | aOR (95% CI) | Yes | No | aOR (95% CI) | Yes | No | aOR (95% CI) | Yes | No | aOR (95% CI) | |
| Pork | 211 | 45 | 216 | 25 | 1.82 (1.08–3.12) | 119 | 10 | 2.59 (1.3–5.68) | 103 | 8 | 2.9 (1.37–6.93) | 55 | 9 | 1.18 (0.55–2.77) |
| Pork chops | 55 | 203 | 71 | 176 | 1.57 (1.04–2.39) | 41 | 90 | 1.91 (1.17–3.11) | 37 | 75 | 2.1 (1.26–3.50) | 16 | 51 | 1.06 (0.54–1.99) |
| Pork shoulder | 17 | 241 | 34 | 213 | 2.23 (1.22–4.22) | 18 | 113 | 1.98 (0.97–4.07) | 16 | 96 | 2.11 (1.00–4.44) | 8 | 59 | 2.08 (0.79–5.13) |
| Pork mince | 15 | 243 | 40 | 207 | 3.04 (1.65–5.85) | 26 | 105 | 3.61 (1.82–7.35) | 25 | 87 | 4.44 (2.21–9.20) | 7 | 60 | 1.77 (0.64–4.57) |
| Pork belly | 17 | 241 | 29 | 218 | 1.86 (1.00–3.55) | 18 | 113 | 2.11 (1.04–4.33) | 16 | 96 | 2.31 (1.10–4.82) | 7 | 60 | 1.72 (0.63–4.33) |
| Pork dumplings | 13 | 245 | 26 | 221 | 2.16 (1.1–4.44) | 15 | 116 | 2.38 (1.08–5.30) | 12 | 100 | 2.17 (0.93–5.01) | 8 | 59 | 2.36 (0.88–6.01) |
| Cooked seafood | 121 | 137 | 127 | 99 | 1.51 (1.05–2.17) | 62 | 62 | 1.18 (0.76–1.83) | 52 | 55 | 1.12 (0.71–1.78) | 41 | 19 | 2.26 (1.24–4.23) |
| Sushi | 46 | 210 | 46 | 171 | 1.23 (0.77–1.95) | 16 | 107 | 0.71 (0.37–1.31) | 14 | 92 | 0.73 (0.37–1.38) | 18 | 36 | 2.18 (1.11–4.20) |
| Tofu | 17 | 239 | 27 | 199 | 1.83 (0.97–3.53) | 11 | 109 | 1.27 (0.55–2.82) | 10 | 94 | 1.35 (0.57–3.06) | 12 | 49 | 3.49 (1.49–8.04) |
| Coleslaw | 83 | 173 | 87 | 131 | 1.44 (0.97–2.14) | 35 | 84 | 1.03 (0.62–1.69) | 32 | 70 | 1.16 (0.68–1.95) | 33 | 24 | 2.45 (1.35–4.51) |
| Prebagged salad | 87 | 169 | 81 | 143 | 1.11 (0.75–1.65) | 32 | 91 | 0.76 (0.46–1.24) | 30 | 76 | 0.86 (0.51–1.44) | 33 | 25 | 2.35 (1.29–4.31) |
| Spinach | 56 | 199 | 66 | 155 | 1.51 (0.99–2.32) | 28 | 95 | 1.15 (0.67–1.96) | 24 | 82 | 1.15 (0.65–2.01) | 24 | 31 | 2.46 (1.32–4.59) |
| Carrots | 151 | 106 | 131 | 90 | 1.01 (0.70–1.46) | 62 | 60 | 0.74 (0.48–1.16) | 53 | 52 | 0.72 (0.45–1.15) | 44 | 15 | 2.2 (1.17–4.33) |
| Sprouts | 25 | 233 | 24 | 203 | 1.06 (0.58–1.94) | 9 | 117 | 0.74 (0.31–1.61) | 8 | 101 | 0.75 (0.30–1.69) | 12 | 46 | 2.3 (1.01–5.08) |
| Spring onions | 43 | 212 | 41 | 183 | 1.16 (0.71–1.89) | 17 | 105 | 0.97 (0.50–1.80) | 15 | 91 | 0.98 (0.49–1.88) | 20 | 39 | 2.23 (1.15–4.27) |
| Fresh herbs | 85 | 170 | 69 | 151 | 0.95 (0.64–1.42) | 27 | 96 | 0.62 (0.37–1.04) | 23 | 83 | 0.60 (0.34–1.03) | 29 | 26 | 2.07 (1.13–3.80) |
aORs and the 95% CI were calculated using all controls and cases within either of the following case definitions: (i) all yersiniosis cases (including cases where an isolate was not referred for typing), (ii) pathogenic YE (collectively defined as bioserotypes 2/3, O:9 and O:5, 27 and 4, O:3), YE multi-locus ST 12 (correlating to bioserotype 2/3; O:9), or YE BT 1A. Odds ratios were adjusted for both gender and age. All cases meeting each definition were included irrespective of onset date of symptoms or dual diagnoses. Risk factors that were observed to be significant for at least one case definition included in this table. Those with statistical significance are shown in boldface. Results for all risk factors are provided in the Supplementary data.
