Abstract
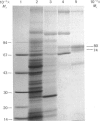
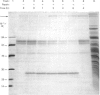
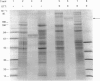
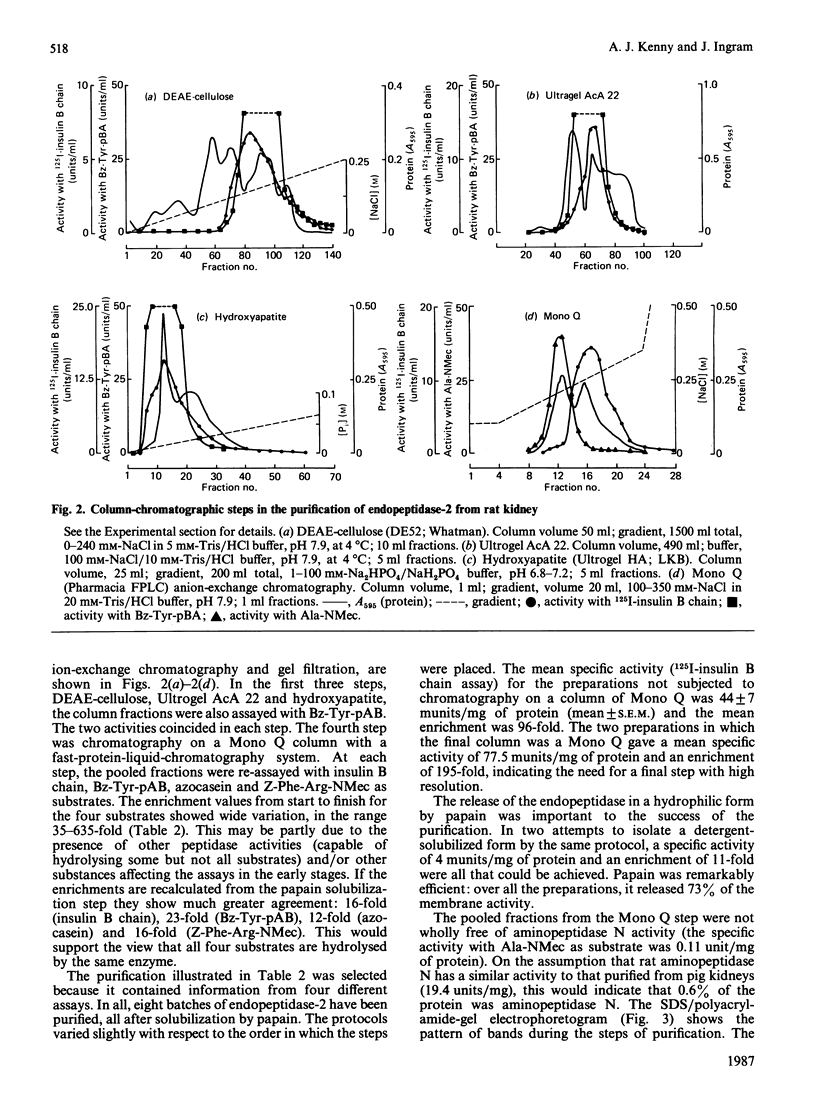
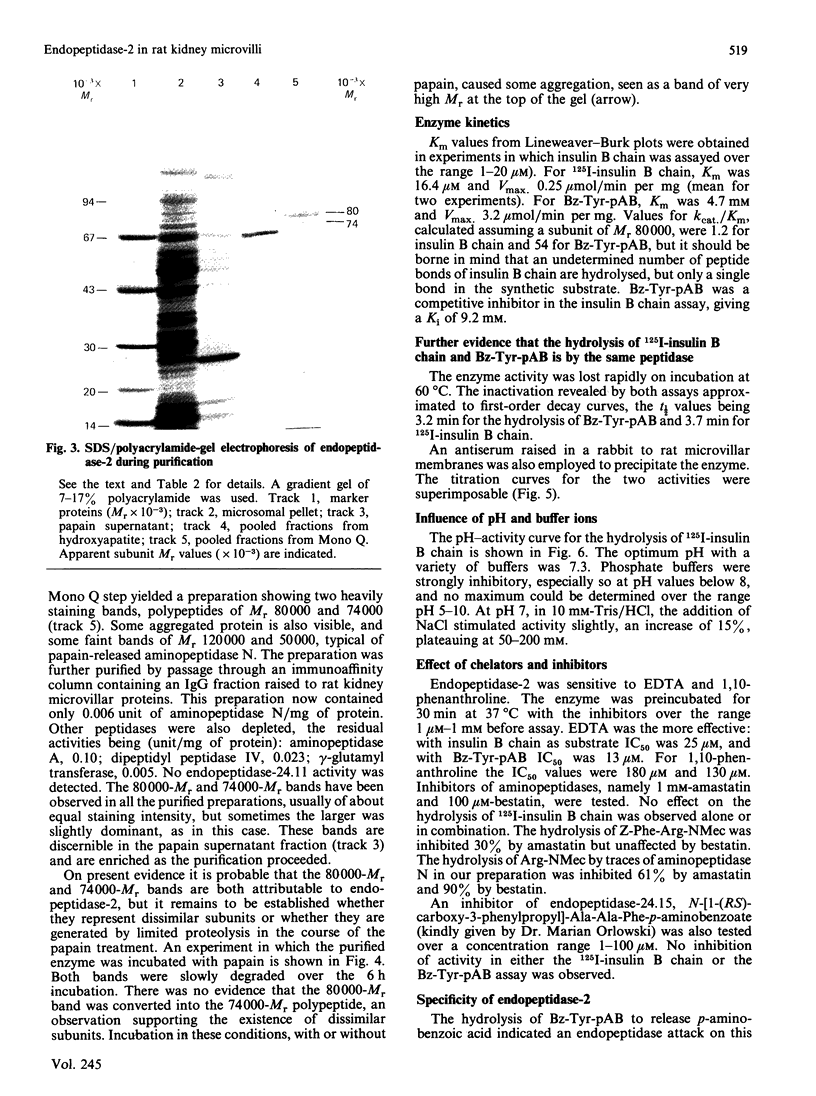
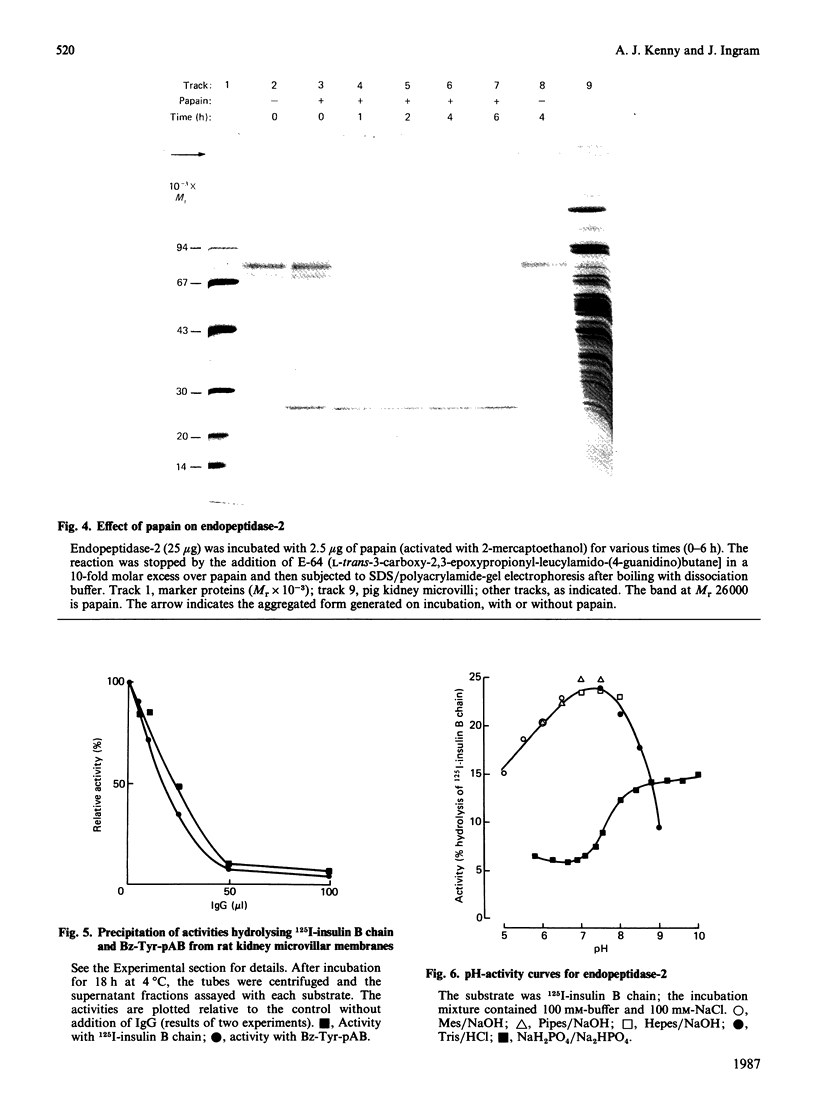
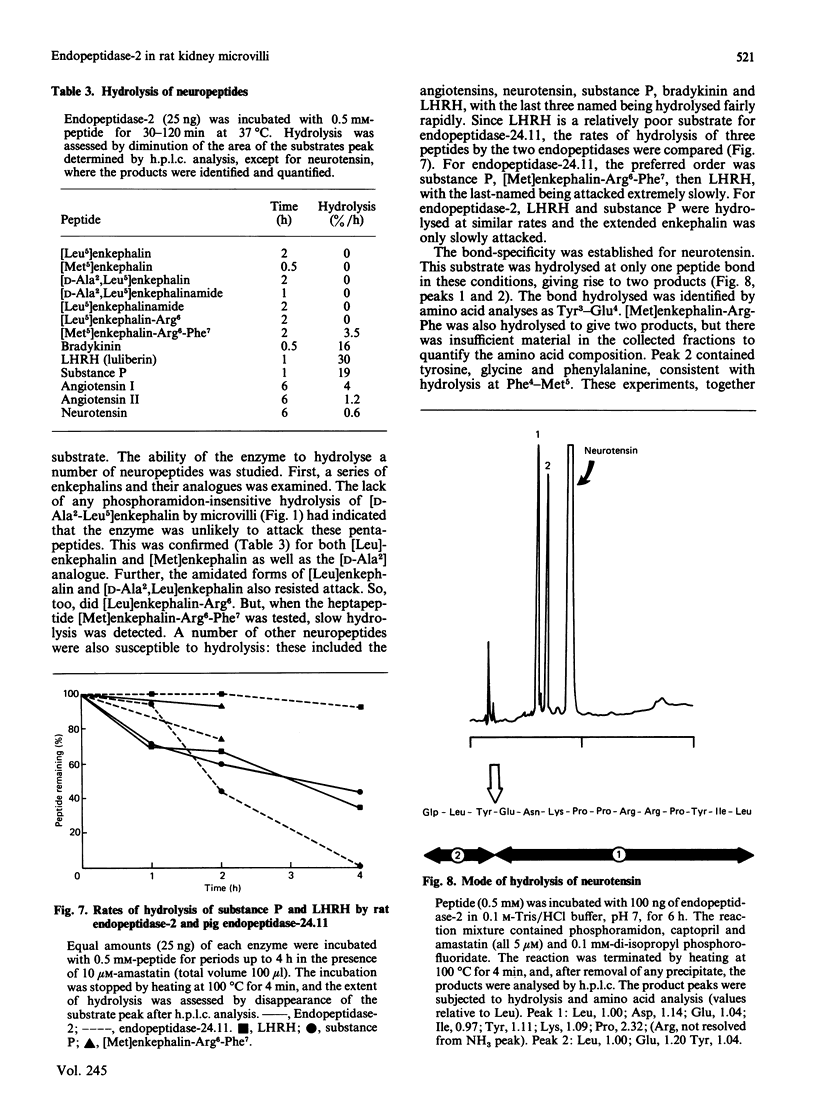
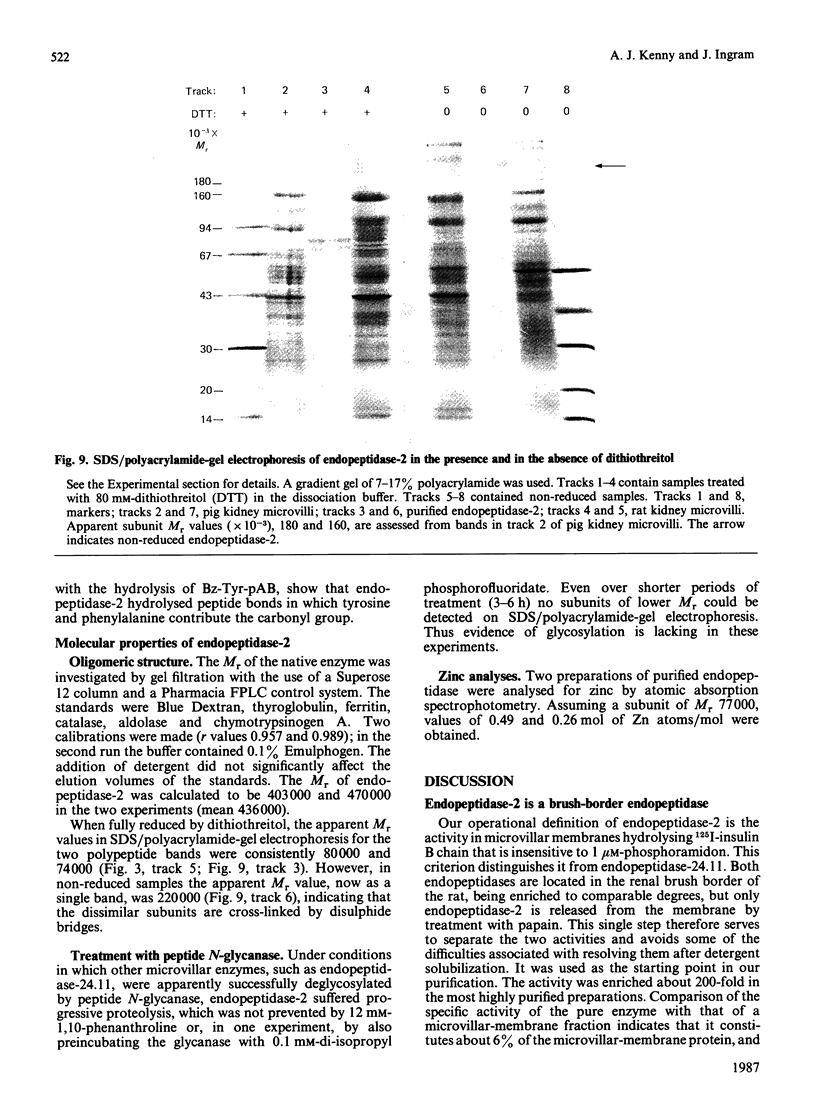
A second endopeptidase is present in the renal microvillar membrane of rats that can be distinguished from endopeptidase-24.11 by its insensitivity to inhibition by phosphoramidon. The purification of this enzyme, referred to as endopeptidase-2, is described. The enzyme was efficiently released from the membrane by treatment with papain. The subsequent four steps depended on ion-exchange and gel-filtration chromatography. These steps were monitored by the hydrolysis of various substrates: 125I-insulin B chain (the normal assay substrate), benzoyl-L-tyrosyl-p-aminobenzoate (Bz-Tyr-pAB), azocasein and benzyloxycarbonyl-L-phenylalanyl-L-arginine 7-amino-4-methylcoumarylamide (Z-Phe-Arg-NMec). All four assays revealed comparable stepwise increases in activity in the main stages of the purification, although it was apparent that the last-named fluorogenic assay depended on traces of aminopeptidase activity present in the preparation. The Km for 125I-insulin B chain was 16 microM and that for Bz-Tyr-pAB was 4.7 