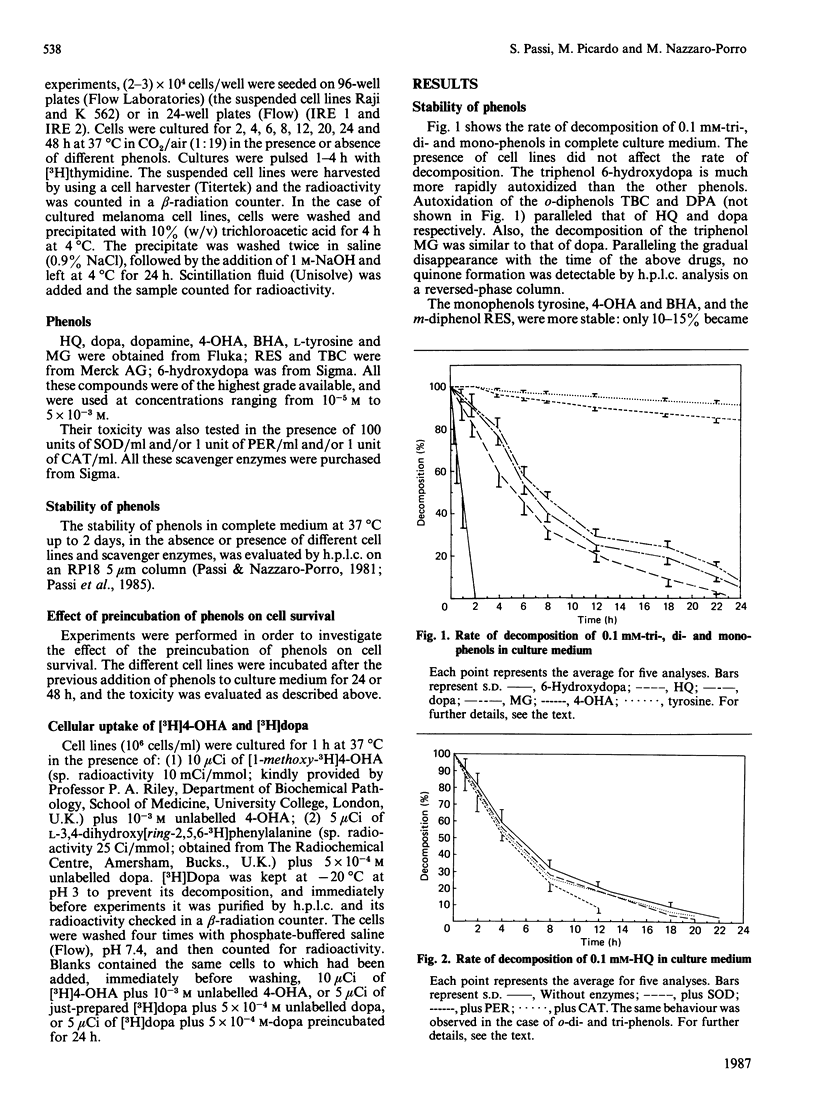
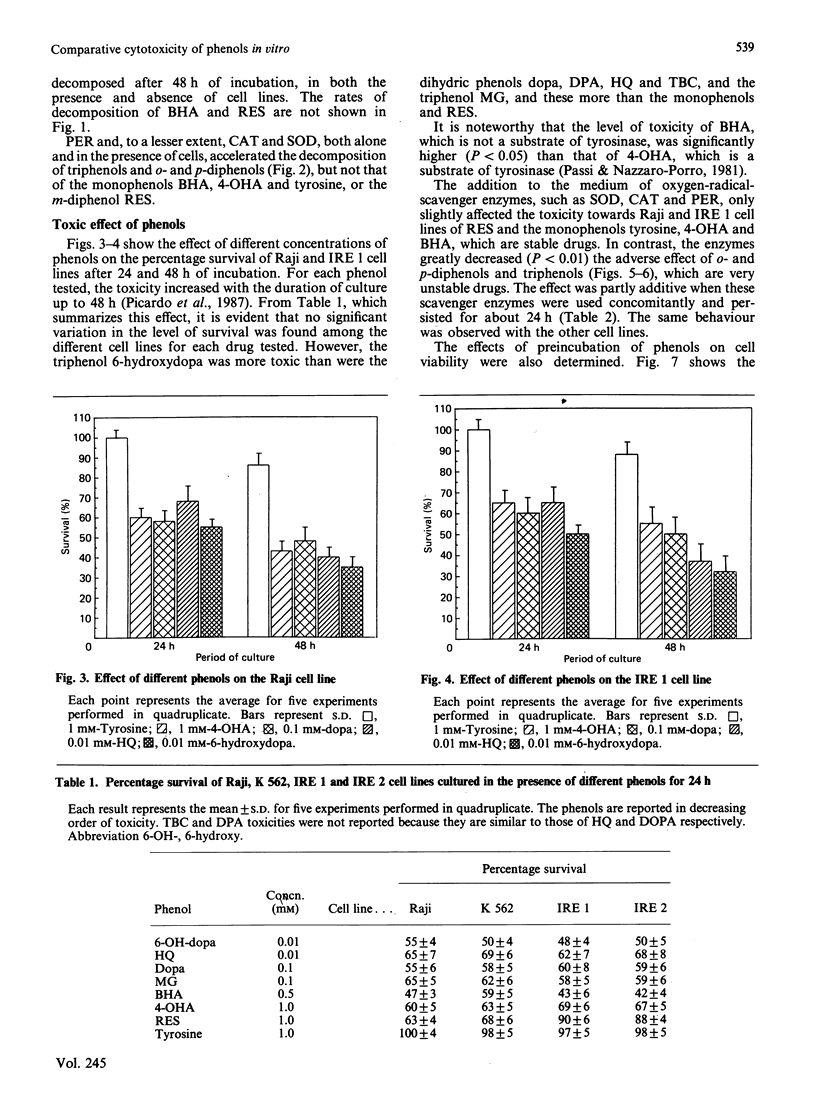
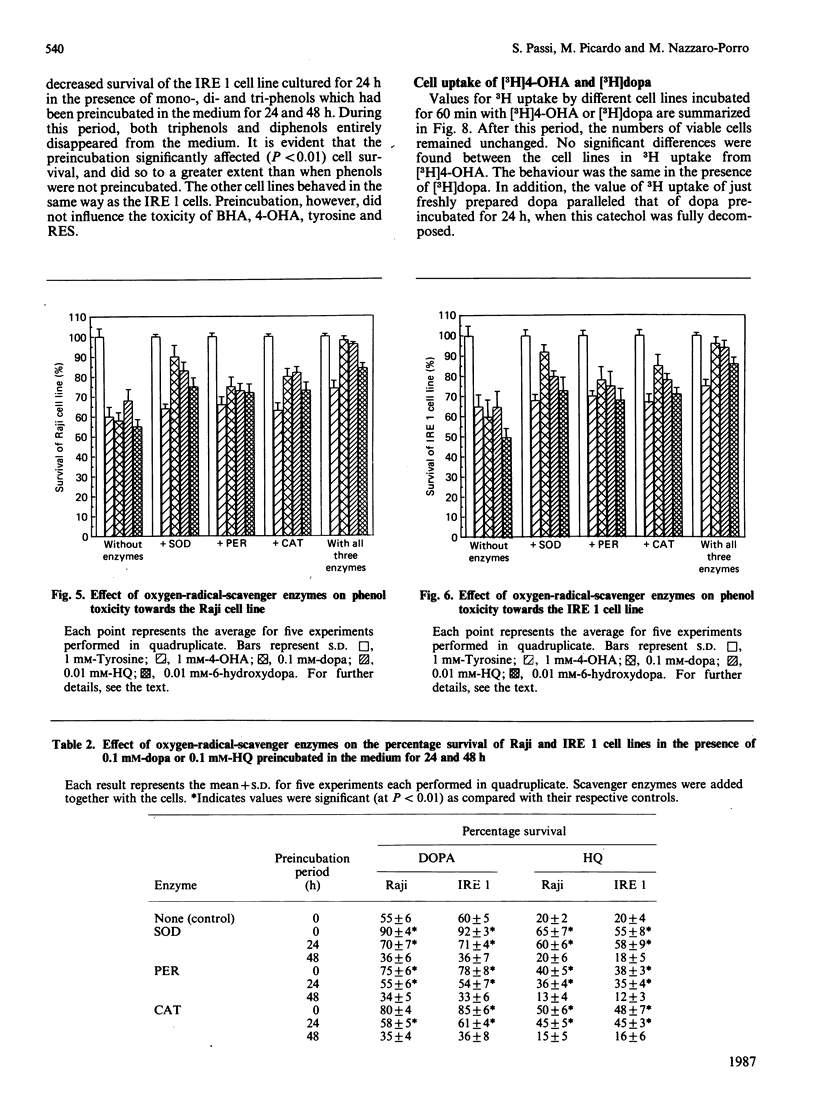
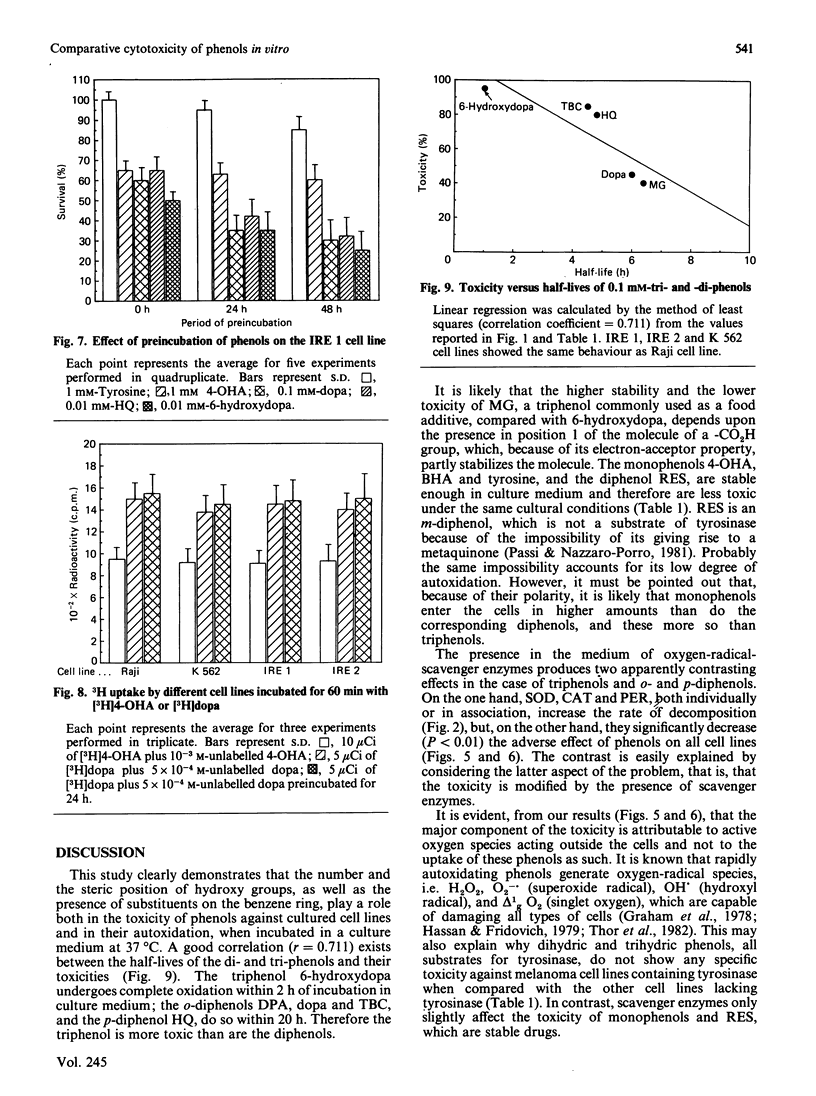
Abstract
Two melanotic human melanoma cell lines, IRE 1 and IRE 2, and the lymphoma- and leukaemia-derived cell lines Raji and K 562, were exposed to different concentrations (from 5 X 10(-3) M to 10(-5) M) of phenols, both substrates (s) and non-substrates (ns) of tyrosinase, in the presence or absence of the oxygen-radical-scavenger enzymes superoxide dismutase, catalase and peroxidase. Monophenols were tyrosine (s), 4-hydroxyanisole (s) and butylated hydroxyanisole (ns); diphenols were L-3,4-dihydroxyphenylalanine (s), dopamine (3,4-dihydroxyphenethylamine) (s), terbutylcatechol (s), hydroquinone (s) and resorcinol (ns); triphenols were 6-hydroxydopa (3,4,6-trihydroxyphenylalanine) (s) and methyl gallate (s). Triphenols and o- and p-diphenols underwent complete oxidation in culture medium within 24 h of incubation and were significantly more toxic than monophenols and the m-diphenol resorcinol, which, under the same cultural conditions, were much more stable. No significant differences in percentage survival were found among the different cell lines for each drug tested. The major component of toxicity up to 24 h of di- and tri-phenols is due to toxic oxygen species acting outside the cells and not to cellular uptake of these phenols as such. In fact the addition of oxygen-radical-scavenger enzymes significantly (P less than 0.01) decreased the adverse effect of these drugs on all cell lines. The