For the purpose of this study, “pathogenic YE” refers collectively to bioserotypes BT 2/3, O:9; BT 2/3, O:5, 27; and BT 4, O:3, which have been previously observed to correlate to ST12, ST14, and ST18, respectively (5). Exposure to pork, either via consumption or being within a household where raw pork was prepared, was a significant risk factor for cases with pathogenic YE (aOR 2.6) or YE ST12 (aOR 2.9), but not for YE BT 1A. Specifically, exposure to pork mince, shoulder, chops, belly, and dumplings were all significant risk factors (aOR 1.9–4.4; Table 1).
For YE BT 1A cases, significant risk factors included vegetables (carrot, coleslaw, prebagged salad greens, spinach, spring onions, sprouts, and fresh herbs), sushi, tofu, and cooked seafood (aOR 2.2–3.5; Table 1). Additional case information provided for cooked seafood included fish, prawns, squid, oysters, and mussels.
Direct animal contact, drinking water source, recreational water exposures, and other food exposures included in the questionnaire were not significant risk factors for either pathogenic YE or YE BT 1A (Table S3).
Exposure to chicken, either via consumption or within the household where chicken was prepared, meets statistical thresholds for all yersiniosis case definitions examined except for YE BT 1A (Table S3). However, the high frequency of this exposure among cases and controls (85%–91%) meant the association could not be robustly assessed. Conversely, exposure to other poultry such as duck, turkey, or wildfowl; offal; mangos; and buying meat from an Asian store met statistical thresholds for pathogenic YE and YE ST12 (Table S3), but these risk factors were observed in a very small number of controls and cases (5–10). Caution must therefore be taken when interpreting the association of these risk factors within the case definitions. Calculating aORs for YP as a separate case definition was not possible due to insufficient case numbers.
Source testing and genomic comparisons
Pathogenic YE (collectively ST12, ST14, and ST18) were isolated from 15% (74/499) of all samples tested with all positive samples from raw meat products (Table 2). The prevalence was significantly (P < 0.001) greater for raw pork (28%; 57/203) compared with raw beef (12%; 10/81) or raw lamb (9%; 5/53). Prevalence was also significantly (P < 0.001) greater for raw pork mince (38%; 26/69) compared with other raw pork products (21%–24%). YE ST12 (n = 53) was the most prevalent pathogenic YE ST isolated, followed by ST14 (n = 25) and ST18 (n = 10). Fourteen raw meat products contained two different pathogenic YE STs (ST12, ST14, and/or ST18).
TABLE 2.
