Abstract
Oral bacteria naturally secrete extracellular vesicles (EVs), which have attracted attention for their promising biomedical applications including cancer therapeutics. However, our understanding of EV impact on tumor progression is hampered by limited in vivo models. In this study, we propose a facile in vivo platform for assessing the effect of EVs isolated from different bacterial strains on oral cancer growth and dissemination using the larval zebrafish model. EVs were isolated from: wild-type Aggregatibacter actinomycetemcomitans and its mutant strains lacking the cytolethal distending toxin (CDT) or lipopolysaccharide (LPS) O-antigen; and wild-type Porphyromonas gingivalis. Cancer cells pretreated with EVs were xenotransplanted into zebrafish larvae, wherein tumor growth and metastasis were screened. We further assessed the preferential sites for the metastatic foci development. Interestingly, EVs from the CDT-lacking A. actinomycetemcomitans resulted in an increased tumor growth, whereas EVs lacking the lipopolysaccharide O-antigen reduced the metastasis rate. P. gingivalis-derived EVs showed no significant effects. Cancer cells pretreated with EVs from the mutant A. actinomycetemcomitans strains tended to metastasize less often to the head and tail compared to the controls. In sum, the proposed approach provided cost- and labor-effective yet efficient model for studying bacterial EVs in oral carcinogenesis, which can be easily extended for other cancer types. Furthermore, our results support the notion that these nanosized particles may represent promising targets in cancer therapeutics.
Keywords: Cancer cell line, Extracellular vesicles, Oral bacteria, Zebrafish larvae, Oral cancer
Introduction
Oral squamous cell carcinoma (OSCC) is among the most common malignancies worldwide, accounting for more than 90% of oral cavity cancers [1, 2]. The majority of OSCC cases are diagnosed at locoregionally advanced stages, leading to high morbidity and mortality rates. Hence, the 5-year survival rate of these patients has remained stagnant at approximately 50% over the past decades [3, 4]. The main risk factors for OSCC are smoking, alcohol abuse and the consumption of tobacco products [5]. In addition, recent evidence suggests that oral microbiota may play a role in oral carcinogenesis [6]. Oral dysbiosis, an imbalance of oral bacteria, can promote various chronic inflammatory diseases including periodontitis, which has been linked to OSCC [7, 8]. On the contrary, some bacteria showed anti-tumorigenic effects and were associated with favorable prognostic outcomes [6]. Furthermore, oral microbiota was shown to differ between OSCC patients with and without lymph node metastasis [9]. Therefore, bacterial species and their role in cancer can vary across different individuals [10].
Oral bacteria actively secrete extracellular vesicles (EVs), which are important immunomodulators carrying multiple virulence factors [11]. Importantly, these nanosized particles have attracted attention for their biomedical applications such as vaccination and cancer therapy [12, 13]. However, despite the recent increasing interest in bacterial EVs, their role in cancer remains elusive with limited studies [14, 15]. EVs from the Gram-negative Aggregatibacter actinomycetemcomitans carry a variety of cargo, including the cytolethal distending toxin (CDT)—a genotoxin with DNase activity that has been implicated in head and neck cancers [16–19]. In addition, the immunomodulator lipopolysaccharide (LPS) is a major constituent of A. actinomycetemcomitans EVs and it has been suggested as a target in cancer therapy [20–22]. Importantly, loss of the LPS O-antigen significantly altered the pathogenic and immunostimulatory features of A. actinomycetemcomitans [23, 24]. Another Gram-negative anaerobic bacterium, Porphyromonas gingivalis, is one of the most studied periodontopathogens in OSCC, revealing mostly pro-tumorigenic effects [25]. Recently, we showed that EVs isolated from A. actinomycetemcomitans and P. gingivalis differentially influenced the behavior of OSCC cells in vitro [26]. Furthermore, P. gingivalis-derived EVs promoted OSCC cell migration and invasion in vitro [27]. However, research exploring the role of bacterial EVs in cancer is still limited, to our knowledge, with only three studies in OSCC to date [26–28].
In vivo studies exploring the bacterial role in cancer are currently conducted using patient-derived murine xenografts [28–32]. However, the utility of these models is dampened by cost, time, and labor challenges, thus hindering advancement in this new field. During recent years, zebrafish larvae have emerged as a favorite organism for wide-ranging studies of cancer [33–36]. Herein, we aimed to assess the utility of zebrafish larvae as a facile and rapid in vivo model for studying the influence of EVs from different A. actinomycetemcomitans strains and wild-type P. gingivalis on OSCC growth and metastasis.
Materials and methods
Bacterial strains and growth conditions
Four different strains of A. actinomycetemcomitans were used in this study (Table 1). A. actinomycetemcomitans D7SS is a serotype a, naturally genetic competent, smooth-colony derivative of wild-type strain D7S, which is isolated from a patient with aggressive periodontitis [37]. D7SS cdtABC is a mutant derivative of D7SS created via a knockout method [38]. Hereafter, they are referred to as D7SS-WT, and D7SS-cdt, respectively. A. actinomycetemcomitans strains SA3138 [39] and SA3139 [39, 40] were recovered from a patient with periodontitis, and the latter strain naturally lacks LPS O-antigen [40]. Hereafter these are referred to as SA3138-WT, and SA3139-LPS-O, respectively. In addition, P. gingivalis ATCC 33277 (American Type Culture Collection) was used [41] (Table 1). Briefly, A. actinomycetemcomitans strains were cultured on blood agar plates (5% defibrinated horse blood, 5 mg hemin/l, 10 mg Vitamin K/l, Columbia agar base; Oxoid Ltd., Basingstoke, Hampshire, UK), in air supplemented with 5% CO2, at 37 °C. The D7SS strains were cultivated for 4 days and SA3138 and SA3139 for 5 days. P. gingivalis was cultured in an anaerobic environment (10% H2, 5% CO2, 85% N2) at 37 °C first on blood agar plates for 3 days and then for additional 48 h in liquid broth fastidious anaerobe agar (FAA; Neogen®, Heywood, UK). Bacterial procedures were conducted according to the guidelines of the local ethics committee at the Medical Faculty of Umeå University.
