Abstract
This systematic review and network meta-analysis aimed to assess the impact of combining professional mechanical plaque removal (PMPR) with probiotics compared to PMPR + placebo on probing pocket depth (PPD) and clinical attachment level (CAL). Randomized controlled trials published until November 2023 were searched across electronic databases, peer-reviewed journals, and grey literature. Two authors independently selected, extracted data, and assessed bias risk. Primary outcomes were mean changes in PPD and CAL. Secondary outcomes included mean changes in bleeding on probing (BOP), plaque index, and colony-forming units. Network meta-analysis with the frequentist weighted least squares approach evaluated the data quantitatively, and CINeMA framework evaluated the quality of evidence. In 33 articles involving 1290 patients, results were stratified by follow-up period (short and long-time studies) and sensitivity analyses conducted based on probiotic therapy duration (1 month reference). Network meta-analysis revealed significant mean differences in PPD for nine probiotic interventions, CAL for eighteen interventions, and BOP for eight interventions, with Lactobacillus demonstrating the most substantial effects. Combining PMPR with probiotics as adjuvants to subgingival instrumentation may be more effective in improving PPD and CAL. Lactobacillus emerged as the most comprehensive and effective among the studied probiotic.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-024-05027-6.
Keywords: Chronic periodontitis, Probiotics, Synbiotics, Network meta-analysis, Systematic review
Introduction
Periodontitis, a chronic inflammatory oral condition, is a major global health concern, ranking as the second leading cause of tooth loss worldwide after dental caries [1]. Approximately 50% of the global population experiences periodontitis, making it the seventh most prevalent disease globally [2, 3].
While periodontitis is multifactorial, the presence of dysbiotic biofilm is crucial for its progression [4]. The primary treatment goal is to reduce harmful microorganisms and restore a healthy flora around teeth, and also to create a biologically compatible root surface for reattachment [5]. Professional mechanical plaque removal (PMPR) are widely accepted methods for achieving this, but their effectiveness can vary due to factors like deep probing depths and difficult-to-reach areas [6]. Probiotics and other adjuvants to subgingival instrumentation [7] have been proposed to address these limitations. While the use of adjunctive antibiotics and other antimicrobials is established, the indications are specific. The primary concern associated with the use of antimicrobials is bacterial resistance [8]. Therefore, there is growing interest in understanding the mechanism of action of probiotics in modifying the microflora of periodontal patients. The mechanisms underlying the potential efficacy of probiotics in periodontal disease are related to biological mechanisms [9]. Probiotics compete with periodontal pathogens, modulating dysbiotic conditions [10]. They can reduce the immunogenicity of the microflora and modulate immunological and inflammatory pathways, resulting in the reduction of the destructive inflammation characteristic of periodontitis [11]. The ultimate outcome is immunological homeostasis, which could persist in the individual for an extended period. Indeed, probiotics can reduce periodontal disease pathogens by producing hydrogen peroxide [12, 13]. Additionally, while plaque is a necessary but not sufficient condition for the development of periodontal disease, probiotics also demonstrate the ability to prevent plaque formation by reducing saliva pH through the production of antioxidants, thereby inhibiting the growth of bacteria [13].
Early systematic reviews (SRs) highlighted the short-term benefits of probiotics as an adjunct to subgingival instrumentation, but no specific regimen was deemed superior [5, 14]. Given the complexity of clinical decisions and the necessity for evidence-based practices, a clear understanding of the relative risks and benefits of probiotic therapy is crucial. To provide a comprehensive understanding of probiotic therapy, a network meta-analysis (NMA) model was implemented. This model, unlike classical meta-analysis, accommodates all available probiotic regimens, allowing for indirect comparisons between interventions not directly assessed in individual trials. This approach enhances accuracy, offers a coherent overview, and enables the ranking of interventions based on their relative risks and benefits.
Therefore, this systematic review and network meta-analysis aimed to answer the following focused question: In adult patients with periodontitis and good general health, what is the effect of the combination of PMPR and different existing probiotics in comparison with PMPR alone on probing pocket depth (PPD) reduction and clinical attachment level (CAL) gain?
