Abstract
Background
Cervical cancer remains a public health problem despite heavy global investment in health systems especially in low-and-middle-income countries (LMIC). Prophylactic vaccines against the most commonly detected human papillomavirus (HPV) types in cervical cancers are available and decisions on the selection of vaccine design depends on the prevalence of high-risk (hr) HPV genotypes for a particular region. In 2015, Botswana adopted the use of a quadrivalent HPV vaccine as a primary prevention strategy. Secondary prevention includes cervical smear screening whose uptake remains notably low among indigenous and marginalized communities despite efforts to improve access.
Aim
To determine the prevalence of hrHPV genotypes and cervical lesions’ burden in women from the indigenous and marginalized communities of Botswana.
Methods
This prospective survey enrolled 171 non-HPV vaccinated women aged 21 years and older. Face-to-face interviews, Pap smear screening, hr-HPV and Human Immuno-deficiency virus (HIV) testing were carried out. Conventional Papanicolau smears were analyzed and cervical brushes were preserved for hrHPV testing using the Ampfire Multiplex HR-HPV protocol which detects the following genotypes: HPV 16, 18, 31, 35, 39, 45, 51, 52, 53, 56, 58, 59 and 68.
Results
In this study, 168/171 (98.6%) of the women consented to HIV testing; 53/171 (31%) were living with HIV and self-reported enrolment on antiretroviral therapy. Among the women examined, 23/171 (13.5%) had cervical dysplasia with most presenting with Atypical Squamous Cells of Undetermined Significance 8/23 (35%), Low-Grade Squamous Intraepithelial Lesions 8/23 (35%), Atypical Squamous Cells-High Grade 4/23 (17%), Atypical Endocervical Cells 2/23 (9%) and Atypical Endocervical Cell favoring neoplasia 1/23(4%). However, no High-Grade Squamous Intraepithelial Lesions (HSIL) or squamous cell carcinoma (SCC) were detected. Overall hrHPV prevalence in this study was at 56/171 (32.7%). The most commonly detected hrHPV genotypes in women with cervical dysplasia were HPV39 (6.25%), HPV51 (14.5%), HPV52 (12.5%) and HPV56 (4%). Notably, HPV 16 and 18 were not found in women with cervical dysplasia.
Conclusions
Our study provides valuable insights into the prevalence and distribution of hrHPV genotypes in indigenous and marginalized communities in Botswana, and the need for further investigation of their potential role in cervical carcinogenesis in this population. These results may also serve as baseline data to facilitate future evaluation of the HPV vaccine needs.
Keywords: High-risk HPV genotypes, Cervical lesions, Indigenous, Marginalized, Botswana
Background
Cervical cancer is the leading cause of cancer morbidity and mortality in women from low- and middle-income countries (LMICs) [1, 2]. Cervical cancer is etiologically linked to persistent genital infection with several oncogenic high-risk (hr) HPV genotypes which include, HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 [3]. According to the working group of the World Health Organization (WHO) International Agency for Research on Cancer (IARC), the most commonly detected HPV genotypes (16, 18, 31,51, 52, 53, 58 and 68) are Group 1 carcinogens [4]. Prophylactic vaccines targeting some common hrHPV genotypes are available and while their potency has been demonstrated, vaccination rates remain low in many LMICs [5, 6]. It is well established that cervical cancer is preceded by a premalignant phase that lasts approximately 10 to 15 years, hence it is preventable and curable with adequate, comprehensive, regular organized community screening and prompt treatment of detected cervical lesions [2]. Therefore, measures to curb cervical cancer burden should continue to enforce improved effective prevention of hrHPV via vaccination and timely detection of associated premalignant cervical lesions [7]. Worth noting is that the prevalence of non-vaccine targeted hrHPV genotypes warrant the need for continued screening efforts for pre-neoplastic cervical lesions surveillance [8].
Botswana, like many other countries in sub-Saharan Africa, has a high HIV prevalence [9]. In addition, cervical cancer is the leading malignancy in both mortality and morbidity among women in Botswana [1, 10]. Botswana ranks fourth highest in adult HIV prevalent countries globally after South Africa, Eswatini and Lesotho [9]. HIV infection has been reported to contribute to HPV acquisition and more rapid cancer progression of cervical dysplastic lesions contributing to poorer patient outcomes [11, 12]. Studies have also reported that despite the introduction of highly active antiretroviral therapy (HAART), HPV-associated cervical cancer cases continue to rise [13, 14]. A study by Dryden-Peterson et al., [15] reported an annual increase in HPV-associated malignancies in people living with HIV in Botswana, despite a successful antiretroviral therapy program. To address the need for continued cervical cancer lesion development surveillance, HPV vaccination, effective screening and prompt treatment of cervical lesions are urgently needed for women living with HIV (WLWH), [16].
The HPV vaccination program in Botswana began in 2015, with the quadrivalent HPV vaccine (HPV 6/11/16/18) targeting all schoolgirls aged 9 to 13 years [17] and is reaching almost 100% coverage of the targeted population [18]. The only hrHPV genotypes targeted through this vaccination drive are HPV 16 and 18. Indigenous and marginalized communities of Botswana are located in Kgalagadi North and Ghanzi Districts and are traditionally a hunting and nomadic community, values that they still cherish today. However, in the 1990s, the government of Botswana embarked on a settlement program, building designated communities to enable better service provision: construction of shelter for elderly persons, provision of food rations, clothing, water and health care services and education. Although these services are provided, indigenous communities still prefer their century-old practices regarding health, food and culture [19, 20]. Indigenous communities tend to prefer their traditional practices for treatment and prevention of disease [21]. The strong cultural beliefs they hold dear make them less inclined to uptake modern healthcare services which are further compounded by the long distances needed to travel to secondary healthcare facilities for delivery and other services not available at the primary healthcare points with hardly any public transport facilities available [22]. The government of Botswana has achieved remarkable success in exceeding the UNAIDS 95–95-95 target: 95% of all individuals living with HIV are aware of their status, 95% of those diagnosed are receiving sustained antiretroviral therapy (ART), and 95% of those on ART have achieved virologic suppression. The current coverage rates for Botswana stand at 95.1%, 98%, and 97.9%, respectively [23–25]. Cervical cancer screening and management using the Pap smear and its alternative, “see and treat” program for cervical cancer lesions using visual inspection with acetic acid, is also well established [25–29].