Table 1.
Characteristics of bacterial extracellular vesicles
| Bacterium | Strain | Source | EV protein concentration (mg/ml)* |
|---|---|---|---|
| Aggregatibacter actinomycetemcomitans | D7SS wild-type | Patient with periodontitis | 1.987 |
| Aggregatibacter actinomycetemcomitans | D7SS cdtABC mutant | Patient with periodontitis | 1.258 |
| Aggregatibacter actinomycetemcomitans | SA3138 wild-type | Patient with periodontitis | 7.813 |
| Aggregatibacter actinomycetemcomitans | SA3139 naturally lacking LPS O-antigen | Patient with periodontitis | 8.732 |
| Porphyromonas gingivalis | ATCC 33277 | ATCC (Gingival sulcus) | 2.132 |
cdtABC cytolethal distending toxin subunit A, B and C gene, LPS lipopolysaccharide, ATCC American Type Culture Collection
*Protein concentration of the vesicle samples was measured with NanoDrop 100 spectrophotometer (Thermo Fisher Scientific)
EV isolation and analyses
The EV isolation was conducted by ultracentrifugation as recently reported [24, 42]. In brief, bacterial cells were harvested from agar plates and suspended in phosphate-buffered saline (PBS) or liquid broth. The optical density (OD) values of the 25 ml suspensions at 600 nm were: 0.76 (D7SS-WT), 0.56 (D7SS-cdt), 1.12 (SA3138-WT), 1.38 (SA3139-LPS-O) and 1.00 (P. gingivalis). The number of agar plates used for harvesting the bacterial cells was 5 (D7SS-cdt), 10 (D7SS-WT, SA3138-WT and SA3139-LPS-O). The suspensions were centrifuged at 12.096×g for 30 min at 4 °C in a JA-25.50 rotor (Beckman Instruments Inc.). Supernatants were filtered through syringe filters (0.45 and 0.2 µm, Filtropur, Sarstedt) and centrifuged at 85.000×g for 2 h at 4 °C in a 70 Ti rotor (Beckman Instruments Inc.). Then pellets were washed with PBS twice (85.000×g for 2 h at 4 °C in a Sw60 Ti rotor (Beckman Instruments Inc.) and suspended in PBS. Absence of contamination was tested by cultivating small EV sample aliquots on blood agar plates in air supplemented with 5% CO2 at 37 °C for 3 days. EV protein concentration was determined by NanoDrop 100 spectrophotometer (Thermo Fisher Scientific) and further analyzed by nanoparticle tracking analysis software Zetaview (Particle Metrix, Germany). A protein gel electrophoresis was conducted with Pierce™ Silver Stain Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions to visualize EV proteins. We used the Criterion™ TGX™ Precast Gels and Precision Plus Protein™ Standard All Blue (Bio-Rad). Images were taken with ChemiDoc™ MP imaging system.
Cancer cell lines and growth conditions
To investigate tumor cell metastasis in vivo, we used the highly metastatic OSCC cell line HSC-3 (JCRB Cell Bank, Japan). Cancer cells were cultured in 1:1 DMEM/F-12 medium which was supplemented with 10% heat-inactivated fetal bovine serum (Gibco), penicillin–streptomycin (Gibco), 250 ng/mL amphotericin B (Sigma-Aldrich, St. Louis, MO, USA), 50 µg/mL ascorbic acid (AppliChem, Chicago, IL, USA), and 0.4 µg/mL hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA). Cell maintenance and incubations were done at 37 °C, 5% CO2 concentration and 95% relative humidity unless otherwise indicated. HSC-3 cell line was authenticated by Technology Centre, Institute for Molecular Medicine Finland FIMM, University of Helsinki.
Zebrafish larvae xenograft
The effect of bacterial EVs on OSCC tumor area and metastasis in vivo was investigated using zebrafish larvae [36]. HSC-3 cells (4 × 106) were challenged with EVs (10 µg/ml) for 12 h. The control cells were cultured in the same DMEM medium but without EVs. The selected EV concentration was based on recent studies [27, 28, 43, 44]. The next day, cells were dyed with CellTrace™ Far Red Cell Proliferation Kit (Thermo Fisher Scientific, Cat. No. C34564) prior to implantation into the zebrafish larvae via microinjection. All zebrafish larvae wild-type (AB strain) were used at two-day post-fertilization (dpf). Fish were dechorionated and anesthetized with 0.04% Tricaine before microinjection to the perivitelline space, mimicking a subcutaneous injection in mouse model, with a 4 nl suspension of HSC-3 cells (1500 cells/4 nl/larva). Fish microinjection and experiments were conducted at the Zebrafish Unit (University of Helsinki) and approved by the ethical permission from the regional state administrative agency (ESAVI/13139/04.10.05/2017). After microinjection, the larvae were transferred to a 24-well plate containing 1000 µl fresh embryonic medium (Merck) and stored at 34 °C. Each group included 19–25 larvae, divided into wells with a maximum of five fish per well. After 72 h, the zebrafish larvae were fixed with 4% paraformaldehyde overnight. The next day, they were washed twice with 1% PBS and mounted on slides with SlowFade™ Gold Antifade Mountant (Thermo Fisher Scientific, S36837).
Imaging and image analysis
The mounted zebrafish larvae were imaged using a Leica Thunder Imager 3D Cell Culture microscope with Plan Fluotar 10×/0.32NA objective at the Biomedicum Imaging Unit, University of Helsinki, Finland. The tumor area was measured using Fiji ImageJ software (Wayne Rasband, National Institute of Health, Bethesda, MD, USA).