Prevalence of YE from source samples
| Sample typea | Number of samples tested | Number of culture-positive samples | ||||
|---|---|---|---|---|---|---|
| YE ST12b | YE ST14b | YE ST18b | Total pathogenic YE (%)b | YE BT 1A (%) | ||
| Raw pork—mince | 69 | 19 | 10 | 6 | 26 (38%)c | 51 (74%) |
| Raw pork—other | 105 | 18 | 7 | 2 | 25 (24%)c | 57 (54%) |
| Raw pork sausages | 29 | 2 | 4 | 1 | 6 (21%)c | 14 (48%) |
| Raw beef | 81 | 7 | 2 | 1 | 10 (12%) | 49 (60%) |
| Raw lamb | 53 | 5 | 2 | 0 | 5 (9%) | 34 (64%) |
| Raw venison | 5 | 1 | 0 | 0 | 1 (20%) | 3 (60%) |
| Raw sausages | 13 | 1 | 0 | 0 | 1 (8%) | 4 (31%) |
| Raw chicken | 21 | 0 | 0 | 0 | 0 | 18 (86%) |
| Raw duck | 5 | 0 | 0 | 0 | 0 | 4 (80%) |
| Raw meat pet food | 4 | 0 | 0 | 0 | 0 | 1 (25%) |
| Bacon | 20 | 0 | 0 | 0 | 0 | 3 (15%) |
| RTE ham | 19 | 0 | 0 | 0 | 0 | 2 (11%) |
| Other RTE meat | 17 | 0 | 0 | 0 | 0 | 0 |
| Dumplings/buns | 14 | 0 | 0 | 0 | 0 | 0 |
| Fruits and vegetables | 17 | 0 | 0 | 0 | 0 | 0 |
| Other foods | 11 | 0 | 0 | 0 | 0 | 0 |
| Animal feces | 11 | 0 | 0 | 0 | 0 | 0 |
| Bore water | 5 | 0 | 0 | 0 | 0 | 0 |
| Total | 499 | 53 (11%) | 25 (5%) | 10 (2%) | 74 (15%) | 240 (48%) |
RTE, ready-to-eat (RTE); fruits and vegetables included fresh baby spinach, cucumber, carrot, grapes, and mung bean sprouts and frozen raspberries and blueberries. Other foods included raw milk, raw prawn, and tofu.
YE multilocus ST12, 14, or 18 inferred using whole-genome sequencing data, which are equivalent to bioserotypes 2/3, O:9, O:5, 27 and 4, O:3, respectively (collectively defined as “pathogenic YE”).
Nine raw pork mince and two each from raw pork other and raw lamb, and one raw pork sausage sample had more than one pathogenic YE isolated.
The prevalence of YE BT 1A across all sample types was 48% (Table 2). YE BT 1A was isolated from various meat types, with no significant (P = 0.295) difference in the prevalence between sample types (raw pork, lamb, or beef) that had sufficient sample numbers for statistical analysis. Of the raw pork containing pathogenic YE, 65% (37/57) also had YE BT 1A. Forty-eight different STs were identified among 245 YE BT 1A isolates obtained from source samples, with the top five most frequently observed including ST3 (n = 53), ST8 (n = 30), ST178 (n = 17), ST147 (n = 12), and ST213 (n = 10) (Table S5).
Whole-genome sequencing was performed on 239 clinical isolates from the Canterbury and Wellington regions (n = 194 and 45, respectively). Of these, 141 were pathogenic YE (ST12, n = 119; ST14, n = 14; and ST18, n = 8), 85 were YE BT 1A, and 13 were YP.
Genomic comparisons using SNP analysis identified multiple clusters containing pathogenic YE from clinical and source isolates (ST12; n = 7, ST14; n = 3; and ST18; n = 2), with each cluster sharing low-level (0–5) SNP differences between them (Fig. 1; Table S6). A total of six clusters had clinical isolates from the two regions, while three of these clusters had isolates from raw pork and other raw meat types. The most predominant cluster was within YE ST12 (YE_ST12_2020_04), which contained clinical isolates (n = 61) from both regions and source isolates (n = 24) from raw pork, beef, and lamb purchased from a variety of retailers throughout the study. In addition, this cluster also contained three pairs of YE ST12 isolates that were genomically indistinguishable (0 SNP) that were obtained from different raw meat type (raw pork, beef, and/or lamb) samples purchased from the same retailer on the same day.
Fig 1.
Minimum spanning tree based on SNP analysis of Y. enterocolitica ST12 (A), ST14 (B), and ST18 (C) [using the McNally scheme (22)]. Branch length scaling is logarithmic with number of SNP differences shown on branches. Isolates sharing ≤5 SNP differences were collapsed into a single node for better visualization and assigned a unique cluster ID (as labeled). Isolates joined within a node or within a pie have zero SNP differences. Cluster YE_ST12_2020_C_04 for YE ST12 contained 85 isolates [54 and 7 from clinicals from Canterbury and Wellington, respectively, 17 raw pork isolates, and 7 from other raw meats (4 beef and 3 lamb)].
For YE BT 1A, 46 different STs were identified among the 85 isolates from clinical cases, with ST192 (n = 6), ST278 (n = 5), and ST291 (n = 5) identified as the most frequently observed STs (Table S5). Twenty-two of the STs identified contained clinical (total of 52) and source isolates (total of 160). There were 24 STs with a total of 33 clinical isolates that did not have a source isolate of the same ST. Conversely, there were 26 STs with a total of 85 source samples that did not have a clinical isolate of the same ST.