While cervical cancer screening tests such as the Pap smear have been successful in reducing cervical cancer incidence and HPV vaccination expected to further reduce HPV infection and related cancers [30], the true extent of cervical disease burden in indigenous communities especially in LMIC [30], remains largely unknown and underestimated due to lack of data emanating from low screening rates [31]. Therefore, in order to achieve the World Health Organization (WHO) goals for elimination of cervical cancer in all women, these health inequities must be addressed, starting with generation of data on the impact of these initiatives in reducing the burden of cervical cancer, particularly in the indigenous and marginalized communities. There is paucity of cervical cancer screening data from indigenous populations in Botswana, with a few studies having been conducted among women from urban areas, who have access to more organized healthcare.
To address this, our study was designed to determine the prevalence of hrHPV genotypes and the burden of cervical pre-malignant and malignant disease in women from isolated indigenous populations in Botswana.
Methods
Study design and population
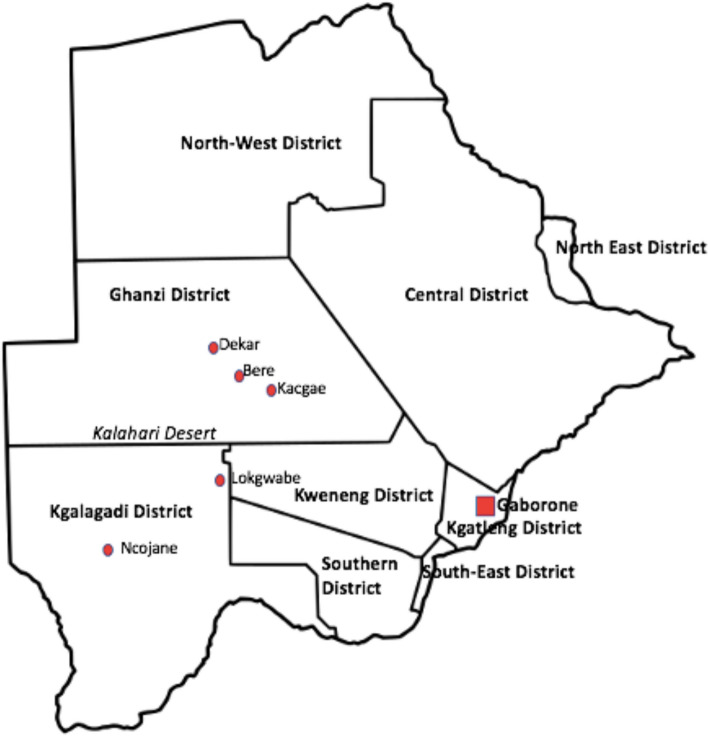
A prospective community survey was undertaken from June 6th to October 18th, 2022, enrolling a total of 171 consenting women aged 21 to 85 years. This study excluded women with a history of hysterectomy and pregnant women. Participants were recruited from five communities (Fig. 1): 3 settlements and 2 small villages of indigenous and marginalized communities. According to the Botswana 2022 Population and Housing census, the settlements and villages chosen for the study had the following populations: Kacgae -776 (380 males & 366 females), Bere – 874 (438 males & 436 females), Dekar -2814 (1382 males & 1432 females), Lokgwabe -1792 (879 males & 913 females), and Ncojane had 2242 (1045 males & 1197 females) people, [32].
Fig. 1.
The map of Botswana showing the 5 study sites located in the Ghanzi and Kgalagadi districts: Kacgae, Bere, Lokgwabe, Dekar highlighted in red dots. Gaborone, the capital city where the National Health Laboratory (NHL) and University of Botswana are located, is depicted by a red square. (Map credit: Patricia S. Rantshabeng)
Study sites
Recent estimates indicate that there are approximately 68 000 people in Botswana who identify as indigenous (marginalized). Many of them have been relocated to settlements in the Ghanzi and Kgalagadi districts. These settlements are remote and found in the Kgalagadi desert, joined together by the Trans Kalahari highway through tarmac and gravel roads which necessitates the use of off-road vehicles for easier access due to limited public transportation. Pap smears collected from women in this region are transported to the NHL in Gaborone, a referral and public health laboratory offering specialist laboratory services including Anatomical Pathology. The NHL also receives samples from the private health sector, with an annual average of 15,000 Pap smears received and resulted. The shortest distance from the study sites to the NHL was from Kacgae settlement (540 km), and the furthest distance was from Ncojane clinic (991 km). The average shortest distance from the cervical cancer screening and treatment facilities was 42 km from Dekar settlement to the nearest See & Treat clinic in Ghanzi. Bere and Kacgae settlements also referred patients to the Ghanzi-based clinic which is 154 km and 172 km respectively for each. Due to limited public transportation in Bere and Kacgae, most patients use the health post ambulance to access the Ghanzi-based care facility. Patients from Lokgwabe access the Kang-based See & Treat clinic which is 120 km away from the village. The furthest study site was Ncojane, which is 385 km from the Ghanzi-based care facilities and requires patients to have lodging arrangements when traveling for treatment.
Data and sample collection
Each community health post/clinic was used as a study site for data and sample collection. Face-to-face interviews using a structured questionnaire developed specifically for this study (see supplementary files), were used to collect demographic data such as age, sexual history, cervical screening history, tobacco and alcohol use history, parity and contraceptive use. Conventional Pap smear collection and rapid HIV testing was conducted by trained research nurses and a medical doctor for the study. The hrHPV samples were collected from cervical smear collection brushes, post smearing and preserved in 10 ml of phosphate buffered saline (PBS). Cervical brushes were preserved at room temperature. All samples collected were transported and analyzed in Gaborone, Botswana.
Rapid HIV testing
Rapid HIV testing was performed using the Maxline HIV 1 & 2 Tri-Line Rapid Test manufactured by Avecon Healthcare Pvt Ltd (Haryana, India-133104). Following aseptic procedures and sanitisation with 70% alcohol swab, a needle prick was made on a participants’ finger to obtain a blood specimen for the HIV test. A blood sample of about 0.5 ml was obtained and placed on the test strip followed by adding a buffer (as per the manufacturer’s instruction), and incubated for 15 min at room temperature. The test was interpreted as negative if only one line appeared on the control test strip, and positive if two lines appeared on both the control and test strips. Pre- and post-testing counseling was conducted by a trained research nurse before results were dispatched on site, to the participants.