Statistical analyses
Statistical analyses were performed with GraphPad Prism Software version 9.4.1 (San Diego, California, USA). The Grubbs` test was used to identify and remove the outlier values, which were considered significant when p < 0.01. The analysis included groups of 18–25 fish per condition which were pooled together. As the variation in tumor area between experiments was significant, the two-way ANOVA with Dunnett`s multiple comparison test was used to determine statistical significance. Differences in metastasis between each condition and control were calculated with Fisher`s exact test with Bonferroni correction. Statistical significance was set to p < 0.05, * indicates p-values < 0.05. Data are represented as mean ± standard deviation (SD) or as quartiles with range from minimum to maximum with median and mean. The experiments were repeated three times independently.
Results
Zebrafish larvae survival
To our knowledge, this is the first study to evaluate the effect of bacterial EVs on OSCC cells using zebrafish larvae model. A total of 389 fish were included in the final analysis from the following testing groups: no-treatment control; D7SS-WT; D7SS-cdt; SA3138-WT; SA3139-LPS-O and P. gingivalis. The survival rate of the zebrafish larvae following xenotransplantation was 98.73%, with only a few fish died (n = 5) before mounting.
Tumor area
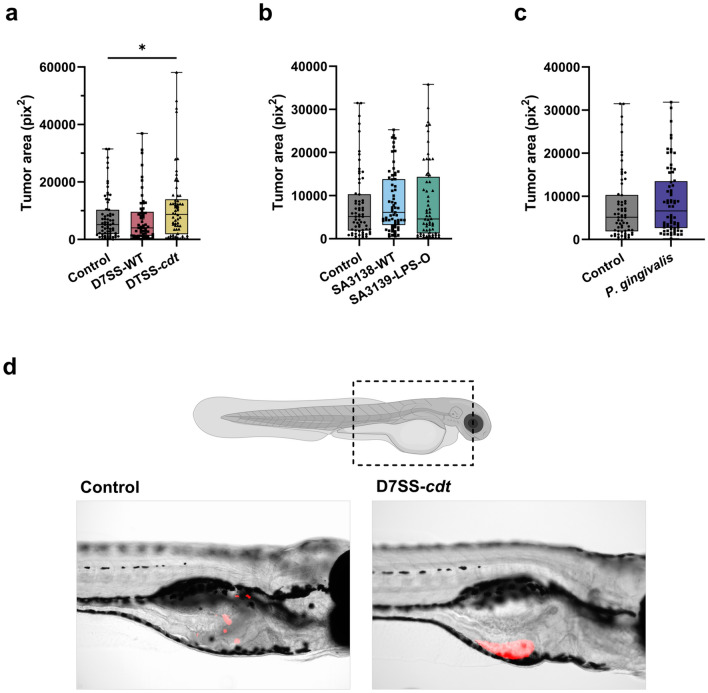
Tumor area was calculated in pix2 using Fiji ImageJ. We compared the effect of EVs from CDT-expressing A. actinomycetemcomitans on tumor area in vivo. Interestingly, while there was no significant difference between D7SS-WT-derived EVs and control, EVs from D7SS-cdt strain increased the tumor area significantly (p < 0.05; Fig. 1a, d). The effect of LPS O-antigen in A. actinomycetemcomitans EVs on OSCC tumor size was also tested using the wild- type strain SA3138-WT and SA3139-LPS-O strain which is naturally lacking LPS O-antigen. Both strains showed variations in the tumor area compared to the control and hence no statistically significant differences were noted (Fig. 1b). To compare A. actinomycetemcomitans strains to another common periodontopathogen, we tested EVs from wild-type P. gingivalis which, however, did not show any effects on tumor area compared to control (Fig. 1c).
Fig. 1.
Tumor area of HSC-3 cells pretreated with bacterial extracellular vesicles (EVs; 10 µg/ml) from: A. actinomycetemcomitans D7SS-WT (wild-type), D7SS-cdt (lacking the cytolethal distending toxin, CDT), SA3138-WT (wild-type), SA3139-LPS-O (lacking the lipopolysaccharide (LPS) O-antigen), and P. gingivalis (wild-type). a HSC-3 cells pretreated with D7SS-cdt-derived EVs formed larger tumors than control cells (p < 0.05). b No statistically significant changes were seen in the tumor area of cells pretreated with EVs from SA3138-WT, SA3139-LPS-O or c EVs from P. gingivalis. d Representative images of tumors formed by control cells and cells pretreated with D7SS-cdt EVs. Red areas represent tumor cells. *p < 0.05. Values are shown as minimum to maximum with all individual values. All experiments were repeated independently three times
Tumor cell metastasis
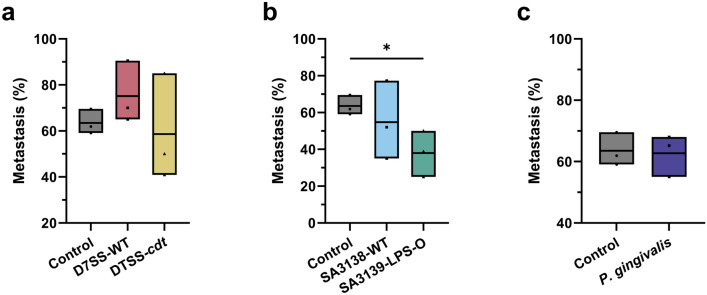
Next, we analyzed the tumor metastasis in zebrafish. Metastasis was analyzed by counting the proportion of fish with tumor cells metastasized outside the perivitelline area, i.e., head or tail, in each treatment group. A cut-off value of ≥1 cell outside the perivitelline area was considered as metastasis. Percentual averages ± SD from the three experiments were: control, 63.52 ± 5.42%; D7SS-WT, 75.16 ± 13.50%; D7SS-cdt, 58.64 ± 23.28; SA3138-WT, 54.76 ± 29.90%; SA3139-LPS-O, 34.63 ± 6.85%; and P. gingivalis, 62.74 ± 5.42%. The CDT expression in A. actinomycetemcomitans EVs did not influence tumor cell metastasis, and neither D7SS-WT nor D7SS-cdt were significantly different from the control (Fig. 2a). EVs from the wild-type A. actinomycetemcomitans strain SA3138-WT did not affect metastasis, but EVs from SA3139-LPS-O strain lacking LPS O-antigen significantly reduced metastasis compared to control (P < 0.05; Fig. 2b). We did not observe difference in metastasis between OSCC cells treated with P. gingivalis-derived EVs and control cells (Fig. 2c).