Core-genome MLST analysis resulted in 29 clusters (0–5 allele differences) of YE BT 1A isolates that either contained YE BT 1A isolates from clinical cases only (10 clusters with 2–4 isolates each) or one or more clinical isolate(s) with source isolate(s) (19 clusters) (Fig. 2; Table S7). An additional 35 cgMLST clusters, encompassing over 64% (158/245) of the total source isolates (across 22 STs), were observed to share low-level (0–5 allele) differences, with 30 of the clusters containing isolates from raw pork and other raw meat types (Fig. 2; Table S7).
Fig 2.
Minimum spanning tree based on core-genome multi-locus sequence typing analysis of Y. enterocolitica BT 1A. Branch length scaling is logarithmic with number of allele differences shown on branches. Isolates sharing ≤5 allele differences were collapsed into a single node for better visualization and assigned a unique cluster ID (as per supplementary data). Isolates joined within a node or within a pie have zero allele differences. Multi-locus ST as per McNally scheme (22) labeled next to isolates/node in blue. Each gray-shaded group represents isolates within the same ST. Isolates within a single node were not shaded.
The use of SNP analysis demonstrated further discrimination within the 29 YE BT 1A cgMLST clusters that contained clinical isolates (Fig. 3). Only four pairs of YE BT 1A clinical isolates shared low-level (0–5) SNP differences, while only one of these pairs (ST754) clustered with a single source isolate. Four additional single-YE BT 1A clinical isolates were observed to each cluster with one or more source isolates, sharing low-level (0–5) SNP differences between isolates (Fig. 3).
Fig 3.
Minimum spanning tree based on SNP analysis of Y. enterocolitica BT 1A isolates. Branch length scaling is logarithmic with number of SNP differences shown. Isolates sharing ≤5 SNP differences were collapsed into a single node for better visualization. Isolates joined within a node or within a pie have zero SNP differences. Cluster ID codes (assigned to isolates sharing ≤5 SNP differences) shown in red for those clusters containing more than one clinical isolate or clinical isolates with source isolates only. Multi-locus ST as per McNally scheme (22) labeled next to isolates/node in blue. Each gray-shaded group represents isolates within the same ST. Isolates within a single node were not shaded.
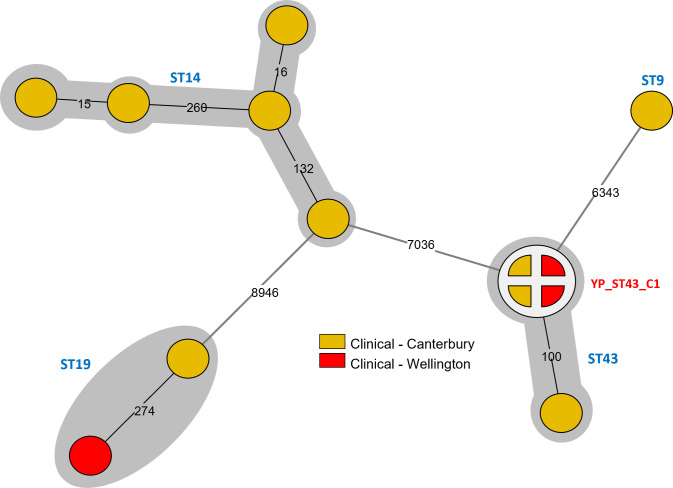
For YP, four different STs (ST9, ST19, ST14, and ST43) were identified among the 13 clinical isolates analyzed. Using SNP analysis, only four clinical isolates (ST43; two each from Canterbury and Wellington) had low-level (0–5) SNP differences between them (Fig. 4).
Fig 4.
Minimum spanning tree based on SNP analysis of Y. pseudotuberculosis isolates. Branch length scaling is logarithmic with number of SNP differences shown on branches. Isolates sharing ≤5 SNP differences were collapsed into a single node and assigned a unique cluster ID (labeled in red). Multi-locus STs as per Achtman scheme (23) labeled next to isolates/node in blue. Each gray-shaded group represents isolates within the same ST.