mM. Several experimental approaches confirmed that both peptides were hydrolysed by the same enzyme. The pH optimum was 7.3. Phosphate buffers were inhibitory and shifted the optimum to above pH 9. Zinc was detected in the purified enzyme; EDTA and 1,10-phenanthroline were strongly inhibitory. SDS/polyacrylamide-gel electrophoresis revealed polypeptides of equal staining intensity of Mr 80,000 and 74,000 in reducing conditions. In non-reducing conditions a single band of apparent Mr 220,000 was seen. Gel filtration yielded an Mr of 436,000. These results are consistent with an oligomeric structure in which the alpha and beta chains are linked by disulphide bridges. Endopeptidase-2 hydrolysed a number of neuropeptides. Enkephalins resisted attack, only the heptapeptide [Met]enkephalin-Arg6-Phe7 being susceptible to slow hydrolysis. Luliberin (luteinizing-hormone-releasing hormone) and bradykinin were rapidly hydrolysed. Neurotensin was shown to be slowly attacked at the Tyr3-Glu4 bond. Thus the specificity appears to be limited to the hydrolysis of bonds involving the carboxy group of aromatic residues, provided that this P1 residue is extended by additional residues, at least to the P3' position. The relationship of this membrane metalloendopeptidase to mouse meprin and human 'PABA peptidase' is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almenoff J., Orlowski M. Membrane-bound kidney neutral metalloendopeptidase: interaction with synthetic substrates, natural peptides, and inhibitors. Biochemistry. 