Methods
Protocol registration and reporting format
This SR and NMA adhered to PRISMA guidelines, including the updated version for network meta-analysis (Appendix 1). It is registered in PROSPERO under trial No. CRD42021250678.
Eligibility criteria
Table 1 shows the main inclusion criteria for the PICO question, including primary and secondary outcomes. Studies lacking essential data required for a meta-analysis; nonrandomized clinical studies, cohort studies, and case series; studies involving patients with systemic diseases (HIV/AIDS or diabetes) or intellectual disabilities; studies focused on forms of periodontal disease other than chronic periodontitis, patients in periodontal supportive therapy, or healthy volunteers; studies examining therapies other than probiotics; studies targeting children, adolescents, or the elderly population; studies failing to meet the transitivity assumption were excluded.
Table 1.
Components of PICOS question
| PICOS question | ||
|---|---|---|
| P | Patients | Adult (≥ 18 years), systemically healthy individuals, untreated patients diagnosed with periodontitis* |
| I | Intervention or exposure | SRP plus single probiotic; SRP plus a combination of probiotics; Lactobacillus rhamanosus; Bifidobacterium Lactis; Lactobacillus reuteri; Lactobacillus brevis; Lactobacillus plantarum; Lactobacillus salivarius; Streptococcus oralis; Streptococcus uberis; Streptococcus rattus; Enterococcus faecalis (S. faecalis); Clostridium butyricum; Bacillus mesentericus; Lactobacillus. sporogenes; Lactobacillus paracasei. |
| C | Comparison | Placebo; absence of probiotic treatment; SRP alone; |
| O | Outcomes |
Primary outcomes: clinical parameters (PPD; CAL) (mean changes in these clinical parameters between baseline and follow-up visits). Secondary outcomes: clinical parameters (BOP; PI and CFU) (mean changes in these parameters between baseline and follow-up visits). |
| S | Study design and duration | RCTs, excluding split-mouth studies, that reported the outcomes of interest; N (patients) ≥ 10; with a minimum follow up time of one month. |
BOP: bleeding on probing; CAL: clinical attachment level; CFU: colony forming units; PI: plaque index; PPD: probing pocket depth; RCTs: randomized controlled trials; SRP: scaling and root planing
* According to the “new classification scheme for periodontal and peri-implant diseases and conditions”. For inclusion, studies must reference diagnostic criteria, with at least one site exhibiting a mean PPD ≥ 5 mm, CAL ≥ 1 mm, and the presence of BOP. Stage II periodontitis; localized or generalized; Grade B. (Caton, J. G. et al. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Clin Periodontol 45 Suppl 20, S1-S8, doi:10.1111/jcpe.12935 (2018).)
Information source and searches
Three electronic databases and three grey literature platforms were searched up to November 2023: MEDLINE (via PubMed), LILACS, and Cochrane Central Registry of Controlled Trials (CENTRAL); and Google Scholar (with the first 300 references retrieved), ClinicalTrials.gov, and a database listing of unpublished studies (DANS EASY Archive, available at 10.17026/dans-xtf-47w5), respectively. Detailed search strategies (Appendix 2) were adopted, supplemented by screening of reference lists (using Research Rabbit, https://www.researchrabbit.ai/) and outreach to corresponding authors via email to inquire about additional research in the field or awareness of any ongoing projects.
Study selection and data extraction
Two reviewers rigorously and independently followed predetermined criteria for screening titles and abstracts for eligibility. Exclusion decisions were meticulously recorded (Table 2, Appendix 3). Full-text reports were obtained for included studies and those lacking sufficient information. Data extraction covered study features, participant details, and outcome measures. Contacting corresponding authors addressed any needed clarifications. Discrepancies were resolved through discussion, with a third reviewer consulted if necessary.
Data items
Table 3 shows the main variables sought in the included studies. Table 4 presents data by group and outcomes (Appendix 4).