DISCUSSION
In this study, only 3% of cases were YE BT 4, O:3 (ST18), which contrasts with the only previous NZ case-control study where 90% of yersiniosis cases was of this bioserotype (32). Instead in the current study, YE BT 2/3, O:9 (ST12) was the most prevalent pathogenic YE bioserotype confirmed among consenting cases (45%) interviewed, followed by YE BT 1A (27%), which is consistent with the national yersiniosis notification percentages for NZ (4).
In this study, exposure to pork, either via consumption or within a household where raw pork was prepared, was identified as a significant risk factor for pathogenic YE and more specifically YE ST12. This risk factor was identified for yersiniosis in the previous NZ case-control study, and several other international studies have also indicated that YE infections are frequently linked to the consumption of undercooked contaminated pork or cross-contamination of other food items during handling and preparation of raw pork (1, 32–35).
SNP analysis of the WGS data identified multiple genomically related clusters of pathogenic YE from clinical (from both regions) and source isolates, particularly from raw pork. In addition, during the case-control study, an increase in YE BT 2/3, O:9 cases occurred in another region of NZ (Auckland) in June–July 2021. A subset of YE isolates from that investigation underwent whole-genome sequencing, and the genomic analysis showed that those clinical isolates were genomically very closely related (0–5 SNP) and clustered among clinical and source isolates from the current study (data not shown). Case interviews undertaken as a part of the outbreak investigation within Auckland identified an association with consumption of pork products. Another 2016 outbreak of YE ST12 from multiple regions in NZ also showed clinical case isolates were genomically related (≤5 SNP), and 68% (39/57) of cases had identified as consuming pork (27). Processors of pork may distribute product across the country, which supports the observation of genomically related clinical cases occurring nationwide.
Source sampling throughout the current study highlighted that pathogenic YE was more frequently isolated from raw pork than other meat types tested and 48% of the isolates from raw pork was observed to genomically cluster with other source isolates. The sampling methodology and the WGS analysis cannot inform on the direction of contamination, but the close genetic relationships between pathogenic YE from raw pork and other meat types from the same retailers may suggest cross-contamination in these environments. Collectively, the evidence to date indicates that pork is the most significant risk factor for yersiniosis, particularly ST12 in NZ.
In contrast to pathogenic YE, pork was not identified as a significant risk factor for cases with YE BT 1A in this study. Instead, the consumption or preparation within the household of cooked seafood, sushi, tofu, and some types of vegetables (carrot, coleslaw, bag greens, spinach, spring onions, sprouts, and herbs) were all significantly associated. Sampling and testing for YE BT 1A of these food types were limited and require further investigation. A Finnish case-control study also found pork not to be associated with YE BT 1A cases, and instead imported fruits and berries, as well as rarely washed vegetables, were associated with increased risk of YE BT 1A (35).
Prior to the introduction of whole-genome sequencing, bioserotyping was useful in NZ for epidemiological investigations for the pathogenic YE BTs, as only a few serotypes correlate with these BTs (5). This was not the case for YE BT 1A, as this BT can include a wide range of serotypes and the limited availability of commercial anti-sera has prevented the accurate identification of a serotype for this BT. Using SNP and wgMLST analyses of WGS data demonstrated that the YE BT 1A isolates collected in this study were genomically very diverse, with only four pairs of clinical isolates showing a close genomic relationship. Of the SNP clusters containing genomically related clinical YE BT 1A and source isolates, only 1–2 clinical isolates were within each cluster. This is despite the higher frequency of isolation of YE BT 1A in comparison to pathogenic YE and that almost two-thirds of raw pork containing pathogenic YE also had YE BT 1A. Multiple ecological niches have been identified for YE BT 1A (36). Considering the risk factor analysis and the lack of overlap of clinical YE BT 1A isolates with those from retail raw meat, an expansion of sources tested would be needed in future investigations. Studies have suggested that YE BT 1A may represent more than one subspecies, with different lineages potentially being more pathogenic than others (8, 37).
YP represented 3% of the consenting clinical yersiniosis cases in this study, which is consistent with previous surveillance rates in NZ (4). Using SNP analysis of WGS data, four clinical isolates identified as ST43 were observed to be genomically closely related, indicating a potential common exposure. However, there was insufficient epidemiological information available from consenting cases and no source sampling for these cases so a potential source could not be identified.