1983 Feb 1;22(3):590–599. doi: 10.1021/bi00272a011. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Bond J. S. Deficiency of a kidney metalloproteinase activity in inbred mouse strains. Science. 1983 Mar 18;219(4590):1351–1353. doi: 10.1126/science.6338590. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Kay J. The inactivation of native enzymes by a neutral proteinase from rat intestinal muscle. Biochem J. 1978 Jul 1;173(1):291–298. doi: 10.1042/bj1730291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon R. J., Shannon J. D., Bond J. S. Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem J. 1981 Dec 1;199(3):591–598. doi: 10.1042/bj1990591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. S., Beynon R. J. Meprin: a membrane-bound metallo-endopeptidase. Curr Top Cell Regul. 1986;28:263–290. doi: 10.1016/b978-0-12-152828-7.50009-3. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Beynon R. J., Reckelhoff J. F., David C. S. Mep-1 gene controlling a kidney metalloendopeptidase is linked to the major histocompatibility complex in mice. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5542–5545. doi: 10.1073/pnas.81.17.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. S., Butler P. E., Beynon R. J. Metalloendopeptidases of the mouse kidney brush border: meprin and endopeptidase-24.11. Biomed Biochim Acta. 1986;45(11-12):1515–1521. [PubMed] [Google Scholar]
- Bond J. S., Shannon J. D., Beynon R. J. Certain mouse strains are deficient in a kidney brush-border metallo-endopeptidase activity. Biochem J. 1983 Jan 1;209(1):251–255. doi: 10.1042/bj2090251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butler P. E., McKay M. J., Bond J. S. Characterization of meprin, a membrane-bound metalloendopeptidase from mouse kidney. Biochem J. 1987 Jan 1;241(1):229–235. doi: 10.1042/bj2410229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checler F., Vincent J. P., Kitabgi P. Purification and characterization of a novel neurotensin-degrading peptidase from rat brain synaptic membranes. J Biol Chem. 1986 Aug 25;261(24):11274–11281. [PubMed] [Google Scholar]
- Chu T. G., Orlowski M. Soluble metalloendopeptidase from rat brain: action on enkephalin-containing peptides and other bioactive peptides. Endocrinology. 1985 Apr;116(4):1418–1425. doi: 10.1210/endo-116-4-1418. [DOI] [PubMed] [Google Scholar]