Fig. 2.
Metastasis of HSC-3 cells pretreated with bacterial extracellular vesicles (EVs; 10 µg/ml) from: A. actinomycetemcomitans D7SS-WT (wild-type), D7SS-cdt (lacking the cytolethal distending toxin, CDT), SA3138-WT (wild-type), SA3139-LPS-O (lacking the lipopolysaccharide (LPS) O-antigen), and P. gingivalis (wild-type). a Metastasis rate was not significantly affected by pretreatment with EVs from D7SS-WT and D7SS-cdt strains. b HSC-3 cells pretreated with SA3139-LPS-O-derived EVs had significantly lower metastatic rate than control cells. c No statistically significant changes were seen in the metastasis rates of HSC-3 cells pretreated with P. gingivalis EVs compared to controls. * p < 0.05. Values are shown as mean values from each experiment and line at mean. All experiments were repeated independently three times
Site of tumor dissemination
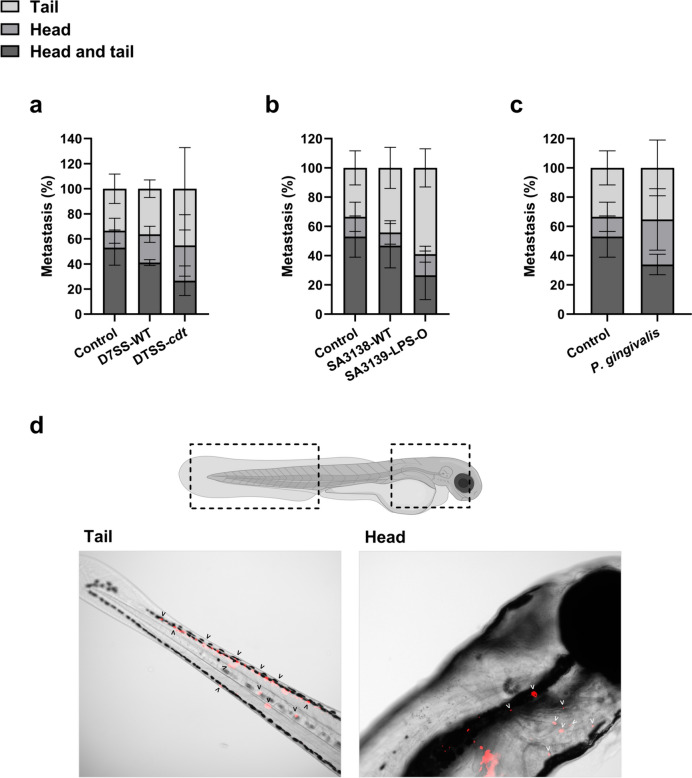
In addition to analyzing metastasis, we further screened whether the metastatic foci were detected in the zebrafish head, tail, or in both head and tail. The differences compared to control were not statistically significant (Fig. 3a–c). Though, an interesting pattern was seen that, OSCC cells pretreated with EVs from the mutant A. actinomycetemcomitans strains D7SS-cdt and SA3139-LPS-O tended to metastasize less often to the head and tail compared to the control. The trend was consistent in all three experiments. Percentual averages ± SD from the three experiments showed that among all metastasis cases, control tumors metastasized more often to head and tail (53.04 ± 14.07%), clearly more than cells pretreated with D7SS-cdt EVs (26.62 ± 11.80%) and SA3139-LPS-O EVs (19.91 ± 23.24%) (Fig. 3a, b). Cells pretreated with P. gingivalis EVs metastasized a little more often to head and tail (33.93 ± 6.97%) but less than the controls, showing a consistent, though non-significant, trend in all three experiments (Fig. 3c).
Fig. 3.
Preferential dissemination sites of the metastatic tumor cells in zebrafish larvae. HSC-3 cells were treated with bacterial extracellular vesicles (EVs; 10 µg/ml) from: A. actinomycetemcomitans D7SS-WT (wild-type), D7SS-cdt (lacking the cytolethal distending toxin, CDT), SA3138-WT (wild-type), SA3139-LPS-O (lacking the lipopolysaccharide (LPS) O-antigen), and P. gingivalis (wild-type). A trend of lower metastasis rate to both head and tail was noted in cells pretreated with EVs from a A. actinomycetemcomitans D7SS-cdt, b A. actinomycetemcomitans SA3139-LPS-O and c P. gingivalis, although the differences were not statistically significant. d Demonstrative images of metastasis in head and tail of zebrafish larvae. Red areas represent tumor cells. Values are shown as mean ± SD. All experiments were repeated independently three times
Discussion
The present study is the first to investigate the interactions between bacterial EVs and OSCC in vivo using zebrafish larvae. Interestingly, we reported that pretreatment with EVs from A. actinomycetemcomitans D7SS-cdt strain resulted in an increased tumor area, while those from the SA3139-LPS-O strain showed lower metastasis rates. No significant changes were observed in cells pre-challenged with P. gingivalis EVs.
