Abstract
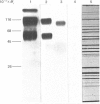
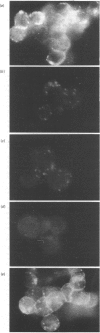
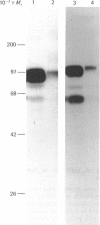
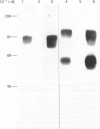
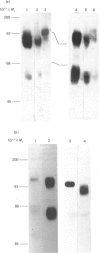
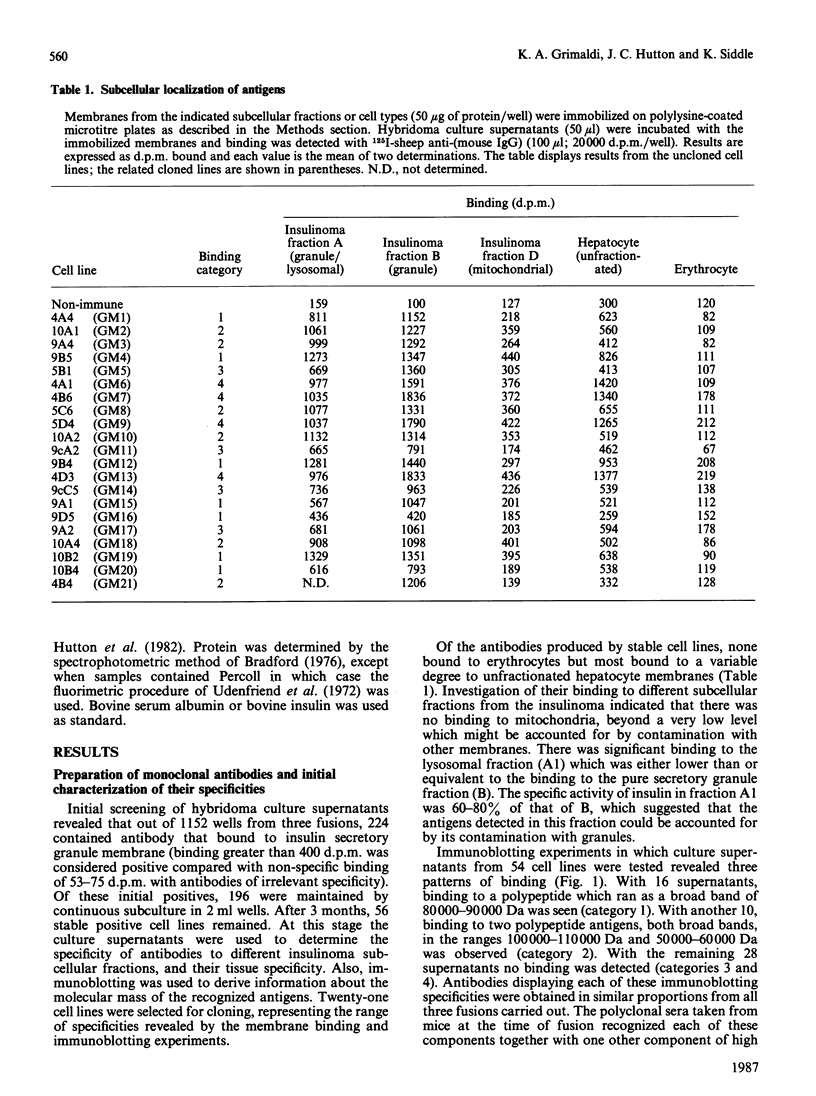
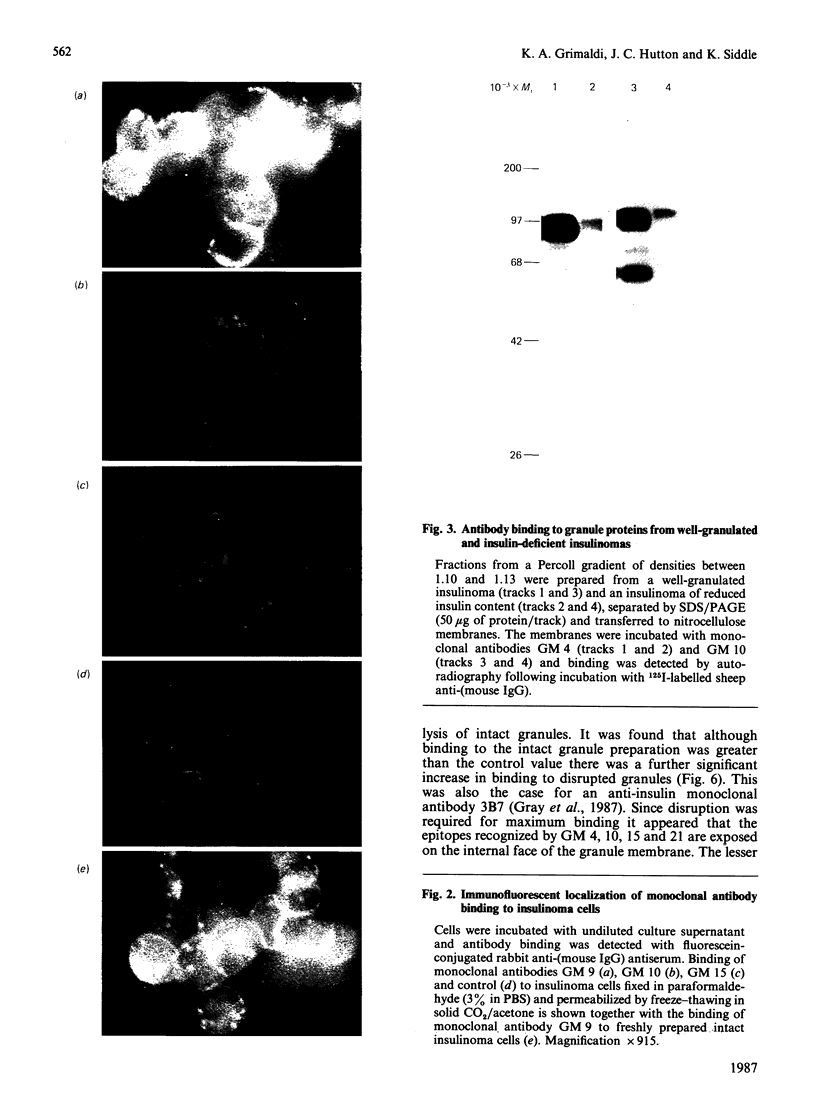
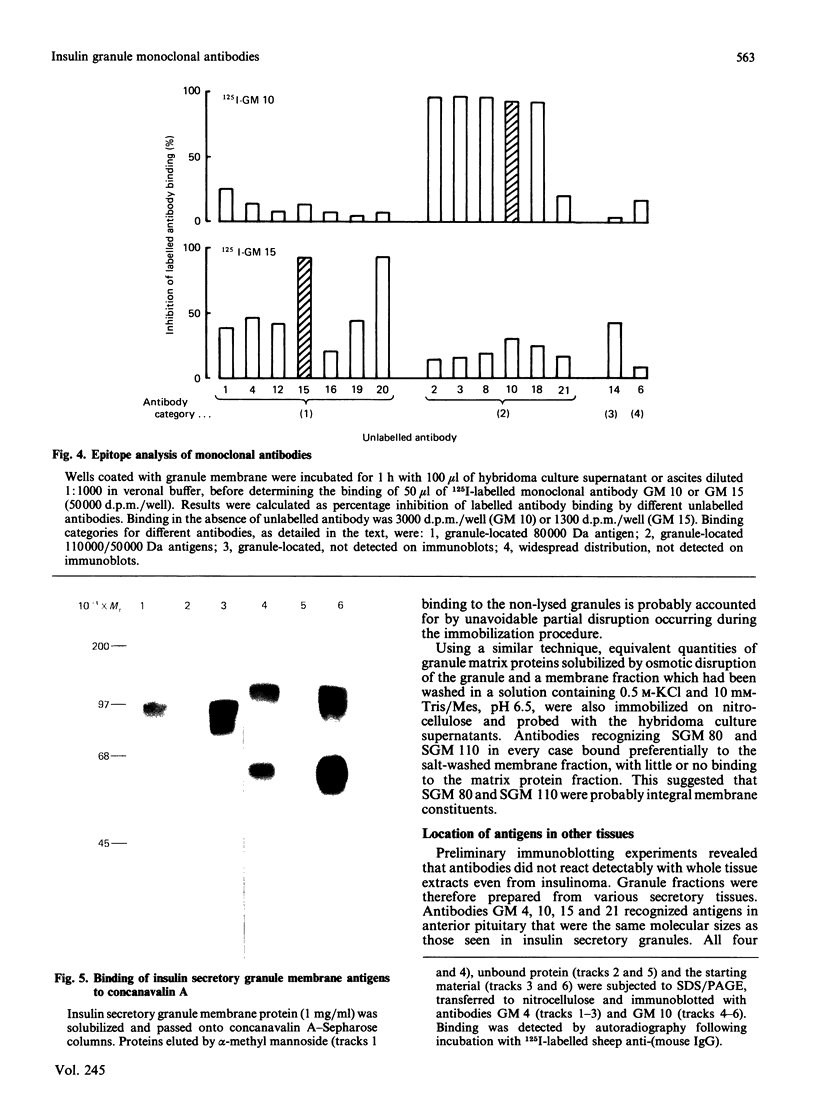
Monoclonal antibodies to insulin secretory granule membranes were obtained following immunization of mice with granule membranes purified from a rat transplantable insulinoma. The specificities of the antibodies were investigated by using binding assays with different insulinoma subcellular fractions, by indirect immunofluorescence studies with intact and permeabilized cells, and by immunoblotting of granule membrane proteins fractionated by SDS/polyacrylamide-gel electrophoresis. Fifty-six antibodies were characterized initially, and 21 representative cell lines were cloned. The antibodies fell into four categories: (1) binding preferentially to secretory granules, and reacting with a component of approx. 80,000 Da on immunoblots (antigen designated SGM 80); (2) binding preferentially to secretory granules, and reacting with components of approx. 110,000 and 50,000 Da on immunoblots (antigen designated SGM 110); (3) binding preferentially to secretory granules but unreactive on immunoblots; (4) binding to membrane antigen(s) with a widespread intracellular distribution which included granules and plasma membranes. The antigens SGM 80 and SGM 110 were studied in more detail and both were shown to be integral membrane glycoproteins with antigenic determinants located on the internal face of the secretory granule membrane. These antigens were also present in normal rat islets of Langerhans and similar components were detected by immunoblotting in secretory granules from anterior pituitary and adrenal medulla. Proteins which were immunologically related to SGM 80 and SGM 110, but distinct in molecular size, were also identified in liver. It is concluded that secretory granules contain specific components which are restricted in subcellular location but widespread in tissue distribution. The antibodies obtained will be valuable reagents in the further investigation of the biogenesis and turnover of insulin secretory granules.