- Fulcher I. S., Kenny A. J. Proteins of the kidney microvillar membrane. The amphipathic forms of endopeptidase purified from pig kidneys. Biochem J. 1983 Jun 1;211(3):743–753. doi: 10.1042/bj2110743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee N. S., Bowes M. A., Buck P., Kenny A. J. An immunoradiometric assay for endopeptidase-24.11 shows it to be a widely distributed enzyme in pig tissues. Biochem J. 1985 May 15;228(1):119–126. doi: 10.1042/bj2280119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedeager-Sørensen S., Kenny A. J. Proteins of the kidney microvillar membrane. Purification and properties of carboxypeptidase P from pig kidneys. Biochem J. 1985 Jul 1;229(1):251–257. doi: 10.1042/bj2290251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny A. J., Fulcher I. S., Ridgwell K., Ingram J. Microvillar membrane neutral endopeptidases. Acta Biol Med Ger. 1981;40(10-11):1465–1471. [PubMed] [Google Scholar]
- Kenny A. J., Maroux S. Topology of microvillar membrance hydrolases of kidney and intestine. Physiol Rev. 1982 Jan;62(1):91–128. doi: 10.1152/physrev.1982.62.1.91. [DOI] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Fulcher I. S., Kenny A. J., Turner A. J. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc Natl Acad Sci U S A. 1983 May;80(10):3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Kenny A. J., Turner A. J. The metabolism of neuropeptides. The hydrolysis of peptides, including enkephalins, tachykinins and their analogues, by endopeptidase-24.11. Biochem J. 1984 Oct 15;223(2):433–440. doi: 