This study demonstrates the utility of combining epidemiological case-control data alongside genomic analysis to elucidate raw pork as a significant risk factor for yersiniosis caused by pathogenic YE, in particular YE ST12 in NZ. It is envisaged that improved pork processing interventions, as well as consumer education on food safety practices of raw meat handling and storage, will help decrease the number of yersiniosis cases and the public health burden in NZ. The implementation of routine whole-genome sequencing and analysis will be valuable for monitoring these improvements and assisting in outbreak investigations.
ACKNOWLEDGMENTS
The authors acknowledge all the individuals that consented to participate in the case-control study, Fiona for all her efforts with interviewing, and Wendy Dallas-Katoa for the assistance with the questionnaire. We are also thankful for the NZ Ministry of Health for the Yersinia surveillance and typing in NZ and for the use of data collected within that process. We thank multiple staff within the Public Health Services for their support for case assessment and follow-up, those within diagnostic laboratories and at ESR who conducted laboratory work (sampling, typing, DNA extractions, and sequencing), and support staff that helped during the study.
This work was supported by the New Zealand Health and Research Council (contract 20/847, 2020–2023) and the ESR Science Strategic Investment Fund of the Ministry of Business, Innovation, and Employment.
Contributor Information
Brent Gilpin, Email: Brent.Gilpin@esr.cri.nz.
Daniel J. Diekema, Maine Medical Center Department of Medicine, Portland, Maine, USA
ETHICS APPROVAL
This study was approved by the New Zealand Southern Health and Disability Ethics (reference 21/STH/84).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/jcm.00754-24.
Epidemiologically linked clusters of consenting cases, dual diagnosis, and additional genomic analysis.
Questionnaires administered to cases and controls.
Tables S1 to S7.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. European Centre for Disease Prevention and Control . 2022. Yersiniosis - annual epidemiology report for 2021. In ECDC (ed), annual epidemiological report for 2021. Stockholm: ECDC [Google Scholar]
- 2. Delahoy MJ, Shah HJ, Weller DL, Ray LC, Smith K, McGuire S, Trevejo RT, Scallan Walter E, Wymore K, Rissman T, McMillian M, Lathrop S, LaClair B, Boyle MM, Harris S, Zablotsky-Kufel J, Houck K, Devine CJ, Lau CE, Tauxe RV, Bruce BB, Griffin PM, Payne DC. 2023. Preliminary incidence and trends of infections caused by pathogens transmitted commonly through food - foodborne diseases active surveillance network, 10 U.S. Sites, 2022. MMWR 72:701–706. doi: 10.15585/mmwr.mm7226a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Šumilo D, Love NK, Manuel R, Dabke G, Paranthaman K, Jenkins C, McCarthy ND. 2023. Forgotten but not gone: Yersinia infections in England, 1975 to 2020. Euro Surveill 28:2200516. doi: 10.2807/1560-7917.ES.2023.28.14.2200516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horn B, Pattis, Cressey P, Armstrong B, Lopez L. 2023. Annual report concerning foodborne diease in New Zealand 2022. Institute of Environmental Science and Research, prepared for New Zealand Food Safety. Wellington [Google Scholar]
- 5. Rivas L, Strydom H, Paine S, Wang J, Wright J. 2021. Yersiniosis in New Zealand. Pathogens 10:191. doi: 10.3390/pathogens10020191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Le Guern AS, Martin L, Savin C, Carniel E. 2016. Yersiniosis in France: overview and potential sources of infection. Int J Infect Dis 46:1–7. doi: 10.1016/j.ijid.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 7. Campioni F, Falcão JP. 2014. Genotyping of Yersinia enterocolitica biotype 1A strains from clinical and nonclinical origins by pulsed-field gel electrophoresis. Can J Microbiol 60:419–424. doi: 10.1139/cjm-2014-0211 [DOI] [PubMed] [Google Scholar]
- 8. Sihvonen LM, Jalkanen K, Huovinen E, Toivonen S, Corander J, Kuusi M, Skurnik M, Siitonen A, Haukka K. 2012. Clinical isolates of Yersinia enterocolitica biotype 1A represent two phylogenetic lineages with differing pathogenicity-related properties. BMC Microbiol 12:208. doi: 10.1186/1471-2180-12-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tennant SM, Grant TH, Robins-Browne RM. 2003. Pathogenicity of Yersinia enterocolitica biotype 1A. FEMS Immunol Med Microbiol 38:127–137. doi: 10.1016/S0928-8244(03)00180-9 [DOI] [PubMed] [Google Scholar]
- 10. Lake R, Hudson A, Cressey P. 2004. Risk profile: Yersinia enterocolitica in pork. Institute of Environmental Science and Research, prepared for the New Zealand Food Safety Authority, Wellington [Google Scholar]
- 11. Fenwick S. 1997. Domestic animals as potential sources of human Yersinia infection. Surveill Well 24:3–4. [Google Scholar]
- 12. Health New Zealand Te Whatu Ora . 2023. Yersiniosis. Available from: https://www.tewhatuora.govt.nz/for-the-health-sector/health-sector-guidance/communicable-disease-control-manual/yersiniosis. Retrieved 8 Apr 2024.