Pap smear analysis
The Pap smear slides were stained in-house at the University of Botswana School of Allied Health Sciences laboratory using routine Papanicolaou stain according to the approved quality control and assurance procedures. Preliminary Pap smear screening was done by a Cytotechnologist and reported following the 2014 Bethesda system for reporting cervical cytology. As part of the quality assurance, the results were reviewed and verified by two Anatomical Pathologists to ensure agreement. All specimens collected were adequate for evaluation. Results for Pap smear analysis and hrHPV testing were returned in a timely manner on the next visit and hand-delivered to participants by the study team together with their health post/clinic nurse. Participants with abnormal lesions were linked to the nearest cervical cancer treatment facility for treatment and further management through their healthcare facility (study site).
High-risk HPV sample preparation, detection and genotyping
Samples were centrifuged at 3500 rpm for 5 min to dislodge the cells from the collection brushes and aliquoted into 1.5 ml eppendorf tubes prior to analysis. Each sample was mixed briefly by vortexing and was concentrated by a centrifugation step, for 30 min at a maximum 3500 rpm. After centrifugation, the supernatant was removed completely and 30 μl of the lysis buffer was added to the tube. The pellet and lysis buffer were then thoroughly mixed using a vortex to resuspend the cell pellet. The resuspended pellet was transferred to a PCR tube, which was incubated at 95 °C for 10 min and immediately used for hrHPV detection after the lysis step. High-risk HPV detection was achieved by using the Atila AmpFire® Multiplex HPV Assay (AmpFire) manufactured by AtilaBioSystems (Mountain View, CA, USA). This is an in vitro nucleic acid isothermal amplification with real-time fluorescence detection assay for the qualitative genotyping of hrHPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66 and 68 from different sample types. The assay uses 4 primer mixes targeting the 15 hrHPV genotypes and has 4 channels (FAM/HEX/ROX/CY5) and specific gene targets which are fluorescently labelled and generate target amplification signals in real-time over 60 min. This assay also uses an internal control targeting the human- β-globin gene for each sample. The lack of amplification curve in the HEX was regarded as invalid and the test has to be repeated. All samples had a detectable internal control and were deemed adequate for analysis.
Statistical analysis
Statistical analysis was performed using R-statistical software version 4.3.1. A sample size of 171 women was calculated as sufficient to determine the burden of disease and hrHPV infection. Owing to their cultural diversity and isolated living arrangements using a power of 0.8 alpha 0.05, with a proportionate sampling of 2 small villages and 3 settlements of differing population sizes provided a population representative sample. Type-specific hrHPV prevalence was calculated with a 95% confidence interval (CI) among women enrolled in the study. Fisher’s Exact tests and Pearson’s Chi-square were used to determine the association between categorical variables and statistical significance was determined by a p-value of ≤ 0.05.
Results
Characteristics of the study population and hr-HPV detected in the study
A total of 171 female participants were enrolled in the study, with ages ranging from 21 to 85 years (mean age 41.5). Among the cohort, 74% (127/171) of the women were ≤ 50 years while 26% (44/171) were > 50 years of age. About 4% (7/171) of the women reported having no children, 16% (28/171) had one child and 80% (136/171) reported having had more than one child in their lifetime. Fifteen percent 15% (25/171) of the women reported having one lifetime sexual partner and 85% (146/171) reported having multiple partners, with a mean age of sexual debut at 18.5 years. The majority of the women (61%) were using hormonal contraceptives at the time of data collection. In addition, 17% of the women were current smokers, and 8.8% had a history of smoking (ex-smokers). High risk HPV was detected in 56/171 (32.7%) participants. Fifty-three out of one hundred and seventy-one (31%) women were living with HIV and self-reported enrolment in the national ART program.
The socio-demographic characteristics and contraception use history of the study participants stratified by hrHPV DNA results are presented in Table 1.
Table 1.
Characteristics of the participants and hrHPV detection in the study
| hrHPV DNA detection | ||||
|---|---|---|---|---|
| Overall (n = 171) | Detected (n = 56) | Not detected (n = 115) | P-value | |
| Age | ||||
| Mean (SD) | 41.5 (12.4) | 41.9 (11.6) | 41.3 (12.8) | 0.75 |
| Age at 1st coitus | ||||
| Mean (SD) | 18.5 (3.16) | 18.5 (3.03) | 18.5 (3.24) | 0.98 |
| HIV status | ||||
| Positive | 53 (31%) | 18 (32.1%) | 35 (30.4%) | 0.06 |
|
Negative Unknown |
115 (67.25%) 3(1.75%) |
35 (62.5%) 3 (5.4%) |
80 (69.6%) 0 |
|
| Lifetime # of sexual partners | ||||
| 1 partner | 25 (14.6%) | 9 (16.1%) | 16 (13.9%) | 0.82 |
| More than 1 partner | 146 (85.4%) | 47 (83.9%) | 99 (86.1%) | |
| Smoking history | ||||
| Current smoker | 29 (17.0%) | 18 (32.1%) | 11 (9.6%) | 0.001 |
| Non-smoker | 127 (74.3%) | 34 (60.7%) | 93 (80.9%) | |
| Ex-smoker | 15 (8.8%) | 4 (7.1%) | 11 (9.6%) | |
| Alcohol use | ||||
| Yes | 56 (32.7%) | 23 (41.1%) | 33 (28.7%) | 0.08 |
| No | 74 (43.3%) | 25 (44.6%) | 49 (42.6%) | |
| Stopped | 41 (24.0%) | 8 (14.3%) | 33 (28.7%) | |
| Family history of cancer | ||||
| Yes | 51 (29.8%) | 14 (25.0%) | 37 (32.2%) | 0.38 |
| None | 120 (70.2%) | 42 (75.0%) | 78 (67.8%) | |
| Parity | ||||
| 0 | 7 (4.1%) | 3 (5.4%) | 4 (3.5%) | |
| 1 | 28 (16.4%) | 9 (16.1%) | 19 (16.5%) | 0.85 |
| > 1 | 136 (79.5%) | 44 (78.6%) | 92 (80.0%) | |
| Previous contraceptive | ||||
| Condom only | 24 (14.0%) | 5 (8.9%) | 19 (16.5%) | |
| Condom, injectables | 2 (1.2%) | 1 (1.8%) | 1 (0.9%) | |
| Condom, pill | 2 (1.2%) | 1 (1.8%) | 1 (0.9%) | |
| Condom, pill, injectables | 3 (1.8%) | 2 (3.6%) | 1 (0.9%) | |
| Injectables only | 56 (32.7%) | 18 (32.1%) | 38 (33.0%) | 0.67 |
| Loop, injectables | 3 (1.8%) | 2 (3.6%) | 38 (33.0%) | |
| Pill only | 27 (15.8%) | 11 (19.6%) | 16 (13.9%) | |
| Pill, injectables | 21 (12.3%) | 8 (14.3%) | 13 (11.3%) | |
| Pill, injectables, loop | 1 (0.6%) | 0 | 1 (0.9%) | |
| Pill, loop | 2 (1.2%) | 1 (1.8%) | 1 (0.9%) | |
| None | 30 (17.5%) | 9 (16.1%) | 21 (18.3%) | |
Cervical cytology analysis and hrHPV results
A total of 23/171 (13.5%) women had abnormal cervical lesions as shown in Table 2. Among the entire cohort, 56/171 (32.7%) tested positive for hrHPV. Of the women with cervical premalignancies, 8/23 (35%) had hrHPV detected. Within this group, 4/23 (17.4%) had Atypical Squamous Cells-High Grade (ASC-H) with hrHPV detected in 1/4 (25%) (Table 2). In addition, 8/23 (35%) women had Atypical Squamous Cells of Undetermined Significance (ASCUS) with hrHPV detected in 4/8 (50%) of the study subjects. Atypical endocervical cells (AEC) were diagnosed in 2/23 (9%) of the women, and hrHPV was not detected in any of these participants. Only 1/23 (4%) of the women had Atypical Endocervical Cells favoring neoplasia (AEC-FN), however she tested negative for hrHPV. Furthermore, 8/23 (35%) of the women had Low-Grade Squamous Intraepithelial Lesions (LSIL) and 3/8 (37.5%) of them had hrHPV while 5/8 (62.5%) of them tested negative for hrHPV.
