Abstract
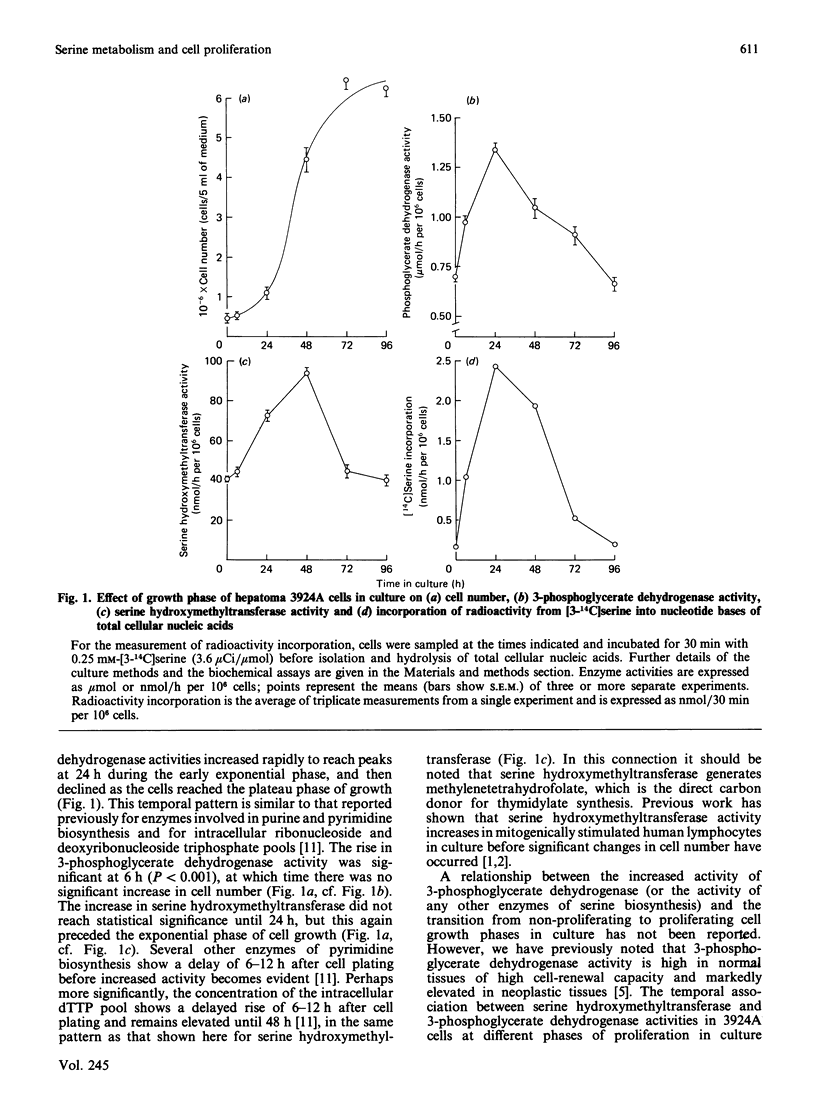
The activities of 3-phosphoglycerate dehydrogenase and serine hydroxymethyltransferase increased markedly during the transition of hepatoma cells from a resting non-proliferating culture into the proliferating growth phase. Activities declined as cells reached confluency and entered the plateau growth phase. This pattern was paralleled by changes in [14C]serine incorporation into nucleic acids. The experiments support the hypothesis that the biosynthesis of serine is metabolically coupled to its utilization for nucleotide precursor formation in cancer cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhme H. J., Belay D., Dettmer D., Goltzsch W., Hofmann E., Lange R., Schubert C., Schulze E., Sparmann G., Weiss E. Interaction of adrenal and pancreatic hormones in the control of hepatic enzymes during development. Adv Enzyme Regul. 1987;26:31–61. doi: 10.1016/0065-2571(87)90005-7. [DOI] [PubMed] [Google Scholar]
- Curtin N. J., Snell K. Enzymic retrodifferentiation during hepatocarcinogenesis and liver regeneration in rats in vivo. Br J Cancer. 1983 Oct;48(4):495–505. doi: 10.1038/bjc.1983.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. L., Fallon H. J., Morris H. P. Two enzymes of serine metabolism in rat liver and hepatomas. Cancer Res. 1970 Dec;30(12):2917–2920. [PubMed] [Google Scholar]
- Eichler H. G., Hubbard R., Snell K. The role of serine hydroxymethyltransferase in cell proliferation: DNA synthesis from serine following mitogenic stimulation of lymphocytes. Biosci Rep. 1981 Feb;1(2):101–106. doi: 10.1007/BF01117006. [DOI] [PubMed] [Google Scholar]
- Greengard O., Dewey H. K. Initiation by glucagon of the premature development of tyrosine aminotransferase, serine dehydratase, and glucose-6-phosphatase in fetal rat liver. J Biol Chem. 1967 Jun 25;242(12):2986–2991. [PubMed] [Google Scholar]
- Knox W. E., Herzfeld A., Hudson J. Phosphoserine phosphatase distribution in normal and neoplastic rat tissues. Arch Biochem Biophys. 1969 Jul;132(2):397–403. doi: 10.1016/0003-9861(69)90381-6. [DOI] [PubMed] [Google Scholar]
- Natsumeda Y., Ikegami T., Weber G. Purine synthetic capacities of de novo and salvage pathways in rat hepatoma 3924A cells. Adv Exp Med Biol. 1986;195(Pt B):371–376. doi: 10.1007/978-1-4684-1248-2_58. [DOI] [PubMed] [Google Scholar]
- Olah E., Lui M. S., Tzeng D. Y., Weber G. Phase and cell cycle specificity of pyrazofurin action. Cancer Res. 1980 Aug;40(8 Pt 1):2869–2875. [PubMed] [Google Scholar]
- Olah E., Weber G. Giemsa-banding karyotype of rat hepatomas of different growth rates. Cancer Res. 1979 May;39(5):1708–1717. [PubMed] [Google Scholar]