lower toxicity of monophenols and resorcinol as compared with that of di- and tri-phenols is due, in our opinion, to the fact that they are less oxidized under the conditions existing in the culture medium, and therefore do not produce sufficient levels of oxygen radicals. For these compounds, a primary intracellular action has to be taken into account to explain their cytotoxicity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breathnach A., Robins E., Ethridge L., Bhasin Y., Gallagher S., Passi S., Nazzaro-Porro M. Ultrastructural and biochemical observations on the effect of 4-hydroxyanisole plus tyrosinase on normal human melanocytes and keratocytes in tissue culture. Br J Cancer. 1983 Jun;47(6):813–822. doi: 10.1038/bjc.1983.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald G. B., Wick M. M. 3,4-Dihydroxybenzylamine: an improved dopamine analog cytotoxic for melanoma cells in part through oxidation products inhibitory to dna polymerase. J Invest Dermatol. 1983 Feb;80(2):119–123. doi: 10.1111/1523-1747.ep12531751. [DOI] [PubMed] [Google Scholar]
- Fitzgerald G. B., Wick M. M. Sequential inhibitory effects of antitumor agents related to levodopa and dopamine upon DNA synthetic enzymes. Biochem Pharmacol. 1986 Jan 15;35(2):271–275. doi: 10.1016/0006-2952(86)90525-3. [DOI] [PubMed] [Google Scholar]
- Fujita K., Ito S., Inoue S., Yamamoto Y., Takeuchi J., Shamoto M., Nagatsu T. Selective toxicity of 5-S-cysteinyldopa, a melanin precursor, to tumor cells in vitro and in vivo. Cancer Res. 1980 Jul;40(7):2543–2546. [PubMed] [Google Scholar]