Table 2.
Proportion of hrHPV DNA detected in final cytology
| Cytology diagnosis | hrHPV DNA | ||
|---|---|---|---|
| Positive | Negative | Total | |
| ASC-H | 1 (25%) | 3 (75%) | 4 (100%) |
| ASCUS | 4 (50%) | 4 (50%) | 8 (100%) |
| AEC | 0 | 2 (100%) | 2 (100%) |
| AEC-FN | 0 | 1 (100%) | 1 (100%) |
| LSIL | 3 (37.5%) | 5 (62.5%) | 8 (100%) |
| NILM | 48 (32.4%) | 100 (67.6%) | 148 (100%) |
| Total | 56 (32.7%) | 115 (67.3%) | 171 (100%) |
Fisher’s Exact test: P-value = 0.861
Distribution of hrHPV genotypes in cervical cytology
Of the 56/171(32.7%) women who tested positive for hrHPV, only 8/56 (14.3%) had cervical pre-malignant lesions. One with ASC-H had HPV58. Of the four women with ASCUS, two tested positive for HPV56, one for HPV35 and the other for HPV51. Three women had Low-grade squamous intraepithelial lesion (LSIL). All three women had co-infection of two hrHPV genotypes: one had HPV18 & HPV39, another had HPV31 & HPV52, while the other had HPV52 & HPV56 respectively. Additionally, 48 women did not have any cytological abnormalities and among those with single infections; six (12.5%) had HPV52, four (8.3%) had HPV31, four (8.3%) had HPV35, three (6.3%) had HPV16, three (6.3%) had HPV51, three (6.3%) had HPV58, three (6.3%) had HPV68, two (4.2%) had HPV18, two (4.2%) had HPV 33, two (4.2%) had HPV39, two (4.2%) had HPV45, two (4.2%) had HPV53, two (4.2%) had HPV66 and one (2.1%) had HPV59. Among those who had two or multiple hrHPV genotypes detected, two women (4.2%) had HPV 16 & 51, one (2.1%) had HPV 33 & 39, one (2.1%) had HPV52 & 53, one (2.1%) had HPV 53 & 68, one (2.1%) had HPV31 & 58, one (2.1%) had HPV18, 35 & 66, one (2.1%) had HPV 31, 35, 58 & 68 and one woman had HPV 16, 31, 51, 59, 45 & 53 co-infection (Table 3). A Pearson’s Chi-square: P-value = 0.61, Fisher’s Exact test: P-value = 0.352 showed that there was an insignificant relationship between hrHPV genotype and cytology results as shown in Table 3.
Table 3.
The distribution of hrHPV genotypes according to cytology results
| Final cytology diagnosis | ||||||
|---|---|---|---|---|---|---|
| ASC- H | ASCUS | LSIL | NILM | TOTAL | ||
| hrHPV TYPE | 16 | 0 | 0 | 0 | 3 (100%) | 3 (100%) |
| 16, 31, 45, 51, 59, 53 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | |
| 16, 51 | 0 | 0 | 0 | 2 (100%) | 2 (100%) | |
| 18 | 0 | 0 | 0 | 2 (100%) | 2 (100%) | |
| 18, 35, 66 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | |
| 18, 39 | 0 | 0 | 1 (100%) | 0 | 1 (100%) | |
| 31 | 0 | 0 | 0 | 4 (100%) | 4 (100%) | |
| 31, 35, 58, 68 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | |
| 31, 52 | 0 | 0 | 1 (100%) | 0 | 1 (100%) | |
| 31, 58 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | |
| 33 | 0 | 0 | 0 | 2 (100%) | 2 (100%) | |
| 33, 39 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | |
| 35 | 0 | 1 (20%) | 0 | 4 (80%) | 5 (100%) | |
| 39 | 0 | 0 | 0 | 2 (100%) | 2 (100%) | |
| 45 | 0 | 0 | 0 | 2 (100%) | 2 (100%) | |
| 51 | 0 | 1 (25%) | 0 | 3 (75%) | 4 (100%) | |
| 52 | 0 | 0 | 0 | 6 (100%) | 6 (100%) | |
| 52, 53 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | |
| 52, 56 | 0 | 0 | 1 (100%) | 0 | 1 (100%) | |
| 53 | 0 | 0 | 0 | 2 (100%) | 2 (100%) | |
| 53, 68 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | |
| 56 | 0 | 2 (100%) | 0 | 0 | 2 (100%) | |
| 59 | 0 | 0 | 0 | 1 (100%) | 1 (100%) | |
| 58 | 1 (25%) | 0 | 0 | 3 (75%) | 4 (100%) | |
| 66 | 0 | 0 | 0 | 2 (100%) | 2 (100%) | |
| 68 | 0 | 0 | 0 | 3 (100%) | 3 (100%) | |
| TOTAL | 1 (1.8%) | 4 (7.1%) | 3 (5.4%) | 48 (87.1%) | 56 (100%) | |
Pearson’s Chi-square: P–value = 0.61, Fisher’s Exact test: P-value = 0.352
Table 4 presents the age distribution of study participants by hrHPV status and final cytology diagnosis. Among those detected with hrHPV (n = 56); there was 1 (0.58%) case of ASCUS and 9 (5.26%) cases of NILM in the 20–30 age group. For those with no hrHPV (n = 115), the 20–30 age group had 1 (0.58%) case of AEC, 2 (1.17%) cases of LSIL, and 19 (11.11%) cases of NILM. In the 31–40 age group there were 2 (1.17%) cases of LSIL and 9 (5.26%) cases of NILM among those with detectable hrHPV. For those with no detectable hrHPV, there was 1 (0.58%) case of ASC-H, 2 (1.17%) cases of ASCUS, 1 (0.58%) case of AEC-FN, 1 (0.58%) case of LSIL, and 31 (18.13%) cases of NILM. In the 41–50 age group, those with detectable hrHPV had 3 (1.75%) cases of ASCUS, 1 (0.58%) case of LSIL, and 16 (9.36%) cases of NILM. Those with no detectable hrHPV had 2 (1.17%) cases of ASC-H, 1 (0.58%) case of ASCUS, 1 (0.58%) cases of AEC, 2 (1.17%) cases of LSIL, and 22 (12.87%) cases of NILM. For participants aged 50 and above, those with detectable hrHPV had 1 (0.58%) case of ASC-H and 14 (8.19%) cases of NILM. In those without hrHPV, there was 1 (0.58%) case of ASCUS and 28 (16.37%) cases of NILM. Fisher’s exact test was used to assess the association between age groups, hrHPV status and cytology diagnosis.