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbs M. T., Phillips J. H. Organisation of the proteins of the chromaffin granule membrane. Biochim Biophys Acta. 1980 Jan 25;595(2):200–221. doi: 10.1016/0005-2736(80)90084-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brocklehurst K. W., Hutton J. C. Involvement of protein kinase C in the phosphorylation of an insulin-granule membrane protein. Biochem J. 1984 May 15;220(1):283–290. doi: 10.1042/bj2200283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck C., Portetelle D., Glineur C., Bollen A. One-step purification of mouse monoclonal antibodies from ascitic fluid by DEAE Affi-Gel blue chromatography. J Immunol Methods. 1982 Sep 30;53(3):313–319. doi: 10.1016/0022-1759(82)90178-8. [DOI] [PubMed] [Google Scholar]
- Buckley K., Kelly R. B. Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J Cell Biol. 1985 Apr;100(4):1284–1294. doi: 10.1083/jcb.100.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chick W. L., Warren S., Chute R. N., Like A. A., Lauris V., Kitchen K. C. A transplantable insulinoma in the rat. Proc Natl Acad Sci U S A. 1977 Feb;74(2):628–632. doi: 10.1073/pnas.74.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran J. J., Lattanzio F. A., Jr, Rubin R. W., Pressman B. C. Subcellular fractions of the adrenal medulla. Comparison by two-dimensional polyacrylamide gel electrophoresis. Biochim Biophys Acta. 1982 Oct 5;707(2):226–235. doi: 10.1016/0167-4838(82)90355-7. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S., Schneider W. J., Hobgood K. K., Tolleshaug H., Brown M. S., Goldstein J. L. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983 Dec 25;258(24):15261–15273. [PubMed] [Google Scholar]
- D'Souza M. P., August J. T. A kinetic analysis of biosynthesis and localization of a lysosome-associated membrane glycoprotein. Arch Biochem Biophys. 1986 Sep;249(2):522–532. doi: 10.1016/0003-9861(86)90030-5. [DOI] [PubMed] [Google Scholar]
- Docherty K., Hutton J. C. Carboxypeptidase activity in the insulin secretory granule. FEBS Lett. 1983 Oct 3;162(1):137–141. doi: 10.1016/0014-5793(83)81065-5. [DOI] [PubMed] [Google Scholar]