- 13. NZ Ministry of Health . 2022. New Zealand health survey. Available from: https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/surveys/new-zealand-health-survey. Retrieved 29 Sep 2022.
- 14. Stats NZ . 2022. Statistical standard for geographic areas 2023. Available from: www.stats.govt.nz. Retrieved 3 Oct 2023.
- 15. International Organization for Standardization . 2017. Microbiology of the food chain — Horizontal method for the detection of pathogenic Yersinia enterocolitica. ISO 10273:2017. [Google Scholar]
- 16. International Organization for Standardization . 2015. Microbiology of the food chain-polymerase chain reaction (PCR) for the detection of food-borne pathogens- detection of pathogenic Yersinia enterocolitica and Yersinia pseudotubculosis. ISO/TS 18867:2015. [Google Scholar]
- 17. Hallanvuo S, Herranen M, Jaakkonen A, Nummela M, Ranta J, Interlaboratory study group, Botteldoorn N, De Zutter L, Fredriksson-Ahomaa M, Hertwig S, Johannessen GS, Ludewig M, Messelhäußer U, Sigvart-Mattila P, Thisted-Lambertz S, Thure T, Vatunen E. 2019. Validation of EN ISO method 10273 - detection of pathogenic Yersinia enterocolitica in foods. Int J Food Microbiol 288:66–74. doi: 10.1016/j.ijfoodmicro.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 18. Garzetti D, Susen R, Fruth A, Tietze E, Heesemann J, Rakin A. 2014. A molecular scheme for Yersinia enterocolitica patho-serotyping derived from genome-wide analysis. Int J Med Microbiol 304:275–283. doi: 10.1016/j.ijmm.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 19. Wang J-Z, Duan R, Liang J-R, Huang Y, Xiao Y-C, Qiu H-Y, Wang X, Jing H-Q. 2014. Real-time TaqMan PCR for Yersinia enterocolitica detection based on the ail and foxA genes. J Clin Microbiol 52:4443–4444. doi: 10.1128/JCM.02528-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hruskova V, Kaclikova E. 2009. Rapid and sensitive detection of Yersinia enterocolitica strains in food using selective enrichment and real-time PCR. J Food Nutr Res 48. https://www.vup.sk/en/download.php?bulID=101. [Google Scholar]
- 21. Seemann T, Goncalves da Silva A, Bulach D, Schultz M, Kwong J, Howden B. 2018. Nullarbor GitHub. https://github.com/tseemann/nullarbor.
- 22. Hall M, Chattaway MA, Reuter S, Savin C, Strauch E, Carniel E, Connor T, Van Damme I, Rajakaruna L, Rajendram D, Jenkins C, Thomson NR, McNally A. 2015. Use of whole-genus genome sequence data to develop a multilocus sequence typing tool that accurately identifies Yersinia isolates to the species and subspecies levels. J Clin Microbiol 53:35–42. doi: 10.1128/JCM.02395-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laukkanen-Ninios R, Didelot X, Jolley KA, Morelli G, Sangal V, Kristo P, Brehony C, Imori PFM, Fukushima H, Siitonen A, Tseneva G, Voskressenskaya E, Falcao JP, Korkeala H, Maiden MCJ, Mazzoni C, Carniel E, Skurnik M, Achtman M. 2011. Population structure of the Yersinia pseudotuberculosis complex according to multilocus sequence typing. Environ Microbiol 13:3114–3127. doi: 10.1111/j.1462-2920.2011.02588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williamson DA, Baines SL, Carter GP, da Silva AG, Ren X, Sherwood J, Dufour M, Schultz MB, French NP, Seemann T, Stinear TP, Howden BP. 2016. Genomic insights into a sustained national outbreak of Yersinia pseudotuberculosis. Genome Biol Evol 8:3806–3814. doi: 10.1093/gbe/evw285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Savin C, Criscuolo A, Guglielmini J, Le Guern AS, Carniel E, Pizarro-Cerdá J, Brisse S. 2019. Genus-wide Yersinia core-genome multilocus sequence typing for species identification and strain characterization. Microb Genom 5:e000301. doi: 10.1099/mgen.0.000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Savin