Table 4.
Age distribution of the study participants by hrHPV status and cytology diagnosis
| hrHPV detected n = 56 | hrHPV not detected n = 115 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age group | ASC-H | ASCUS | AEC | AEC-FN | LSIL | NILM | ASC-H | ASCUS | AEC | AEC-FN | LSIL | NLM | Total |
| 20–30 | 0 | 1 (0.58) | 0 | 0 | 0 | 9 (5.26) | 0 | 0 | 1 (0.58) | 0 | 2 (1.17) | 19 (11.11) | 32 (18.71) |
| 31–40 | 0 | 0 | 0 | 0 | 2 (1.17) | 9 (5.26) | 1 (0.58) | 2 (1.17) | 0 | 1 (0.58) | 1 (0.58) | 31 (18.13) | 47 (27.49) |
| 41–50 | 0 | 3 (1.75) | 0 | 0 | 1 (0.58) | 16 (9.36) | 2 (1.17) | 1 (0.58) | 1 (0.58) | 0 | 2 (1.17) | 22 (12.87) | 48 (28.07) |
| 50 < | 1 (0.58) | 0 | 0 | 0 | 0 | 14 (8.19) | 0 | 1 (0.58) | 0 | 0 | 0 | 28 (16.37) | 44 (25.73) |
| Total | 1 (0.58) | 4 (2.34) | 0 | 0 | 3 (1.75) | 48 (28.07) | 3 (1.75) | 4 (2.34) | 2 (1.17) | 1 (0.58) | 5 (2.92) | 100 (58.48) | 171 (100) |
Fisher's exact test: Age group by hrHPV status, P-value = 0.304
Fisher's exact test: Age group by cytology diagnosis, P-value = 0.633
The p-value for the age groups by hrHPV status was 0.304, indicating no statistically significant association between age groups and hrHPV status. Similarly, the p-value for the age groups by final cytology diagnosis was 0.633, indicating no statistically significant association between age groups and final cytology diagnosis.
Table 5 presents an unadjusted logistic regression (adding risk factors individually to the model). Results shows that for every unit increase in age, women are equally likely to have hrHPV [95% CI = (0.96, 1.02), p-value = 0.754]. Women living with HIV were 0.85 less likely to have HPV [95% CI = (0.43, 1.70), p-value = 0.984]. Women who had more than 1 partner were 1.18 more likely to get hrHPV [95% CI = (0.49, 2.88), p-value = 0.708]. However, all p-values were reported to be more than 0.05, meaning these associations were statistically insignificant. After adjusting the logistic regression for multicollinearity by adding all risk factors in the model, the table shows that for every unit increase in age, women were still equally likely to have hrHPV [95% CI = (0.97, 1.03), p-value = 0.903]. Women living with HIV were 0.85 less likely to have hrHPV [95% CI = (0.35, 2.08), p-value = 0.647]. Women who had more than 1 partner were 1.28 more likely to get hrHPV [95% CI = (0.19, 8.83), p-value = 0.587]. All p-values were reported to be more than 0.05, meaning these associations were statistically insignificant.
Table 5.
hrHPV risk association with age, HIV status and number of lifetime sexual partners
| Risk factor | ODDS RATIO | 95% CI | P-value | |
|---|---|---|---|---|
| Age | 1.00 | 0.96, 1.02 | 0.754 | |
| Unadjusted | HIV positive | 0.85 | 0.43, 1.70 | 0.984 |
| More than 1 sexual partner | 1.18 | 0.49, 2.88 | 0.708 | |
| Age | 1.00 | 0.97, 1.03 | 0.903 | |
| Adjusted | HIV positive | 0.85 | 0.35, 2.08 | 0.647 |
| More than 1 sexual partner | 1.28 | 0.19, 8.83 | 0.587 |
Discussion
This study reports hrHPV prevalence of 32.7% (56/171) in HPV vaccine-naïve women who were 21 years and above from the indigenous and marginalized communities of Ghanzi and Kgalagadi regions in western Botswana. This is the first study to report hrHPV genotypes detected from cervical screening in a unique population of indigenous (marginalized) persons residing in the western region of Botswana. The median age of the participants was 42 years (Table 1); 74% (127/171) were aged 50 years and below while 26% (44/171) were above 50 years. Prevalence of pre-malignant cervical lesions in this study was 13.5% (Table 2) and the prevalence of hrHPV DNA in premalignant cervical lesions was 35%. Overall, the hrHPV prevalence was lower than that reported by a previous study in Botswana in unvaccinated young college women with a prevalence rate of 60.2% [33]. Other studies reported varying hrHPV prevalence rates between 30–64% mainly focused on women with premalignant and malignant cervical lesions and living with HIV [27, 34–36]. However, these studies focused on women living in and around Gaborone (see map above).