Risk of bias within individual studies
The methodological quality of the included studies was assessed using Cochrane Collaboration’s Tool Assessment Risk of Bias 5.1.0 [15]. Two independent reviewers assigned ‘low risk,’ ‘unclear risk,’ or ‘high risk’ of bias to each question. Discrepancies were resolved through discussion or consultation with a third reviewer if needed. Cohen’s kappa coefficient evaluated interrater agreement, with interpretations ranging from poor to almost perfect. Final scores were determined based on the percentage of ‘low risk of bias’ responses. Study bias was categorized as high (≤ 49%), moderate (50–69%), or low (≥ 70%).
Data synthesis
Summary treatment effect measures
Clinical parameters for continuous primary and secondary outcomes were derived from included studies. Mean differences (MD) and standard errors were presented for all studies. Effect sizes within and between groups at baseline and last follow-up were calculated using MedCalc® Software Ltd (available at https://www.medcalc.org/calc/comparison_of_means.php) (Appendix 5, Tables 5, 6, 7, 8 and 9).
Planned methods of analysis
Network meta-analyses, incorporating direct and indirect comparisons, were conducted using a frequentist weighted least-squares approach with the “netmeta package” in Rstudio (R. Rstudio, PBC, Boston, MA). Random-effects models were applied, categorizing results by follow-up periods: ≤3 months (short-term) and > 3 months (long-term). A single common approach was used to assess heterogeneity within studies.
Assessment of inconsistency
Both local (SIDE method) and global (incoherence models) approaches were employed to assess inconsistency. The netsplit function separated indirect from direct evidence, while incoherence models assessed inconsistency across the entire network. No inconsistency was considered for p > 0.05.
Confidence in the results of the network meta-analysis
The CINeMA framework (Confidence in Network Meta-Analysis (https://cinema.ispm.unibe.ch/) assessed confidence in results and certainty of evidence, covering within-study bias, reporting bias, indirectness, imprecision, heterogeneity, and incoherence. Confidence was graded as high, moderate, low, or very low (Appendix 6, Tables 10, 11 and 12).
Additional analyses
A sensitivity analysis was conducted, considering the duration of probiotic therapy, categorized as either ≤ 1 month or > 1 month.
Assessment of transitivity across comparisons
To evaluate transitivity, systematic information on patient and study characteristics was provided. This allowed the empirical assessment of potential effect modifiers’ distribution across trials, including periodontal disease severity, diagnostic criteria, smoking habits, and follow-up period.
Network geometry
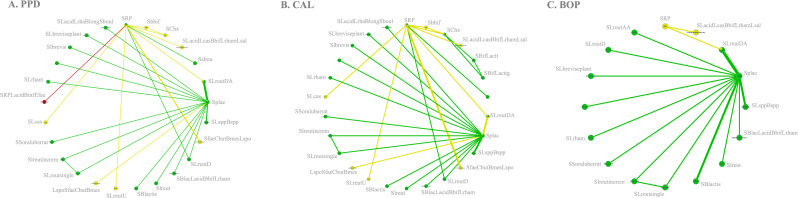
Illustrated as spider web-like plots, network geometry portrays connections between studies employing diverse periodontal therapies. Plots, categorized by outcomes, interpret geometry based on parameters like patient count, study numbers, nodes, edges, strong edges percentage, common comparators percentage, density, and median thickness.
Results
Study selection
A flow diagram in Appendix 7 outlines the article screening process. From 2,599 articles, 104 underwent full-text review, with 33 meeting the inclusion criteria for qualitative assessment. Quantitative analysis included 28 studies for PPD, 26 for CAL, and 18 for BOP; other outcomes (PI and CFU) lacked sufficient data for network meta-analysis.