Previously, several studies utilized zebrafish larvae in bacterial research [45–48]. For instance, zebrafish larvae were used to study the effect of P. gingivalis on vascular permeability and systemic dissemination [45–47]. However, to our knowledge, this model has not been employed for studying the influence of bacterial EVs on cancer cell growth and metastasis to date. Currently, the interactions between bacterial EVs and cancer are studied in vivo using patient-derived murine xenografts [28, 44, 49, 50]. In these studies, EVs were administered for immunization prior cancer cell implantation in a model of murine melanoma [50]; intravenously after implantation of murine mammary, adenocarcinoma and melanoma cells [44]; subcutaneously after implantation of murine lung carcinoma cells [49]; or intratumorally in OSCC tumors formed by human tongue cancer cells [28]. In zebrafish larvae, the studied compounds can be administered via immersion (i.e. from embryonic medium), microinjection or through pretreatment of cancer cells prior implantation [51]. We opt herein for the latter approach and pretreated the OSCC cells with the EVs, based on previous in vitro and in vivo studies [27, 28, 52]. Alternatively, in a previous study, Escherichia coli cells were added to the embryonic medium to study hepatic and breast cancer in zebrafish model [48]. Adding EVs to the embryonic medium could also be considered, however, they might not be easily immersed due to the hydrophobic nature of EVs [51].
A. actinomycetemcomitans is the only known oral bacterium that produces CDT [53], which is delivered into host cells via EVs [42]. CDT from A. actinomycetemcomitans showed antitumorigenic potential in leukemia, oral, prostate and lung cancers [16, 17, 19, 54–56] but CDT has also been suggested to promote carcinogenesis via DNA damage [18, 57]. We showed that HSC-3 cells pretreated with EVs from the CDT-lacking strain formed larger tumors in vivo. Interestingly, our recent in vitro findings revealed that HSC-3 cell proliferation was not affected by EVs from the D7SS-cdt strain; while only the wild strain EVs significantly reduced the proliferation of the metastatic tumor cells [26]. However, despite their larger sizes, they did not exhibit a higher metastasis rate. In this regard, OSCC tumor size does not always correlate with metastasis [58] but rather with the depth of invasion and tumor budding [58, 59].
The periodontopathogen P. gingivalis has been shown to mainly promote pro-tumorigenic effects in OSCC [25, 26, 30, 60]. Importantly, P. gingivalis promoted key features for metastasis including oral epithelial cell “stemness” and epithelial-mesenchymal transition (EMT) [61–64]. Furthermore, P. gingivalis and its EVs promoted OSCC cell migration and invasion [27, 65, 66]. In agreement, P. gingivalis promoted OSCC tumor growth [31, 32] and metastasis [29] in mice. Our findings did not reveal any significant effects of P. gingivalis EVs on tumor growth or metastasis. The reason is not clear; however, this encourages further studies using different EV doses and treatment durations.
Metastasis is the main cause of morbidity and cancer-related deaths in OSCC patients [67, 68]. Unlike its wild-type equivalent, EVs from the A. actinomycetemcomitans strain lacking O-antigen in LPS showed reduced dissemination of the highly metastatic HSC-3 cells. The A. actinomycetemcomitans O-antigen is an immunodominant, serotype-specific polysaccharide of LPS [69]. One of the key benefits of using zebrafish larvae in cancer research is their compatibility with xenograft transplantation. During the first 30 dpf, the fish larvae have only innate immune cells and lack an adaptive immune response, thus eliminating concerns of immune rejection in larval xenografts [70, 71]. In this context, considering the immunodeficient nature of this model, it is improbable that the findings are due to LPS-mediated immune modulation as commonly seen in other cancer studies [72]. Of note, only LPS from periodontopathogens, but not LPS from commensal bacteria, influenced oral cancer cells directly in vitro [32]. Thus, it is logical to assume that the structural variation of EVs, mediated by different LPS structure, could affect cell metastasis. LPS consists of hydrophobic lipid A, hydrophilic core polysaccharide and hydrophilic O-antigen [73]. Lack of O-antigen reduces the hydrophilicity of EVs and possibly affects EV properties in vivo.
In conclusion, we explored the feasibility of zebrafish larvae as a simple yet efficient model for studying bacterial EVs and OSCC in vivo. Our findings revealed both pro- and anti-tumorigenic effects of EVs from A. actinomycetemcomitans strains, depending in part on their expression of CDT and LPS O-antigen. Given the promising utility of bacterial EVs as potential therapeutic targets in cancer, we encourage further research on these nanosized molecules using the zebrafish larvae, which overcome many limitations associated with the traditional murine models.
Acknowledgements
Authors would like to thank Henri Koivula from the Zebrafish unit at the University of Helsinki for the technical assistance.
Author contributions
Marjut Metsäniitty performed the experiments and collected data. Saika Hasnat and Carina Öhman assisted with the experiments and data collection. Marjut Metsäniitty, Jan Oscarsson, Kari Eklund and Abdelhakim Salem performed data analysis and interpretation. Marjut Metsäniitty and Abdelhakim Salem wrote the manuscript. Abdelhakim Salem is the main supervisor and corresponding author of this work. All authors read, commented and approved the final manuscript.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). This research has been funded by the Research Council of Finland (Academy of Finland; 362035); the Doctoral Program in Oral Sciences (FINDOS); Suomen Naishammaslääkärit ry; Minerva Foundation, Selma and Maja-Lisa Selander`s Fund; Ida Montinin Säätiö; University of Helsinki, the Medicine Fund; Päivikki and Sakari Sohlberg Foundation; Odontologiska Samfundet i Finland r.f; The Finnish Dental Society, Apollonia; TUA grants (7003193 and 7003766) from the County Council of Västerbotten, Sweden; Insamlingsstiftelsen, Medical Faculty, Umeå University.