10.1042/bj2230433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski M., Michaud C., Chu T. G. A soluble metalloendopeptidase from rat brain. Purification of the enzyme and determination of specificity with synthetic and natural peptides. Eur J Biochem. 1983 Sep 1;135(1):81–88. doi: 10.1111/j.1432-1033.1983.tb07620.x. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Wilk S. Purification and specificity of a membrane-bound metalloendopeptidase from bovine pituitaries. Biochemistry. 1981 Aug 18;20(17):4942–4950. doi: 10.1021/bi00520a021. [DOI] [PubMed] [Google Scholar]
- Semenza G. Anchoring and biosynthesis of stalked brush border membrane proteins: glycosidases and peptidases of enterocytes and renal tubuli. Annu Rev Cell Biol. 1986;2:255–313. doi: 10.1146/annurev.cb.02.110186.001351. [DOI] [PubMed] [Google Scholar]
- Sterchi E. E., Green J. R., Lentze M. J. Non-pancreatic hydrolysis of N-benzoyl-l-tyrosyl-p-aminobenzoic acid (PABA-peptide) in the human small intestine. Clin Sci (Lond) 1982 May;62(5):557–560. doi: 10.1042/cs0620557. [DOI] [PubMed] [Google Scholar]
- Sterchi E. E., Green J. R., Lentze M. J. Nonpancreatic hydrolysis of N-benzoyl-L-tyrosyl-p-aminobenzoic acid (PABA peptide) in the rat small intestine. J Pediatr Gastroenterol Nutr. 1983;2(3):539–547. doi: 10.1097/00005176-198302030-00024. [DOI] [PubMed] [Google Scholar]