- Graham D. G., Tiffany S. M., Bell W. R., Jr, Gutknecht W. F. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol. 1978 Jul;14(4):644–653. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Parsons P. G. Modification of dopa toxicity in human tumour cells. Biochem Pharmacol. 1985 May 15;34(10):1801–1807. doi: 10.1016/0006-2952(85)90652-5. [DOI] [PubMed] [Google Scholar]
- Parsons P. G., Morrison L. E. DNA damage and selective toxicity of dopa and ascorbate:copper in human melanoma cells. Cancer Res. 1982 Sep;42(9):3783–3788. [PubMed] [Google Scholar]
- Passi S., Nazzaro-Porro M. Molecular basis of substrate and inhibitory specificity of tyrosinase: phenolic compounds. Br J Dermatol. 1981 Jun;104(6):659–665. doi: 10.1111/j.1365-2133.1981.tb00752.x. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M., Lerner A. B. 5,6-Dihydroxyindole is a melanin precursor showing potent cytotoxicity. Nature. 1978 Dec 7;276(5688):626–628. doi: 10.1038/276627a0. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M., Murray M. Increase in melanin formation and promotion of cytotoxicity in cultured melanoma cells caused by phosphorylated isomers of L-dopa. Cancer Res. 1986 Feb;46(2):493–497. [PubMed] [Google Scholar]
- Picardo M., Passi S., Nazzaro-Porro M., Breathnach A., Zompetta C., Faggioni A., Riley P. Mechanism of antitumoral activity of catechols in culture. Biochem Pharmacol. 1987 Feb 15;36(4):417–425. doi: 10.1016/0006-2952(87)90345-5. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
- Wick M. M. An experimental approach to the chemotherapy of melanoma. J Invest Dermatol. 1980 Feb;74(2):63–65. doi: 10.1111/1523-1747.ep12519812. [DOI] [PubMed] [Google Scholar]
