Abstract
Background
Cancer survivors (CS) comprise a particularly high-risk group for both de-novo and recurrent malignancies after solid organ transplantation.
Case presentation
We report a case of relapsed melanoma, presented as metastatic disease seven months after heart transplantation in a patient who had an early-stage melanoma resected 25 years prior. Treatment with a combination of dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase (MEK) inhibitor resulted in a near-complete metabolic response, without major adverse effects.
Conclusion
This case demonstrates the increased risk of recurrence in CS with melanoma, which can persist decades after cancer diagnosis. These patients may be amenable to treatment using modern treatment modalities in oncology.
Keywords: Heart transplantation, Malignant melanoma, Dabrafenib, Trametinib
Background
Due to an aging population and improvements in cancer therapies, there are currently over 18 million cancer survivors (CS) in the United States [1]. Concurrently, there has been a steady increase in the number of CS undergoing heart transplantation (HT) with roughly 8% of current HT patients in the US having a history of malignancy [2].
Melanoma is among the most common cancers in the United States [3]. According to recent consensus statements, patients with a history of early-stage melanoma, which has an excellent long-term prognosis, may be candidates for HT immediately following resection of the disease, while patients with advanced stages may require a 5-year wait time interval prior to HT [4]. Long-term outcomes of this change in approach to HT in CS are unknown.
Herein we describe a case of relapsed melanoma that presented as metastatic disease seven months after HT in a patient who had an early-stage melanoma resected 25 years prior, and report the safe and efficacious treatment with a combination of BRAF and MEK inhibitors (dabrafenib and trametinib).
Case presentation
A 69-year-old male with non-ischemic cardiomyopathy due to a sarcomere protein titin (TTN) gene mutation, underwent HT nine months after HeartMate 3 LVAD placement for advanced heart failure. Oncologic history was significant for a 1-mm lower back melanoma for which the patient underwent a wide local excision and sentinel lymph node biopsy 25 years prior, without evidence of residual melanoma or lymph node involvement. No adjuvant therapy was administered, and no further oncologic evaluation was obtained. The patient underwent HT with intraoperative administration of methylprednisolone and mycophenolate mofetil. Immunosuppression regimen post-HT included tacrolimus, mycophenolic acid, and prednisone per our institutional protocol.
Seven months following HT, the patient presented with a rapidly growing, painful right groin mass, limiting his ambulation. The patient also noted weight loss and poor appetite. On physical examination, a 5-cm well-circumscribed, firm, fixed mass was noted, and right inguinal adenopathy was palpated. A core needle biopsy of the mass revealed a poorly differentiated neoplasm composed of sheets of atypical epithelioid cells and frequent and atypical mitoses. Immunohistochemistry and exome sequencing were consistent with a diagnosis of BRAFV600E cutaneous melanoma. Positron emission tomography-computed tomography (PET/CT) demonstrated an FDG-avid superficial soft tissue mass in the right groin measuring 5.1 × 4.6 cm. Multiple hypermetabolic pulmonary, liver, and bone lesions, were consistent with multi-site metastatic disease. Brain MRI showed no evidence of mass or metastasis.
The patient was treated with a combination of dabrafenib, a BRAF inhibitor, and trametinib, a mitogen-activated protein kinase (MEK) inhibitor. Following treatment initiation, tacrolimus dosage was increased to overcome dabrafenib’s CYP3A4-inducing effect. Despite increasing the dose, tacrolimus serum level decreased. As early as one week after treatment initiation, the patient had a substantial reduction in the mass size, allowing for improved ambulation.
A routine transthoracic echocardiogram that was performed a month after initiation of the melanoma treatment demonstrated a decrease in left ventricular ejection fraction (LVEF) from 60 to 45% over one month. Differential diagnoses at this time included MEK inhibitor-related cardiotoxicity or acute graft rejection. An endomyocardial biopsy was consistent with acute cellular rejection ISHLT grade 1R/1B. The patient was treated with oral steroids with normalization in LVEF and had no evidence of rejection on a follow-up biopsy, three weeks later. His clinical course was consistent with graft rejection, rather than MEK-inhibitor-related cardiomyopathy, and therapy with dabrafenib and trametinib was continued at the full dose.
