Abstract
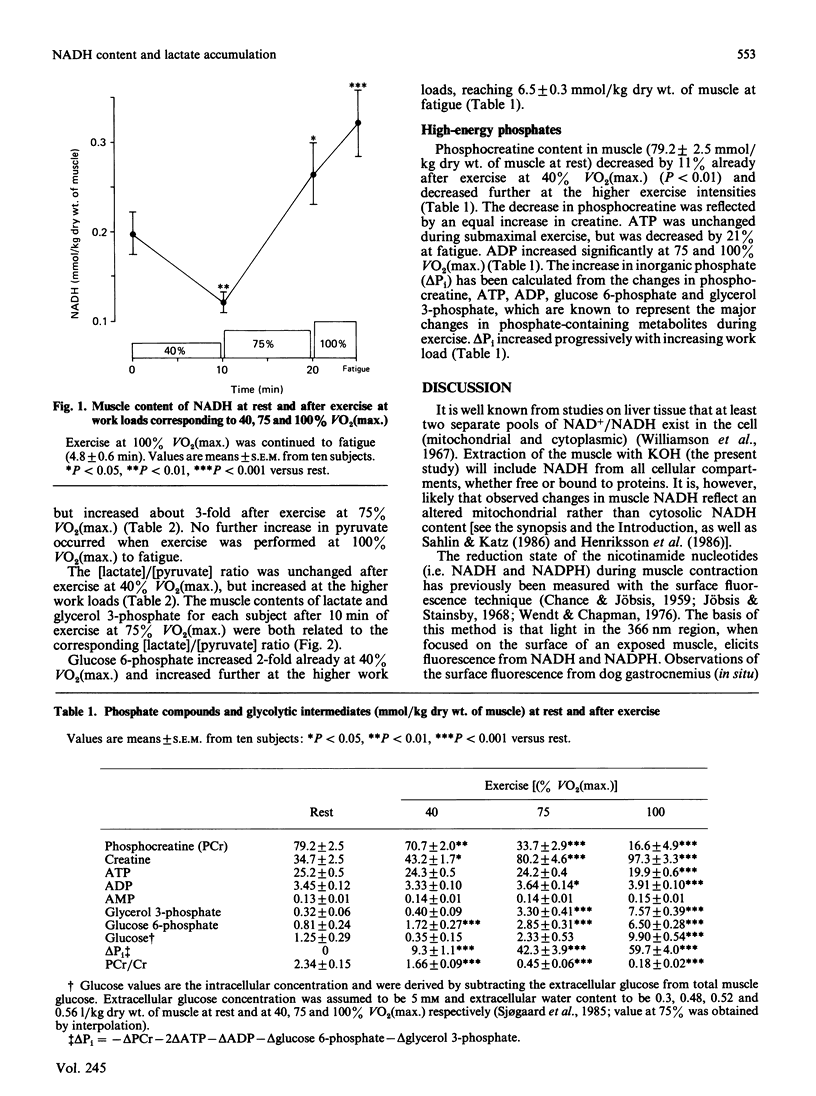
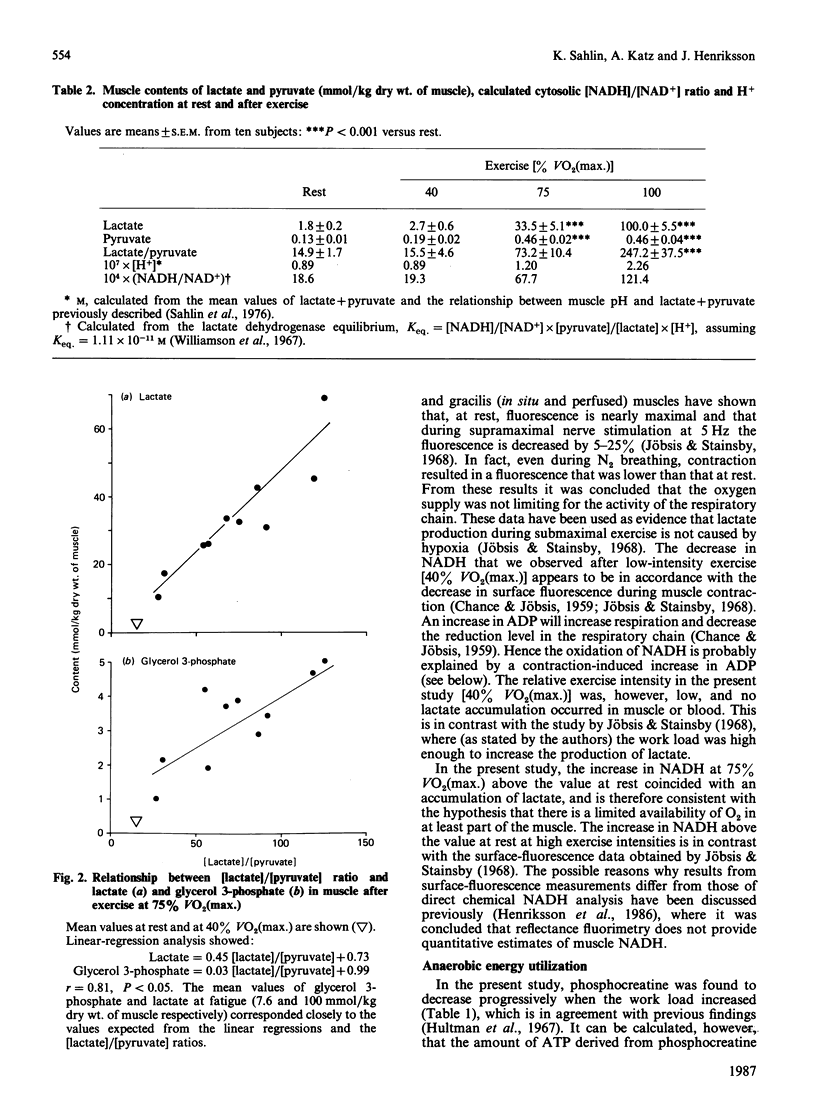
The relationship between the redox state and lactate accumulation in contracting human skeletal muscle was investigated. Ten men performed bicycle exercise for 10 min at 40 and 75% of maximal oxygen uptake [VO2(max.)], and to fatigue (4.8 +/- 0.6 min; mean +/- S.E.M.) at 100% VO2(max.). Biopsies from the quadriceps femoris muscle were analysed for NADH, high-energy phosphates and glycolytic intermediates. Muscle NADH was 0.20 +/- 0.02 mmol/kg dry wt. of muscle at rest, and decreased to 0.12 +/- 0.01 (P less than 0.01) after exercise at 40% VO2(max.), but no change occurred in the [lactate]/[pyruvate] ratio. These data, together with previous results on isolated cyanide-poisoned soleus muscle, where NADH increased while [lactate]/[pyruvate] ratio was unchanged [Sahlin & Katz (1986) Biochem. J. 239, 245-248], suggest that the observed changes in muscle NADH occurred within the mitochondria. After exercise at 75 and 100% VO2(max.), muscle NADH increased above the value at rest to 0.27 +/- 0.03 (P less than 0.05) and 0.32 +/- 0.04 (P less than 0.001) mmol/kg respectively. Muscle lactate was unchanged after exercise at 40% VO2(max.), but increased substantially at the higher work loads. At 40% VO2(max.), phosphocreatine decreased by 11% compared with the values at rest, and decreased further at the higher work loads. The decrease in phosphocreatine reflects increased ADP and Pi. It is concluded that muscle NADH decreases during low-intensity exercise, but increases above the value at rest during high-intensity exercise. The increase in muscle NADH is consistent with the hypothesis that the accelerated lactate production during submaximal exercise is due to a limited availability of O2 in the contracting muscle. It is suggested that the increases in NADH, ADP and Pi are metabolic adaptations, which primarily serve to activate the aerobic ATP production, and that the increased anaerobic energy production (phosphocreatine breakdown and lactate formation) is a consequence of these changes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chance B. Reaction of oxygen with the respiratory chain in cells and tissues. J Gen Physiol. 