The hrHPV genotypes detected in premalignant cervical lesions included the quadrivalent vaccine targeted HPV18, (6.25%) and non-quadrivalent HPV 31, 35, 39, 51, 52 and 56 at 10.42%, 4.7%, 6.25%, 14.5%, 12.5% and 4.17% respectively (Table 3). HPV16 was only detected in women with normal cytology at a prevalence rate of 10.42%. This finding is in contrast with the observations that HPV16 is the most common genotype despite geographic variation for other HPV genotypes [37, 38]. High-risk HPV infection was also detected more in women with normal cervical cytology, followed by LSIL and ASCUS (Table 4). In addition, more hrHPV associated cervical premalignant lesions were detected in women less that 50 years (Table 4). There was an increased detection of non-quadrivalent targeted hrHPV genotypes in the ASCUS lesion as compared to other cervical premalignant lesions with HPV 35, 51 and 56 being the most commonly detected in this category. In 56 of the 171 women (32.7%) who tested positive for hrHPV DNA; seven women living without HIV had cervical premalignant lesions and 2/7 (14.2%) had hrHPV (HPV18, HPV39 and HPV56) with only one woman out of the two, having hrHPV genotypes that are targeted by the current quadrivalent vaccine that is in use in Botswana. Six out of seven (85.7%) women with cervical premalignant lesions had hrHPV genotypes which are non-quadrivalent vaccine targeted. Although the predominance of HPV genotypes 51, 52 and 56 in our study is not consistent with global findings, previous studies in Botswana have also reported a higher prevalence of HPV genotype 51; 15.9%[39], 15.9%[40] 12.6% [36] highlighting the importance of HPV51 genotype circulation in the general population. Similarly, quadrivalent-targeted hrHPV genotype prevalence in these studies ranged from 21% to 34.3% [36, 40]. It is also important to note that these studies have been conducted among women from urban and peri-urban areas (Gaborone and surrounding areas), who have access to better healthcare services. In our study quadrivalent vaccine targeted hrHPV genotype prevalence was 16.67% vs 83.33% of the non-quadrivalent vaccine targeted genotypes.
Risk factors including the number of lifetime partners, age, parity, smoking and contraceptive use were also assessed for association with hrHPV positivity. Our results indicate that smoking history was the only statistically significant risk factor for hrHPV detection in this cohort (Table 1) with a p-value of 0.01. Studies have reported smoking as a risk factor for HPV infection [41, 42] and this has been supported by our findings. Association between HIV and hrHPV positivity showed a marginal significance (p-value 0.06) in Table 1, with the data indicating that WLWH were 0.85 less likely to have HPV [95% CI = (0.43, 1.70), p-value = 0.984] as indicated in Table 5. However, all p-values were more than 0.05, meaning that the association between living with HIV and hrHPV positivity were statistically insignificant. Our study also reports a slightly higher HIV prevalence (31%), than the national prevalence estimated to be at 26.2% for adult women (15 – 49 years) living with HIV in Botswana, [23]. This prevalence however, is also higher than that reported by the Botswana AIDS Impact Survey for both Kgalagadi and Ghanzi regions, which ranged between 15.2 – 21.1% for women aged between 15–64 years [23]. In our study, hrHPV/ HIV co-infection was 37.2% (Table 1); cervical premalignant lesions were detected in 13.3% of WLWH in this study and 30.2% of the women with hrHPV had normal cytology. Another study by Luckett et al. [43], in a subset of WLHW and diagnosed with CIN2 + in Gaborone reported a hrHPV prevalence of 29% which was lower than that reported by our study. Women living with HIV have been reported to be more likely to have hrHPV infection than those living without HIV [44, 45]. Since HIV and hrHPV infections share a common route of transmission, it is to be expected that these findings corroborate those of previous studies of the duality of existence in their host [46]. HIV infection is a well-known risk factor for HPV persistence, cervical cancer development and its progression [47–50], with the burden of cervical cancer in Botswana reported to be largely driven by the high HIV prevalence [43]. This has been attributed to immune suppression which supports hrHPV persistence and ultimate cancer development [45, 48].
In this study, 23/171 (13.5%) of the women had cervical premalignant lesions. Of the 23, only 8 had hrHPV (8/23). In addition, 9/23 (39%) women with premalignant lesion were living with HIV. Nine women living with HIV had cervical premalignant lesions and 4/9 (44%) had hrHPV (HPV 31, HPV 52, HPV 56 and HPV 58) DNA. HPV 58 has been previously reported as the common type in WLWH with cervical premalignant lesions in Botswana [39]. The high hrHPV prevalence and low detection of cervical premalignant lesion in our study population could be explained by the success of highly active antiretroviral therapy (HAART) in Botswana. Although we did not assess the viral suppression, recent reports indicate that 98.4% of WLWH aged 15–64 years in Botswana are on antiretroviral treatment and 98.6% have viral suppression [23].
A total of 12/56 (21.4%) women in our study who were co-infected with more than one hrHPV genotype and 50% of them had HIV infection. In this study, the most common hrHPV genotypes detected were 52, 51, 31, and 16 respectively, in WLWH with only HPV 16 as the genotype being targeted by the current vaccine being administered in Botswana. A study by Kim et al., [51] reported that “uncommon and rare HPV genotypes may provide incremental etiologic contributions in cervical carcinogenesis.” The predominance of this pattern of hrHPV genotypes in this population corroborates findings from previous studies in Botswana, especially for people living with HIV and warrants further attention. The findings of this study highlight the geographic disparity of understudied and underrepresented populations in Botswana and add the dynamics of region-specific hrHPV distribution of varying carcinogenicity. The high HIV prevalence in this community also calls for consideration of healthcare policies to address cervical cancer surveillance, screening and treatment monitoring which may include region-specific HPV vaccine designs for prevention strategies in isolated populations. These hrHPV genotypes are not covered by the current prophylactic vaccine in use, which is achieving 90 -100% nationwide coverage and is expected to reduce cervical lesions caused by HPV16 and HPV18. Other studies in Botswana have also reported low hrHPV prevalence for quadrivalent targeted hrHPV genotypes [36, 40]. However, these studies were conducted on a younger cohort (18-22 years) for vaccine impact monitoring and lacked cervical cytology results for comparison.