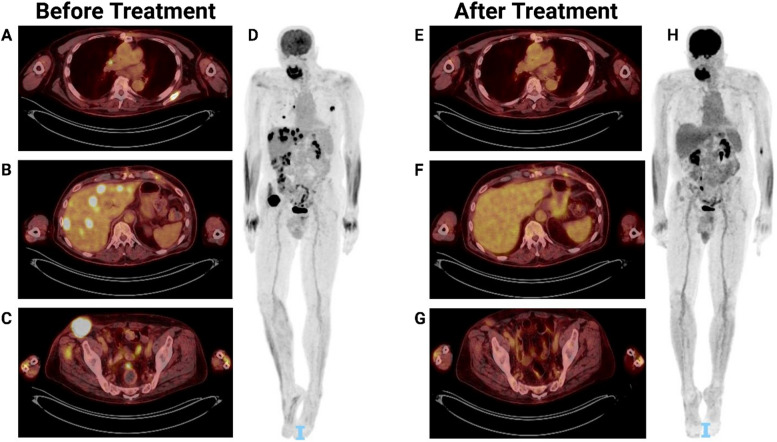
A follow-up PET/CT, two months after treatment initiation, demonstrated a near-complete metabolic response, with a dramatic decrease in the right inguinal mass size and FDG avidity, and resolution of the metabolic activity of the other metastatic sites (Fig. 1). The patient has since been treated with dabrafenib and trametinib, without major adverse effects, and is currently 33 months after HT with normal graft function and without evidence of other transplant-related complications. He has been maintained on tacrolimus at a goal of 6–8 ng/ml, mycophenolic acid 360 mg twice daily.
Fig. 1.
PET/CT scans before and after treatment. Positron emission tomography-computed tomography (PET/CT) scans prior to (A, B, C, D) and two months following (E, F, G, H) treatment with dabrafenib and trametinib. A Initial lung and scapula lesions. B Initial liver lesions. C Initial right inguinal lesion. D Initial whole-body scan demonstrating diffuse metastasis. E Resolution of the lung and scapula lesions. F Resolution of the liver lesions. G Near-complete resolution of the right inguinal lesion. H Whole body scan demonstrating complete resolution
Discussion and conclusions
Cancer survivors who meet criteria for HT remain at risk for recurrent malignancies post-HT [2, 5]. While non-melanoma skin cancer confers the highest risk of malignancy following HT, melanoma pre-HT is associated with one of the highest rates of recurrency post-HT, together with lung and breast cancers [6, 7]. According to the 2016 ISHLT listing criteria when recurrence risk, assessed by its type, response to treatment, and metastatic status, is low, HT should be considered. Time from neoplasm to HT depends on patient and tumor-specific factors and there is no strict period for observation before qualifying for HT. Similarly, according to the American Society for Transplantation (AST), no wait time is necessary in patients with a history of in-situ melanoma [4]. 1 year wait time is recommended for stages IA-IIA, 1–2 years for stage IIIA, 2–4 years for stages IIB-IIC and III, and “at least 5 years” wait time is recommended for stage IIIC-IV. Yet, data regarding the risk of cancer recurrence in transplant recipients is limited, specifically in the long-term survivors group.
In our case, the recurrence risk from the primary melanoma was very low, given the thin nature of the melanoma and the absence of lymph node involvement. Therefore, no further oncologic workup was performed before HT. Despite this low risk, the patient developed metastatic melanoma seven months post-HT. This highlights the limitations of the current understanding of pre-transplant risk assessment for cancer recurrence in CS.
Although we were unable to obtain the histology sample from the initial tumor that was resected 25 years ago for comparison, it is most likely that the relapsed melanoma arose from individual tumor cells that had disseminated 25 years prior, remained dormant, and underwent metastatic reactivation and progression in the context of immunosuppression, and therefore impaired immune surveillance, following HT. There are alternative, but extremely unlikely, explanations for this patient’s presentation. It is possible, that he developed an independent primary melanoma that underwent spontaneous involution and metastatic dissemination closer to the HT, which is unlikely because the patient had routine skin checks that did not yield such lesions. Another alternative is that the patient had an undiscovered primary mucosal melanoma that metastasized, however, the molecular pattern of BRAF and TERT promoter mutations would be unusual for mucosal melanomas and is more consistent with a typical cutaneous melanoma origin. Lastly, it is possible that the donor graft harbored dormant cells that reactivated and underwent secondary dissemination. While melanoma is one of the few cancers that may metastasize to the heart, this possibility is also unlikely.