1965 Sep;49(1 Suppl):163–195. doi: 10.1085/jgp.49.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasiotis D., Sahlin K., Hultman E. Regulation of glycogenolysis in human muscle at rest and during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1982 Sep;53(3):708–715. doi: 10.1152/jappl.1982.53.3.708. [DOI] [PubMed] [Google Scholar]
- Connett R. J., Gayeski T. E., Honig C. R. Lactate accumulation in fully aerobic, working, dog gracilis muscle. Am J Physiol. 1984 Jan;246(1 Pt 2):H120–H128. doi: 10.1152/ajpheart.1984.246.1.H120. [DOI] [PubMed] [Google Scholar]
- Davis E. J., Bremer J., Akerman K. E. Thermodynamic aspects of translocation of reducing equivalents by mitochondria. J Biol Chem. 1980 Mar 25;255(6):2277–2283. [PubMed] [Google Scholar]
- Henriksson J., Katz A., Sahlin K. Redox state changes in human skeletal muscle after isometric contraction. J Physiol. 1986 Nov;380:441–451. doi: 10.1113/jphysiol.1986.sp016296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D. S., Bache R. J. Transmural distribution of myocardial blood flow during systole in the awake dog. Circ Res. 1976 Jan;38(1):5–15. doi: 10.1161/01.res.38.1.5. [DOI] [PubMed] [Google Scholar]
- Holloszy J. O., Booth F. W. Biochemical adaptations to endurance exercise in muscle. Annu Rev Physiol. 1976;38:273–291. doi: 10.1146/annurev.ph.38.030176.001421. [DOI] [PubMed] [Google Scholar]
- Hultman E., Bergström J., Anderson N. M. Breakdown and resynthesis of phosphorylcreatine and adenosine triphosphate in connection with muscular work in man. Scand J Clin Lab Invest. 1967;19(1):56–66. doi: 10.3109/00365516709093481. [DOI] [PubMed] [Google Scholar]
- JACOBSON K. B., KAPLAN N. O. Pyridine coenzymes of subcellular tissue fractions. J Biol Chem. 1957 Jun;226(2):603–613. [PubMed] [Google Scholar]