- FALLON H. J., FREI E., 3rd, DAVIDSON J. D., TRIER J. S., BURK D. Leukocyte preparations from human blood: evaluation of their morphologic and metabolic state. J Lab Clin Med. 1962 May;59:779–791. [PubMed] [Google Scholar]
- Formby B., Capito K., Egeberg J., Hedeskov C. J. Ca-activated ATPase activity in subcellular fractions of mouse pancreatic islets. Am J Physiol. 1976 Feb;230(2):441–448. doi: 10.1152/ajplegacy.1976.230.2.441. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Galfrè G., Milstein C. Preparation of monoclonal antibodies: strategies and procedures. Methods Enzymol. 1981;73(Pt B):3–46. doi: 10.1016/0076-6879(81)73054-4. [DOI] [PubMed] [Google Scholar]
- Grimaldi K. A., Siddle K., Hutton J. C. Biosynthesis of insulin secretory granule membrane proteins. Control by glucose. Biochem J. 1987 Jul 15;245(2):567–573. doi: 10.1042/bj2450567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. N., Woodhead J. S. Labeled antibodies and their use in the immunoradiometric assay. Methods Enzymol. 1980;70(A):334–355. doi: 10.1016/s0076-6879(80)70063-0. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Jackson P., Hales C. N. Isolation and characterization of calmodulin from an insulin-secreting tumour. Biochem J. 1981 Mar 1;193(3):875–885. doi: 10.1042/bj1930875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Isolation and characterisation of insulin secretory granules from a rat islet cell tumour. Diabetologia. 1982 Oct;23(4):365–373. doi: 10.1007/BF00253746. [DOI] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983 Feb 15;210(2):297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Peshavaria M. Proton-translocating Mg2+-dependent ATPase activity in insulin-secretory granules. Biochem J. 1982 Apr 15;204(1):161–170. doi: 10.1042/bj2040161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leach B. S., Collawn J. F., Jr, Fish W. W. Behavior of glycopolypeptides with empirical molecular weight estimation methods. 1. In sodium dodecyl sulfate. Biochemistry. 1980 Dec 9;19(25):5734–5741. doi: 10.1021/bi00566a011. [DOI] [PubMed] [Google Scholar]