Study characteristics
Appendix 8’s table 13 summarizes details from the 33 RCTs, featuring patients diagnosed with moderate to severe chronic periodontitis [16]. All participants were untreated patients, and smoking habits varied, with one RCT exclusive to smokers and 22 involving non-smokers. Follow-up periods ranged from 1 month to 1 year, and probiotic therapy durations spanned from a single application to 6 months. The probiotics included Bifidobacterium, Bacillus, Lactobacillus, Streptococci, Saccharomyces, alone or in combination, administered through various routes like gel, lozenges, paste, gum, powder, tablets, capsules, drops, mouthwash, sachets, and yogurt.
Summary of network geometry
In analyzing periodontal outcomes, PPD reduction data were extracted from 28 studies (85%), encompassing 1,056 participants [17–44], revealing a network diagram with 25 nodes and 27 edges, 11.11% strong edges, 32% common comparators, and a median network connection with a density of 0.09 and a mean thickness of 1.04 (Fig. 1A). For CAL, 26 RCTs [17–26,28−33,35–40,42−45] (79%) with 906 patients showcased a network diagram (Fig. 1B) containing 26 nodes and 29 edges, 10.34% strong edges, 38.46% common comparators, a median network connection with a density of 0.09, and a mean thickness of 0.90. Additionally, BOP data from 18 RCTs (55%) [18, 20, 21, 24, 26–28, 31, 32, 35, 36, 39, 41–46] with 682 patients exhibited a network diagram (Fig. 1C) featuring 16 nodes and 16 edges, 18.75% strong edges, 31.25% common comparators, a median network connection with a density of 0.13, and a mean thickness of 1.13.
Fig. 1.
Network plot for primary outcomes [PPD (1 A), CAL (1B)] and secondary outcome [BOP (1 C)]. The nodes have the same size. Treatments with direct comparisons are linked with a line. These star networks emphasize two major comparators—SRP and SRP + placebo (Splac)—relying heavily on indirect evidence. Node color indicates risk of bias (RoB) of the intervention, while edge color signifies average RoB. Edge width corresponds to the number of studies comparing treatments. The PPD network includes 300 potential comparisons with 9% direct evidence, and the CAL network has 325 possible comparisons with 8.92% direct evidence. The BOP network comprises 120 potential comparisons, with 13% direct evidence