Limitations of the study
A smaller sample size of cervical premalignant lesions in our study could have introduced a bias. However, these are naturally smaller communities and isolated in nature. We also note that our findings may not be generalizable to other indigenous (marginalized) populations in Botswana accounting for the fact that there is cultural diversity among indigenous communities including those in Botswana. A major limitation of this study is that no high-grade lesions were detected, therefore there were no biopsies collected and analyzed. This study was conducted alongside routine Ministry of Health mobile screening outreach service guidelines, therefore all women with abnormal cytology results were referred for further management through their healthcare facility which also served as a study site. However, there are plans for a follow-up study in the future to assess treatment uptake in this very same community. To our knowledge, this is the first study to report hrHPV prevalence, diversity and cervical disease burden focused on a specific population of marginalized and indigenous populations of Botswana.
Conclusions
Our study found a predominance of non-quadrivalent vaccine targeted hrHPV genotypes in the indigenous and marginalized communities in Botswana and this is atypical of what has been observed in other populations. Although our study has a small sample size of abnormal cervical cytology, we note that these hrHPV genotypes detected are epidemiologically significant. Marginalized communities’ geographical isolation and territorial relationships could provide a niche for these less common hrHPV genotypes to increase the burden of non-quadrivalent targeted vaccine hrHPV-associated cervical carcinogenesis. Therefore, there is a need to monitor and further investigate these findings and determine their potential impact on cervical carcinogenesis in this isolated population. Furthermore, these results support those of previous studies on the consideration of broadening protection against hrHPV genotypes that are not targeted by the current vaccine in use.
Acknowledgements
We acknowledge the District Health Management Team leaders from the Ministry of Health (MOH), various Healthcare workers in the Ghanzi and Kgalagadi MoH Health facilities, the participants, Mr Nichodimus Cooper from the NAMA community in Lokgwabe and various community leaders for supporting this study. We also acknowledge Mr Leema A. Hiri from The University of Botswana-based San Research Center for his assistance in this study. We also wish to acknowledge the support of the department of Pathology, University of Botswana and Botswana Harvard Health Partnership Research Laboratory staff.
Authors' contributions
PSR, study concept, study design, data collection, laboratory investigations, data analysis & manuscript write-up, review and approval of final draft. AKN: study concept, study design, laboratory investigations, data analysis & manuscript write-up. TG: study concept, study design, data collection, data analysis, & manuscript write-up. KM: study concept, study design, data collection, data analysis, & manuscript write-up. KS: study concept, study design, data analysis & manuscript write-up. CMB: laboratory investigations, data collection & analysis, manuscript write-up. AKTMM: laboratory investigations, data collection & analysis, manuscript write-up. ABE: laboratory investigations, data collection & manuscript write-up. LT: laboratory investigations, data analysis & manuscript write-up and review, review and approval of final draft. NOM: laboratory investigation, data analysis & manuscript write-up. KM: laboratory investigations, data analysis & manuscript write-up, review and approval of final draft. SM: laboratory investigations, data analysis & manuscript write-up, review and approval of final draft. BMT: study concept, study design, data collection, data analysis & manuscript write-up, review and approval of final draft. LTK: study concept, study design, data collection, laboratory investigation, data analysis & manuscript write-up, review and approval of final draft.
Funding
The study was funded by the University of Botswana Office of Research and Development (ORD) Round 39 call and grant number R1271. SM was funded by the NIH Fogarty International Center (grants K43TWO12350 and 3D43TW009610). PSR, SM and NOM were partially supported by the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE) which is funded by the Science for Africa Foundation [Del-22-007] with support from Wellcome Trust and the UK Foreign, Commonwealth & Development Office and is part of the EDCPT2 programme supported by the European Union; the Bill & Melinda Gates Foundation [INV-033558]; and Gilead Sciences Inc., [19275]. All content contained within is that of the authors and does not necessarily reflect positions or policies of any SANTHE funder. For the purpose of open access, the authors have applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. The funders had no role in role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of this manuscript.
Availabilityof data and materials
All relevant data is available upon request from the corresponding author, Patricia S. Rantshabeng.
Declarations
Ethics approval and consent to participate
This study was approved by the University of Botswana Institutional Review Board (reference number UBR/RES/IRB/BIO/241) and a permit issued by the Ministry of Health Research and Development Committee (reference number HPDME/13/8/1). Informed consent to participate in the study was obtained from the participants before data collection. All procedures pertaining to the clinical data and sample collection were explained in native Setswana and translated into local languages where needed.
Consent for publication
The participants in this study consented to de-identified data dissemination exercises.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn M, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008;110(3 Suppl 2):S4-7. [DOI] [PubMed] [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt B):1–441. [PMC free article] [PubMed]
- 5.Schiller J, Lowy D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 2018;36(32 Pt A):4768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallagher KE, LaMontagne DS, Watson-Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine. 2018;36(32 Pt A):4761–7. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick MB, et al. hrHPV prevalence and type distribution in rural Zimbabwe: A community-based self-collection study using near-point-of-care GeneXpert HPV testing. Int J Infect Dis. 2019;82:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paz-Zulueta M, et al. Prevalence of high-risk HPV genotypes, categorised by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC Cancer. 2018;18(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dwyer-Lindgren L, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature. 2019;570(7760):189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botswana Human Papillomavirus and Related Cancers, Fact Sheet 2023. ICO/IARC Information Center on HPV and Cancer. Barcelona: Institut Catalàd’ Oncologia.