- Lernmark A. The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia. 1974 Oct;10(5):431–438. doi: 10.1007/BF01221634. [DOI] [PubMed] [Google Scholar]
- Lewis V., Green S. A., Marsh M., Vihko P., Helenius A., Mellman I. Glycoproteins of the lysosomal membrane. J Cell Biol. 1985 Jun;100(6):1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Lysosomal membrane dynamics: structure and interorganellar movement of a major lysosomal membrane glycoprotein. J Cell Biol. 1986 May;102(5):1593–1605. doi: 10.1083/jcb.102.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y. P., Tam W. W., Russell J. T. Measurement of delta pH and membrane potential in secretory vesicles isolated from bovine pituitary intermediate lobe. J Biol Chem. 1984 Jul 10;259(13):8238–8245. [PubMed] [Google Scholar]
- Maguire G. A., Luzio J. P. The presence and orientation of ecto-5'-nucleotidase in rat liver lysosomes. FEBS Lett. 1985 Jan 21;180(1):122–126. doi: 10.1016/0014-5793(85)80244-1. [DOI] [PubMed] [Google Scholar]
- Mehler P. S., Sussman A. L., Maman A., Leitner J. W., Sussman K. E. Role of insulin secretagogues in the regulation of somatostatin binding by isolated rat islets. J Clin Invest. 1980 Dec;66(6):1334–1338. doi: 10.1172/JCI109986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos M., Siddle K. Characterization of monoclonal antibodies directed against human thyroid stimulating hormone. J Immunol Methods. 1982;51(1):57–68. doi: 10.1016/0022-1759(82)90382-9. [DOI] [PubMed] [Google Scholar]
- Sussman K. E., Leitner J. W. Conversion of ATP into other adenine nucleotides within isolated islet secretory vesicles. Effect of cyclic AMP on phosphorus translocation. Endocrinology. 1977 Sep;101(3):694–701. doi: 10.1210/endo-101-3-694. [DOI] [PubMed] [Google Scholar]
- Tooke N. E., Hales C. N., Hutton J. C. Ca2+-sensitive phosphatidylinositol 4-phosphate metabolism in a rat beta-cell tumour. Biochem J. 1984 Apr 15;219(2):471–480. doi: 10.1042/bj2190471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Watkins D. T., Moore M. Uptake of NADPH by islet secretion granule membranes. Endocrinology. 1977 May;100(5):1461–1467. doi: 10.1210/endo-100-5-1461. [DOI] [PubMed] [Google Scholar]