Risk of bias within included studies
Cohen’s kappa for the 33 studies assessed using the Cochrane Collaboration’s Tool was 0.96 (p = 0.018), indicating almost perfect agreement. No study was excluded after overall appraisal, but 30% had a high risk of bias, primarily in selection, performance, and detection domains (Fig. 2) (Appendix 9).
Fig. 2.
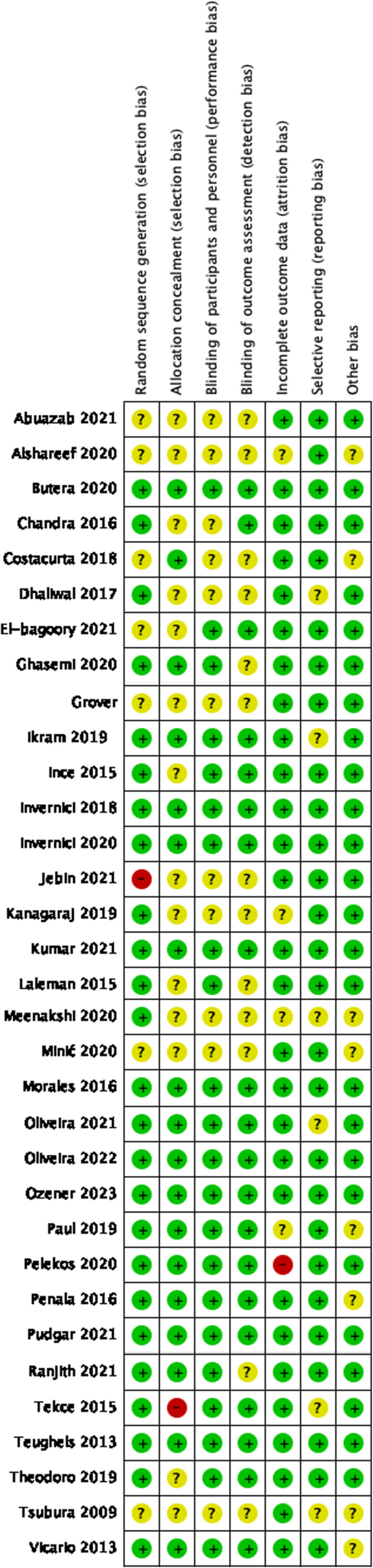
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study
Synthesis of results
PPD (short-term studies)
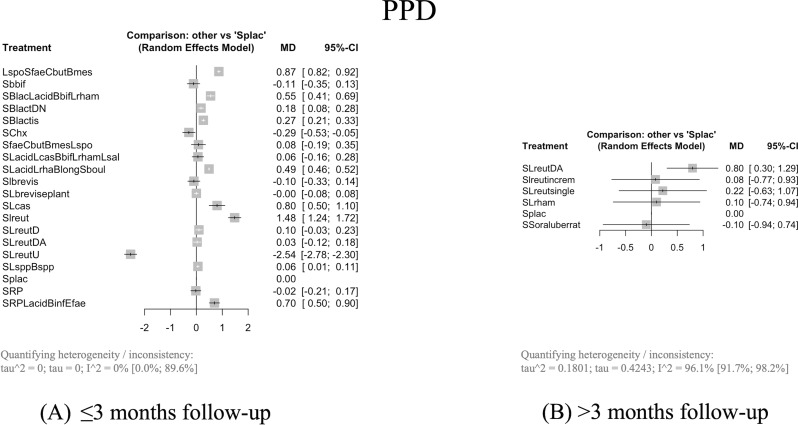
When combined with SRP, eight probiotic interventions resulted in significantly greater PPD reduction compared to Splac with a MD from 0.18 mm (95% confidence interval [CI]: 0.08–0.28, p = 0.0004, 95% prediction intervals [PdI]: -0.4680; 0.8280) with SRP + Bifidobacterium lactis DN (SBlactDN) to 1.48 mm (95% CI: 1.24–1.72, p = 0.0001, 95% PdI: -0.0829; 3.0429) with SRP + Lactobacillus reuteri (SLreut) (Fig. 3A).
Fig. 3.
Changes in PPD (3A; 3B). Effect sizes are presented as mean differences with a 9% confidence interval. MD = mean difference; CI = confidence interval
PPD (long-term studies)
When combined with SRP, Lactobacillus reuteri DA (SLreutDA) significantly reduced PPD with a MD of 0.80 (95% CI: 0.30–1.29, p = 0.0016, 95% PdI: -5.4763; 7.0680) compared with Splac (Fig. 3B).
CAL (short-term studies)
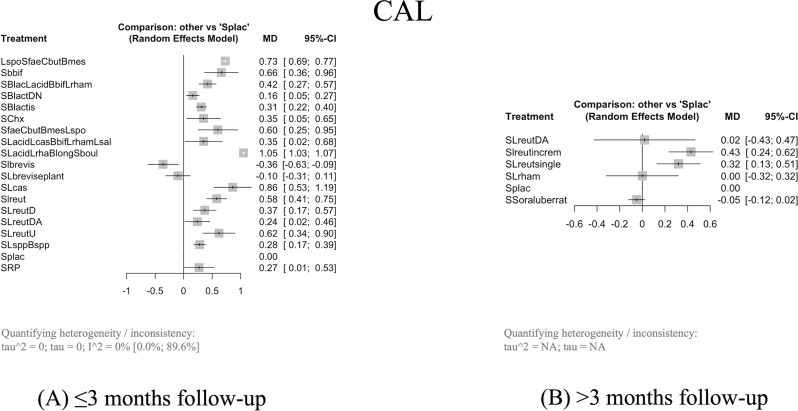
Sixteen probiotic interventions combined with SRP caused significantly more CAL gain than Splac, with a MD from 0.16 mm (95% CI: 0.05–0.27, p = 0.0050, 95% PdI: -0.5643; 0.8843) with SRP + Bifidobacterium lactis DN (SBlactDN) to 1.05 mm (95% CI: 1.03–1.07, p = 0.0001, 95% PdI: 0.9102; 1.1898) with SRP + Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum and Saccharomyces boulardii (SLacidLrhamBlongSboul) (Fig. 4A).