- Oliver I. T., Martin R. L., Fisher C. J., Yeoh G. C. Enzymic differentiation in cultured foetal hepatocytes of the rat. Induction of serine dehydratase activity by dexamethasone and dibutyryl cyclic AMP. Differentiation. 1983;24(3):234–238. doi: 10.1111/j.1432-0436.1983.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Rowe P. B., Sauer D., Fahey D., Craig G., McCairns E. One-carbon metabolism in lectin-activated human lymphocytes. Arch Biochem Biophys. 1985 Jan;236(1):277–288. doi: 10.1016/0003-9861(85)90627-7. [DOI] [PubMed] [Google Scholar]
- Snell K. Enzymes of serine metabolism in normal and neoplastic rat tissues. Biochim Biophys Acta. 1985 Dec 13;843(3):276–281. doi: 10.1016/0304-4165(85)90149-7. [DOI] [PubMed] [Google Scholar]
- Snell K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv Enzyme Regul. 1984;22:325–400. doi: 10.1016/0065-2571(84)90021-9. [DOI] [PubMed] [Google Scholar]
- Snell K. Liver enzymes of serine metabolism during neonatal development of the rat. Biochem J. 1980 Aug 15;190(2):451–455. doi: 10.1042/bj1900451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K. Pathways of gluconeogenesis from L-serine in the neonatal rat. Biochem J. 1974 Aug;142(2):433–436. doi: 10.1042/bj1420433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Walker D. G. Regulation of hepatic L-serine dehydratase and L-serine-pyruvate aminotransferase in the developing neonatal rat. Biochem J. 1974 Dec;144(3):519–531. doi: 10.1042/bj1440519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Weber G. Enzymic imbalance in serine metabolism in rat hepatomas. Biochem J. 1986 Jan 15;233(2):617–620. doi: 10.1042/bj2330617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike J., Pelliniemi T. T., Beck W. S. Serine hydroxymethyltransferase activity and serine incorporation in leukocytes. Cancer Res. 1979 Sep;39(9):3435–3440. [PubMed] [Google Scholar]
- Weber G. Biochemical strategy of cancer cells and the design of chemotherapy: G. H. A. Clowes Memorial Lecture. Cancer Res. 1983 Aug;43(8):3466–3492. [PubMed] [Google Scholar]
- Weber G., Olah E., Denton J. E., Lui M. S., Takeda E., Tzeng D. Y., Ban J. Dynamics of modulation of biochemical programs in cancer cells. Adv Enzyme Regul. 1980;19:87–102. doi: 10.1016/0065-2571(81)90010-8. [DOI] [PubMed] [Google Scholar]
- Weber G., Olah E., Lui M. S., Kizaki H., Tzeng D. Y., Takeda E. Biochemical commitment to replication in cancer cells. Adv Enzyme Regul. 1980;18:3–26. doi: 10.1016/0065-2571(80)90005-9. [DOI] [PubMed] [Google Scholar]