C, Le Guern AS, Chereau F, Guglielmini J, Heuzé G, Demeure C, Pizarro-Cerdá J. 2022. First description of a Yersinia pseudotuberculosis clonal outbreak in France, confirmed using a new core genome multilocus sequence typing method. Microbiol Spectr 10:e0114522. doi: 10.1128/spectrum.01145-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strydom H, Wang J, Paine S, Dyet K, Cullen K, Wright J. 2019. Evaluating sub-typing methods for pathogenic Yersinia enterocolitica to support outbreak investigations in New Zealand. Epidemiol Infect 147:e186. doi: 10.1017/S0950268819000773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stevens MJA, Horlbog JA, Diethelm A, Stephan R, Nüesch-Inderbinen M. 2024. Characteristics and comparative genome analysis of Yersinia enterocolitica and related species associated with human infections in Switzerland 2019-2023. Infect Genet Evol 123. doi: 10.1016/j.meegid.2024.105652:105652 [DOI] [PubMed] [Google Scholar]
- 29. Le Guern AS, Savin C, Chereau F, Tessier S, Guglielmini J, Brémont S, Pizarro-Cerdá J. 2024. A novel cgMLST for genomic surveillance of Yersinia enterocolitica infections in France allowed the detection and investigation of outbreaks in 2017-2021. Microbiol Spectr 12:e0050424. doi: 10.1128/spectrum.00504-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. R Development Core Team . 2021. R: a language and environment for statistical computing, R foundation for statistical computing. Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- 31. Wickham H, Averick M, Bryan J, Chang W, McGowan L, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen T, Miller E, Bache S, Müller K, Ooms J, Robinson D, Seidel D, Spinu V, Takahashi K, Vaughan D, Wilke C, Woo K, Yutani H. 2019. Welcome to the Tidyverse. JOSS 4:1686. doi: 10.21105/joss.01686 [DOI] [Google Scholar]
- 32. Satterthwaite P, Pritchard K, Floyd D, Law B. 1999. A case-control study of Yersinia enterocolitica infections in Auckland. Aust N Z J Public Health 23:482–485. doi: 10.1111/j.1467-842X.1999.tb01303.x [DOI] [PubMed] [Google Scholar]
- 33. Ostroff SM, Kapperud G, Hutwagner LC, Nesbakken T, Bean NH, Lassen J, Tauxe RV. 1994. Sources of sporadic Yersinia enterocolitica infections in Norway: a prospective case-control study. Epidemiol Infect 112:133–141. doi: 10.1017/s0950268800057496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rosner BM, Stark K, Höhle M, Werber D. 2012. Risk factors for sporadic Yersinia enterocolitica infections, Germany 2009-2010. Epidemiol Infect 140:1738–1747. doi: 10.1017/S0950268811002664 [DOI] [PubMed] [Google Scholar]
- 35. Huovinen E, Sihvonen LM, Virtanen MJ, Haukka K, Siitonen A, Kuusi M. 2010. Symptoms and sources of Yersinia enterocolitica-infection: a case-control study. BMC Infect Dis 10:122. doi: 10.1186/1471-2334-10-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Terentjeva M, Ķibilds J, Gradovska S, Alksne L, Streikiša M, Meistere I, Valciņa O. 2022. Prevalence, virulence determinants, and genetic diversity in Yersinia enterocolitica isolated from slaughtered pigs and pig carcasses. Int J Food Microbiol 376:109756. doi: 10.1016/j.ijfoodmicro.2022.109756 [DOI] [PubMed] [Google Scholar]
- 37. Murros A, Säde E, Johansson P, Korkeala H, Fredriksson-Ahomaa M, Björkroth J. 2016. Characterization of European Yersinia enterocolitica 1A strains using restriction fragment length polymorphism and multilocus sequence analysis. Lett Appl Microbiol 63:282–288. doi: 10.1111/lam.12626 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Epidemiologically linked clusters of consenting cases, dual diagnosis, and additional genomic analysis.
Questionnaires administered to cases and controls.
Tables S1 to S7.