Data availability
Data is provided within the manuscript and also available from the lead authors upon a reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Conflict of interest
No potential conflict of interest was reported by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24(3):491–508. 10.1016/j.soc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Mauceri R, Bazzano M, Coppini M, Tozzo P, Panzarella V, Campisi G. Diagnostic delay of oral squamous cell carcinoma and the fear of diagnosis: a scoping review. Front Psychol. 2022;13:1009080. 10.3389/fpsyg.2022.1009080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Economopoulou P, de Bree R, Kotsantis I, Psyrri A. Diagnostic tumor markers in head and neck squamous cell carcinoma (HNSCC) in the clinical setting. Front Oncol. 2019;9:827. 10.3389/fonc.2019.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway DI, Purkayastha M, Chestnutt IG. The changing epidemiology of oral cancer: definitions, trends, and risk factors. Br Dent J. 2018;225(9):867–73. 10.1038/sj.bdj.2018.922. [DOI] [PubMed] [Google Scholar]
- 6.Metsäniitty M, Hasnat S, Salo T, Salem A. Oral microbiota—a new frontier in the pathogenesis and management of head and neck cancers. Cancers. 2021;14(1):46. 10.3390/cancers14010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colonia-García A, Gutiérrez-Vélez M, Duque-Duque A, de Andrade CR. Possible association of periodontal disease with oral cancer and oral potentially malignant disorders: a systematic review. Acta Odontol Scand. 2020;78(7):553–9. 10.1080/00016357.2020.1774076. [DOI] [PubMed] [Google Scholar]
- 8.Javed F, Warnakulasuriya S. Is there a relationship between periodontal disease and oral cancer? A systematic review of currently available evidence. Crit Rev Oncol Hematol. 2016;97:197–205. 10.1016/j.critrevonc.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Eun YG, Lee JW, Kim SW, Hyun DW, Bae JW, Lee YC. Oral microbiome associated with lymph node metastasis in oral squamous cell carcinoma. Sci Rep. 2021;11(1):23176. 10.1038/s41598-021-02638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mager DL. Bacteria and cancer: cause, coincidence or cure? A review. J Transl Med. 2006;4:14. 10.1186/1479-5876-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015;40:97–104. 10.1016/j.semcdb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 12.Fazal S, Lee R. Biomimetic bacterial membrane vesicles for drug delivery applications. Pharmaceutics. 2021;13(9):1430. 10.3390/pharmaceutics13091430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Zhou H, Yang C, Wu Y, Zhou X, Liu H, Wang Y. Bacterial outer membrane vesicles as a platform for biomedical applications: an update. J Control Release. 2020;323:253–68. [DOI] [PubMed] [Google Scholar]
- 14.Chronopoulos A, Kalluri R. Emerging role of bacterial extracellular vesicles in cancer. Oncogene. 2020;39(46):6951–60. 10.1038/s41388-020-01509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amatya SB, Salmi S, Kainulainen V, Karihtala P, Reunanen J. Bacterial extracellular vesicles in gastrointestinal tract cancer: an unexplored territory. Cancers. 2021;13(21):5450. 10.3390/cancers13215450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damek-Poprawa M, et al. Targeted inhibition of CD133+ cells in oral cancer cell lines. J Dent Res. 2011;90(5):638–45. 10.1177/0022034510393511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwanaga K, et al. Local delivery system of cytotoxic agents to tumors by focused sonoporation. Cancer Gene Ther. 2007;14(4):354–63. 10.1038/sj.cgt.7701026. [DOI] [PubMed] [Google Scholar]
- 18.Teshima R, Hanada K, Akada J, Kawano K, Yamaoka Y. Aggregatibacter actinomycetemcomitans infection causes DNA double-strand breaks in host cells. Genes Cells. 2018;23(4):264–73. 10.1111/gtc.12570. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K, Tominaga K, Sukedai M, Okinaga T, Iwanaga K, Nishihara T, Fukuda J. Delivery of cytolethal distending toxin B induces cell cycle arrest and apoptosis in gingival squamous cell carcinoma in vitro. Eur J Oral Sci. 2004;112(5):445–51. 10.1111/j.1600-0722.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- 20.Jain S, et al. Lipopolysaccharide (LPS) enhances prostate cancer metastasis potentially through NF-κB activation and recurrent dexamethasone administration fails to suppress it in vivo. Prostate. 2019;79(2):168–82. 10.1002/pros.23722. [DOI] [PubMed] [Google Scholar]
- 21.Song W, et al. Trapping of Lipopolysaccharide to Promote Immunotherapy against colorectal cancer and attenuate liver metastasis. Adv Mater. 2018;30(52):e1805007. 10.1002/adma.201805007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shetab Boushehri MA, Lamprecht A. TLR4-based Immunotherapeutics in cancer: a review of the achievements and shortcomings. Mol Pharm. 2018;15(11):4777–800. 10.1021/acs.molpharmaceut.8b00691. [DOI] [PubMed] [Google Scholar]
- 23.Monasterio G, et al. O-Polysaccharide plays a major role on the virulence and immunostimulatory potential of Aggregatibacter actinomycetemcomitans during periodontal infection. Front Immunol. 2020;11:591240. 10.3389/fimmu.2020.591240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindholm M, Metsäniitty M, Granström E, Oscarsson J. Outer membrane vesicle-mediated serum protection in Aggregatibacter actinomycetemcomitans. J Oral Microbiol. 2020;12(1):1747857. 10.1080/20002297.2020.1747857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh S, Singh AK. Porphyromonas gingivalis in oral squamous cell carcinoma: a review. Microbes Infect. 2022;24(3):104925. 10.1016/j.micinf.2021.104925. [DOI] [PubMed] [Google Scholar]