- Jorfeldt L., Juhlin-Dannfelt A., Karlsson J. Lactate release in relation to tissue lactate in human skeletal muscle during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1978 Mar;44(3):350–352. doi: 10.1152/jappl.1978.44.3.350. [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F., Stainsby W. N. Oxidation of NADH during contractions of circulated mammalian skeletal muscle. Respir Physiol. 1968 May;4(3):292–300. doi: 10.1016/0034-5687(68)90035-2. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Harris R. C., Nylind B., Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976 Dec 28;367(2):143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Sahlin K., Katz A. The content of NADH in rat skeletal muscle at rest and after cyanide poisoning. Biochem J. 1986 Oct 1;239(1):245–248. doi: 10.1042/bj2390245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K. NADH and NADPH in human skeletal muscle at rest and during ischaemia. Clin Physiol. 1983 Oct;3(5):477–485. doi: 10.1111/j.1475-097x.1983.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Sahlin K. NADH in human skeletal muscle during short-term intense exercise. Pflugers Arch. 1985 Feb;403(2):193–196. doi: 10.1007/BF00584099. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G., Adams R. P., Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol. 1985 Feb;248(2 Pt 2):R190–R196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Lawson J. W., Cornell N. W., Krebs H. A. Cytosolic phosphorylation potential. J Biol Chem. 1979 Jul 25;254(14):6538–6547. [PubMed] [Google Scholar]
- Wendt I. R., Chapman J. B. Fluorometric studies of recovery metabolism of rat fast- and slow-twitch muscles. Am J Physiol. 1976 Jun;230(6):1644–1649. doi: 10.1152/ajplegacy.1976.230.6.1644. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Erecińska M., Drown C., Silver I. A. Effect of oxygen tension on cellular energetics. Am J Physiol. 1977 Nov;233(5):C135–C140. doi: 10.1152/ajpcell.1977.233.5.C135. [DOI] [PubMed] [Google Scholar]