- Wick M. M., Byers L., Frei E., 3rd L-dopa: selective toxicity for melanoma cells in vitro. Science. 1977 Jul 29;197(4302):468–469. doi: 10.1126/science.877570. [DOI] [PubMed] [Google Scholar]
- Wick M. M., Frei E., 3rd Selective incorporation of L-3,4-dihydroxyphenylalanine by S-91 Cloudman melanoma in vitro. Cancer Res. 1977 Jul;37(7 Pt 1):2123–2125. [PubMed] [Google Scholar]
- Wick M. M. L-Dopa methyl ester: prolongation of survival of neuroblastoma-bearing mice after treatment. Science. 1978 Feb 17;199(4330):775–776. doi: 10.1126/science.622565. [DOI] [PubMed] [Google Scholar]
- Wick M. M. Levodopa and dopamine analogs as DNA polymerase inhibitors and antitumor agents in human melanoma. Cancer Res. 1980 May;40(5):1414–1418. [PubMed] [Google Scholar]
- Wick M. M. Levodopa and dopamine analogs: melanin precursors as antitumor agents in experimental human and murine leukemia. Cancer Treat Rep. 1979 Jun;63(6):991–997. [PubMed] [Google Scholar]
- Wick M. M., Rosowsky A., Ratliff J. Antitumor effects of L-glutamic acid dihydroxyanilides against experimental melanoma. J Invest Dermatol. 1980 Feb;74(2):112–114. doi: 10.1111/1523-1747.ep12520030. [DOI] [PubMed] [Google Scholar]
- Wick M. M. Therapeutic effect of dopamine infusion on human malignant melanoma. Cancer Treat Rep. 1982 Aug;66(8):1657–1659. [PubMed] [Google Scholar]
- Wick M. M. l-Dopa methyl ester as a new antitumour agent. Nature. 1977 Oct 6;269(5628):512–513. doi: 10.1038/269512a0. [DOI] [PubMed] [Google Scholar]