- 26.Metsäniitty M, Hasnat S, Öhman C, Salo T, Eklund KK, Oscarsson J, Salem A. Extracellular vesicles from Aggregatibacter actinomycetemcomitans exhibit potential antitumorigenic effects in oral cancer: a comparative in vitro study. Arch Microbiol. 2024;206(6):244. 10.1007/s00203-024-03976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu D, Liu S, Liu J, Miao L, Zhang S, Pan Y. sRNA23392 packaged by Porphyromonas gingivalis outer membrane vesicles promotes oral squamous cell carcinomas migration and invasion by targeting desmocollin-2. Mol Oral Microbiol. 2021;36(3):182–91. 10.1111/omi.12334. [DOI] [PubMed] [Google Scholar]
- 28.Chen G, et al. Fusobacterium nucleatum outer membrane vesicles activate autophagy to promote oral cancer metastasis. J Adv Res. 2023. 10.1016/j.jare.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo BH, et al. Oral cancer cells sustainedly infected with Porphyromonas gingivalis exhibit resistance to Taxol and have higher metastatic potential. Oncotarget. 2017;8(29):46981–92. 10.18632/oncotarget.16550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan Z, Zou KL, Cui H, Zhao YY, Yu GT. Porphyromonas gingivalis suppresses oral squamous cell carcinoma progression by inhibiting MUC1 expression and remodeling the tumor microenvironment. Mol Oncol. 2023. 10.1002/1878-0261.13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binder Gallimidi A, et al. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget. 2015;6(26):22613–23. https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med12&AN=26158901. [DOI] [PMC free article] [PubMed]
- 32.Kamarajan P, et al. Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of Integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PLoS Pathog. 2020;16(10):e1008881. 10.1371/journal.ppat.1008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murali Shankar N, et al. Preclinical assessment of CAR-NK cell-mediated killing efficacy and pharmacokinetics in a rapid zebrafish xenograft model of metastatic breast cancer. Front Immunol. 2023;14:1254821. 10.3389/fimmu.2023.1254821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Astell KR, Sieger D. Zebrafish in vivo models of cancer and metastasis. Cold Spring Harb Perspect Med. 2020;10(8):37077. 10.1101/cshperspect.a037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hujanen R, Almahmoudi R, Salo T, Salem A. Comparative analysis of vascular mimicry in head and neck squamous cell carcinoma: in vitro and in vivo approaches. Cancers. 2021;13(19):4747. 10.3390/cancers13194747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karinen S, et al. Tumour cells express functional lymphatic endothelium-specific hyaluronan receptor in vitro and in vivo: lymphatic mimicry promotes oral oncogenesis? Oncogenesis. 2021;10(3):23–33. 10.1038/s41389-021-00312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Goodman SD, Redfield RJ, Chen C. Natural transformation and DNA uptake signal sequences in Actinobacillusactinomycetemcomitans. J Bacteriol. 2002;184(13):3442–9. 10.1128/jb.184.13.3442-3449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nalbant A, Chen C, Wang Y, Zadeh HH. Induction of T-cell apoptosis by Actinobacillusactinomycetemcomitans mutants with deletion of ltxA and cdtABC genes: possible activity of GroEL-like molecule. Oral Microbiol Immunol. 2003;18(6):339–49. 10.1046/j.0902-0055.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- 39.Asikainen S, Chen C, Slots J. Actinobacillusactinomycetemcomitans genotypes in relation to serotypes and periodontal status. Oral Microbiol Immunol. 1995;10(2):65–8. 10.1111/j.1399-302x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 40.Kanasi E, Doğan B, Karched M, Thay B, Oscarsson J, Asikainen S. Lack of serotype antigen in A.actinomycetemcomitans. J Dent Res. 2010;89(3):292–6. 10.1177/0022034509358865. [DOI] [PubMed] [Google Scholar]
- 41.American Type Culture Collection. Porphyromonas gingivalis (Coykendall et al.) Shah and Collins 33277™. https://www.atcc.org/products/33277?nt=wobj-20-q. Accessed 2023.
- 42.Rompikuntal PK, et al. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect Immun. 2012;80(1):31–42. 10.1128/IAI.06069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhuang WR, et al. Bacterial outer membrane vesicle based versatile nanosystem boosts the efferocytosis blockade triggered tumor-specific immunity. Nat Commun. 2023;14(1):1675. 10.1038/s41467-023-37369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim OY, et al. Bacterial outer membrane vesicles suppress tumor by interferon-γ-mediated antitumor response. Nat Commun. 2017;8(1):626. 10.1038/s41467-017-00729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farrugia C, Stafford GP, Murdoch C. Porphyromonas gingivalis outer membrane vesicles increase vascular permeability. J Dent Res. 2020;99(13):1494–501. 10.1177/0022034520943187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Widziolek M, Prajsnar TK, Tazzyman S, Stafford GP, Potempa J, Murdoch C. Zebrafish as a new model to study effects of periodontal pathogens on cardiovascular diseases. Sci Rep. 2016;6:36023. 10.1038/srep36023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farrugia C, Stafford GP, Potempa J, Wilkinson RN, Chen Y, Murdoch C, Widziolek M. Mechanisms of vascular damage by systemic dissemination of the oral pathogen Porphyromonas gingivalis. FEBS J. 2021;288(5):1479–95. 10.1111/febs.15486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi L, et al. Combination therapy of TGF-β blockade and commensal-derived probiotics provides enhanced antitumor immune response and tumor suppression. Theranostics. 2019;9(14):4115–29. 10.7150/thno.35131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S, et al. Engineered outer membrane vesicle is potent to elicit HPV16E7-specific cellular immunity in a mouse model of TC-1 graft tumor. Int J Nanomedicine. 2017;12:6813–25. 