- 11.Looker KJ, et al. Evidence of synergistic relationships between HIV and Human Papillomavirus (HPV): systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J Int AIDS Soc. 2018;21(6): e25110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chirenje ZM. HIV and cancer of the cervix. Best Pract Res Clin Obstet Gynaecol. 2005;19(2):269–76. [DOI] [PubMed] [Google Scholar]
- 13.Shmakova A, Germini D, Vassetzky Y. HIV-1, HAART and cancer: A complex relationship. Int J Cancer. 2020;146(10):2666–79. [DOI] [PubMed] [Google Scholar]
- 14.Palefsky JM. Cervical human papillomavirus infection and cervical intraepithelial neoplasia in women positive for human immunodeficiency virus in the era of highly active antiretroviral therapy. Curr Opin Oncol. 2003;15(5):382–8. [DOI] [PubMed] [Google Scholar]
- 15.Dryden-Peterson S, et al. Cancer Incidence following Expansion of HIV treatment in Botswana. PLoS One. 2015;10(8): e0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Black E, Richmond R. Prevention of Cervical Cancer in Sub-Saharan Africa: The Advantages and Challenges of HPV Vaccination. Vaccines (Basel). 2018;6(3):61. 10.3390/vaccines6030061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raesima MM, et al. Human papillomavirus vaccination coverage among school girls in a demonstration project - Botswana, 2013. MMWR Morb Mortal Wkly Rep. 2015;64(40):1147–9. [DOI] [PubMed] [Google Scholar]
- 18.Ministry of Health Child and Adolescent Immunisation report (2023). Unpublished Botswana Government reports. 2023.
- 19.Ohenjo N, et al. Health of indigenous people in Africa. Lancet. 2006;367(9526):1937–46. [DOI] [PubMed] [Google Scholar]
- 20.Hitchcock RK, Sapignoli M, Babchuk WA. What about our rights? Settlements, subsistence and livelihood security among Central Kalahari San and Bakgalagadi. The International Journal of Human Rights. 2011;15:62–88. [Google Scholar]
- 21.Twyman C. Rethinking community resource management: managing resources or managing people in western Botswana? Third World Quarterly. 1998;19(4):745–70. [Google Scholar]
- 22.Chipfakacha V. Attitudes of women towards traditional midwives–a survey in the Kgalagadi (Kalahari) region. S Afr Med J. 1994;84(1):30–2. [PubMed] [Google Scholar]
- 23.Botswana AIDS Impact Survey V 2021 (BAIS V): Botswana Government Health Reports. Gaborone: National AIDS & Health Promotion Agency; 2023.
- 24.Makhema J, et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med. 2019;381(3):230–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Organisation, W.H. Global strategy towards the elimination of cervical cancer as a public health problem [WHO cervical cancer elimination initiative]. 2020; WHO reports. Cited 2023 18/08/23.
- 26.Grover S, et al. Cervical cancer in Botswana: current state and future steps for screening and treatment programs. Front Oncol. 2015;5: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramogola-Masire D, et al. Cervical cancer prevention in HIV-infected women using the “see and treat” approach in Botswana. J Acquir Immune Defic Syndr. 2012;59(3):308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organisation Cervical Cancer Elimination Initiative. World Health Organisation Reports; 2020.
- 29.World Health Organisation prequalifies additional HPV test, expanding options as countries pursue cervical cancer elimination. World Health Organisation reports; 2023.
- 30.Sethi S, et al. Working towards a comprehensive understanding of HPV and cervical cancer among Indigenous women: a qualitative systematic review. BMJ Open. 2021;11(6): e050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shannon GD, et al. Cervical cancer in Indigenous women: The case of Australia. Maturitas. 2011;70(3):234–45. [DOI] [PubMed] [Google Scholar]
- 32.Botswana S. Population, housing census 2022: population of cities, towns, villages, associated localities. Gaborone: Statistics Botswana; 2022. [Google Scholar]
- 33.Ramatlho P, et al. Human papillomavirus prevalence among unvaccinated young female college students in Botswana: A cross-sectional study. S Afr Med J. 2022;112(5):335–40. [PubMed] [Google Scholar]
- 34.Tawe L, et al. Cervical human papillomavirus genotypes in a high HIV setting: A scoping review of a decade of human papillomavirus epidemiological research in Botswana. Front Med (Lausanne). 2022;9:1020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castle PE, et al. High-risk human papillomavirus prevalence in self-collected cervicovaginal specimens from human immunodeficiency virus (HIV)-negative women and women living with HIV living in Botswana. PLoS One. 2020;15(2): e0229086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClung N, et al. HPV prevalence among young adult women living with and without HIV in Botswana for future HPV vaccine impact monitoring. BMC Infect Dis. 2022;22(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruni L, et al. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789–99. [DOI] [PubMed] [Google Scholar]
- 38.Bruni L, et al. Global and regional estimates of genital human papillomavirus prevalence among men: a systematic review and meta-analysis. Lancet Glob Health. 2023;11(9):e1345–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Macleod IJ, et al. Prevalence of human papillomavirus genotypes and associated cervical squamous intraepithelial lesions in HIV-infected women in Botswana. J Med Virol. 2011;83(10):1689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramogola-Masire D, et al. Human papillomavirus prevalence in male and female university students in Gaborone. Botswana Epidemiol Infect. 2022;150:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schabath MB, et al. Smoking and human papillomavirus (HPV) infection in the HPV in Men (HIM) study. Cancer Epidemiol Biomarkers Prev. 2012;21(1):102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaccarella S, et al. Smoking and human papillomavirus infection: pooled analysis of the International Agency for Research on Cancer HPV Prevalence Surveys. Int J Epidemiol. 2008;37(3):536–46. [DOI] [PubMed] [Google Scholar]
- 43.Luckett R, et al. Performance of two-stage cervical cancer screening with primary high-risk human papillomavirus testing in women living with human immunodeficiency virus. Obstet Gynecol. 2019;134(4):840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denslow SA, et al. Incidence and progression of cervical lesions in women with HIV: a systematic global review. Int J STD AIDS. 2014;25(3):163–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clifford GM, et al. Immunodeficiency and the risk of cervical intraepithelial neoplasia 2/3 and cervical cancer: A nested case-control study in the Swiss HIV cohort study. Int J Cancer. 2016;138(7):1732–40. [DOI] [PubMed] [Google Scholar]
- 46.Liu G, et al. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS. 2018;32(6):795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harris TG, et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA. 2005;293(12):1471–6. [DOI] [PubMed] [Google Scholar]
- 48.Strickler HD, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus-positive women. J Natl Cancer Inst. 2005;97(8):577–86. [DOI] [PubMed] [Google Scholar]
- 49.Schiffman M, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. [DOI] [PubMed] [Google Scholar]
- 50.Louie KS, de Sanjose S, Mayaud P. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: a comprehensive review. Trop Med Int Health. 2009;14(10):1287–302. [DOI] [PubMed] [Google Scholar]
- 51.Kim NR, et al. Uncommon and rare human papillomavirus genotypes relating to cervical carcinomas. Korean J Pathol. 2014;48(1):43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data is available upon request from the corresponding author, Patricia S. Rantshabeng.