New developments in oncogene-directed therapeutics have improved outcomes in patients with druggable driver mutations. Specifically, BRAF gene mutation, present in nearly 50% of invasive melanomas, sensitizes the cancer to inhibition with BRAF/MEK inhibitor combinations and improves survival. The possibility to utilize these medications after transplant provides an essential tool in the management of malignancy post-HT, however, in the context of immunosuppression, this confers challenges as drug-to-drug interactions.
In this case, the use of dabrafenib, a CYP3A4 inducer, was anticipated to lower the effective levels of tacrolimus, a CYP3A4 substrate. Therefore, tacrolimus levels gradually increased. Yet, a concomitant reduction in graft function had occurred and was concerning for either insufficient increase in tacrolimus levels, leading to graft rejection, or manifestation of trametinib-induced cardiotoxicity, which occurs in 11% of patients [8]. The dilemma of whether to increase immunosuppression, discontinue trametinib and thus risk melanoma exacerbation, or continue trametinib and risk cardiotoxicity exacerbation, is an example of the complex decision-making specific to the management of the cardio-oncology patient undergoing HT. Furthermore, it emphasizes the need for a multidisciplinary team to provide high-quality care for these patients. Further studies are needed to assess long-term outcomes of HT in CS, including optimal patient selection and optimal immunosuppression.
Acknowledgments
Author disclosures
B.I. is supported by National Institute of Health grants, R37CA258829, R01CA280414, R01CA266446, and additionally by the Pershing Square Sohn Cancer Research Alliance Award, the Burroughs Wellcome Fund Career Award for Medical Scientists; a Tara Miller Melanoma Research Alliance Young Investigator Award; the Louis V. Gerstner, Jr. Scholars Program; and the V Foundation Scholars Award.
B.I. is a consultant for or received honoraria from Volastra Therapeutics, Johnson & Johnson/Janssen, Novartis, Eisai, AstraZeneca and Merck, and has received research funding to Columbia University from Agenus, Alkermes, Arcus Biosciences, Checkmate Pharmaceuticals, Compugen, Immunocore, Regeneron, and Synthekine. A.F.V has received grant support from Alfonso Martin Escudero Foundation.
All other authors have reported that they have no relationships relevant to the contents of this.
Abbreviations
- HT
Heart transplantation
- CS
Cancer survivors
- BRAF
V-RAF murine sarcoma viral oncogene homolog B1
- MEK
Mitogen-activated protein kinase
Authors’ contributions
All authors contributed significantly to the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72(5):409–36. 10.3322/CAAC.21731. [DOI] [PubMed] [Google Scholar]
- 2.Batra J, Defilippis EM, Golob S, et al. Impact of pretransplant malignancy on heart transplantation outcomes: contemporary united network for organ sharing analysis amidst evolving cancer therapies. Circ Hear Fail. 2022;15(4):E008968. 10.1161/CIRCHEARTFAILURE.121.008968. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. 10.3322/CAAC.21820. [DOI] [PubMed] [Google Scholar]
- 4.Al-Adra DP, Hammel L, Roberts J, et al. Preexisting melanoma and hematological malignancies, prognosis, and timing to solid organ transplantation: a consensus expert opinion statement. Am J Transplant. 2021;21(2):475–83. 10.1111/AJT.16324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Youn JC, Kim D, Kim KA, et al. Characteristics and outcomes of heart transplant recipients with a pretransplant history of malignancy. Am J Transplant. 2022;22(12):2942–50. 10.1111/AJT.17186. [DOI] [PubMed] [Google Scholar]
- 6.Velleca A, Shullo MA, Dhital K, et al. The International Society for Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients. J Hear Lung Transplant. 2022;0(0). 10.1016/J.HEALUN.2022.09.023. [DOI] [PubMed]
- 7.Crow LD, Jambusaria-Pahlajani A, Chung CL, et al. Initial skin cancer screening for solid organ transplant recipients in the United States: Delphi method development of expert consensus guidelines. Transpl Int. 2019;32(12):1268–76. 10.1111/TRI.13520. [DOI] [PubMed] [Google Scholar]
- 8.Beck TC, Arhontoulis DC, Morningstar JE, et al. Cellular and molecular mechanisms of MEK1 inhibitor-induced cardiotoxicity. JACC CardioOncol. 2022;4(4):535–48. 10.1016/J.JACCAO.2022.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.