10.2147/ijn.S143264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grandi A, et al. Synergistic protective activity of tumor-specific epitopes engineered in bacterial outer membrane vesicles. Front Oncol. 2017;7:253. 10.3389/fonc.2017.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Hua X, Xu K, Song Y, Lv T. Zebrafish in lung cancer research. Cancers. 2023;15(19):4721. 10.3390/cancers15194721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, et al. Genetic depletion and pharmacological targeting of αv integrin in breast cancer cells impairs metastasis in zebrafish and mouse xenograft models. Breast Cancer Res. 2015;17(1):28. 10.1186/s13058-015-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Belibasakis GN, et al. Virulence and pathogenicity properties of Aggregatibacter actinomycetemcomitans. Pathogens. 2019;8(4):222. 10.3390/pathogens8040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ohara M, Hayashi T, Kusunoki Y, Nakachi K, Fujiwara T, Komatsuzawa H, Sugai M. Cytolethal distending toxin induces caspase-dependent and -independent cell death in MOLT-4 cells. Infect Immun. 2008;76(10):4783–91. 10.1128/iai.01612-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaghoobi H, Bandehpour M, Kazemi B. Apoptotic effects of the B subunit of bacterial cytolethal distending toxin on the A549 lung cancer cell line. Asian Pac J Cancer Prev. 2016;17(S3):299–304. 10.7314/apjcp.2016.17.s3.299. [DOI] [PubMed] [Google Scholar]
- 56.Yaghoobi H, Kazemi B, Bandehpour M. Sensitization of radio-resistant lung cancer cells with a B subunit of bacterial cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Iran J Cancer Prev. 2017;10(2):e5792. 10.5812/ijcp.5792. [Google Scholar]
- 57.Tremblay W, et al. Cytolethal distending toxin promotes replicative stress leading to genetic instability transmitted to daughter cells. Front Cell Dev Biol. 2021;9:656795. 10.3389/fcell.2021.656795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haksever M, Inançlı HM, Tunçel U, Kürkçüoğlu SS, Uyar M, Genç O, Irkkan C. The effects of tumor size, degree of differentiation, and depth of invasion on the risk of neck node metastasis in squamous cell carcinoma of the oral cavity. Ear Nose Throat J. 2012;91(3):130–5. 10.1177/014556131209100311. [DOI] [PubMed] [Google Scholar]
- 59.Almangush A, Pirinen M, Heikkinen I, Mäkitie AA, Salo T, Leivo I. Tumour budding in oral squamous cell carcinoma: a meta-analysis. Br J Cancer. 2018;118(4):577–86. 10.1038/bjc.2017.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cho TJ, et al. Porphyromonas gingivalis-induced autophagy suppresses cell proliferation through G1 arrest in oral cancer cells. Arch Oral Biol. 2014;59(4):370–8. https://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=med11&AN=24606908. [DOI] [PubMed]
- 61.Sztukowska MN, et al. Porphyromonas gingivalis initiates a mesenchymal-like transition through ZEB1 in gingival epithelial cells. Cell Microbiol. 2016;18(6):844–58. 10.1111/cmi.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee J, Roberts JS, Atanasova KR, Chowdhury N, Han K, Yilmaz Ö. Human primary epithelial cells acquire an epithelial-mesenchymal-transition phenotype during long-term infection by the oral opportunistic pathogen, Porphyromonas gingivalis. Front Cell Infect Microbiol. 2017;7:493. 10.3389/fcimb.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ha NH, et al. Prolonged and repetitive exposure to Porphyromonas gingivalis increases aggressiveness of oral cancer cells by promoting acquisition of cancer stem cell properties. Tumour Biol. 2015;36(12):9947–60. 10.1007/s13277-015-3764-9. [DOI] [PubMed] [Google Scholar]
- 64.Salem A, Salo T. Identity matters: cancer stem cells and tumour plasticity in head and neck squamous cell carcinoma. Expert Rev Mol Med. 2023;25:e8. 10.1017/erm.2023.4. [DOI] [PubMed] [Google Scholar]
- 65.Cho BH, et al. Acetylshikonin suppresses invasion of Porphyromonas gingivalis-infected YD10B oral cancer cells by modulating the interleukin-8/matrix metalloproteinase axis. Mol Med Rep. 2018;17(2):2327–34. 10.3892/mmr.2017.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdulkareem AA, Shelton RM, Landini G, Cooper PR, Milward MR. Periodontal pathogens promote epithelial-mesenchymal transition in oral squamous carcinoma cells in vitro. Cell Adh Migr. 2018;12(2):127–37. 10.1080/19336918.2017.1322253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin X, et al. Analysis of clinicopathological characteristics associated with the outcome of oral squamous cell carcinoma and the establishment of tissue microarrays. Oncol Lett. 2016;12(5):3175–82. 10.3892/ol.2016.5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol. 2003;39(2):130–7. 10.1016/s1368-8375(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 69.Oscarsson J, Claesson R, Lindholm M, Höglund Åberg C, Johansson A. Tools of Aggregatibacter actinomycetemcomitans to evade the host response. J Clin Med. 2019;8(7):1079. 10.3390/jcm8071079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miao KZ, Kim GY, Meara GK, Qin X, Feng H. Tipping the scales with zebrafish to understand adaptive tumor immunity. Front Cell Dev Biol. 2021;9:660969. 10.3389/fcell.2021.660969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fontana CM, Van Doan H. Zebrafish xenograft as a tool for the study of colorectal cancer: a review. Cell Death Dis. 2024;15(1):23. 10.1038/s41419-023-06291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lundin JI, Checkoway H. Endotoxin and cancer. Environ Health Perspect. 2009;117(9):1344–50. 10.1289/ehp.0800439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zanoni I, et al. Similarities and differences of innate immune responses elicited by smooth and rough LPS. Immunol Lett. 2012;142(1–2):41–7. 10.1016/j.imlet.2011.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript and also available from the lead authors upon a reasonable request.