Fig. 4.
Changes in CAL (4A; 4B). Effect sizes are presented as mean differences with a 95% confidence interval. MD = mean difference; CI = confidence interval
CAL (long-term studies)
When combined with SRP, two probiotic interventions significantly cause more CAL gain than Splac with a MD from 0.32 mm (95% CI: 0.13–0.51, p = 0.0011) with SRP + Lactobacillus reuteri single (SLreutsingle) to 0.43 mm (95% CI: 0.24–0.62, p = 0.0001) with SRP + Lactobacillus reuteri incremental (Slreutincrem) (Fig. 4B).
BOP (short-term studies)
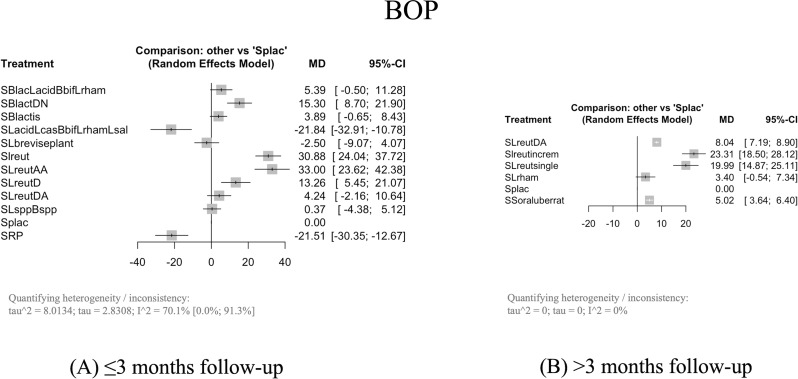
In four probiotic combinations with SRP, it is verified the significant reduction of BOP compared to Splac, with a MD from 13.26% (95% CI: 5.45–21.07, p = 0.0009, 95% PdI: -48.8436; 75.3636 ) with SRP + Lactobacillus reuteri D (SlreutD) to 33.00% (95% CI: 23,62–42.38, p = 0.0001, 95% PdI: -37.6700; 103.6700 ) with SRP + Lactobacillus reuteri AA (SLreutAA) (Fig. 5A).
Fig. 5.
Changes in BOP (5A; 5B). Effect sizes are presented as mean differences with a 95% confidence interval. MD = mean difference; CI = confidence interval
BOP (long-term studies)
When combined with SRP, four probiotic interventions significantly cause more BOP reduction than Splac with a MD from 5.02% (95% CI: 3.64–6.40, p = 0.0001) with SRP + Streptococcus oralis, uberis and rattus (SSoraluberrat) to 23.31% (95% CI: 18.50–28.12, p = 0.0001,) with Slreutincrem (Fig. 5B).
Exploration for inconsistency
SIDE analysis revealed no inconsistency (0%) for all the studied outcomes. Global inconsistency was not identified for any outcome as well: PPD (Q = 0, p = NA, for both short- and long-term studies), CAL (Q = 0, p = NA, for both short- and long-term studies), and BOP (Q = 0, p = NA, for both short- and long-term studies) (Appendix 10).
Results of additional analyses
To reduce heterogeneity (for PPD long-term studies and BOP short-term studies), we implemented the following strategies: removing studies with a high risk of bias and conducting a subgroup analysis based on the duration of antibiotic therapy (with 1 month as the reference). Since there were no differences in the final results when excluding studies with a high risk of bias, we decided to retain them to increase the sample size and enhance the robustness of the results. For the other outcomes, the heterogeneity was not significant (I²=0%).
PPD (long-term studies)
Therapy ≤ 1 m
Considering the studies with a duration of therapy ≤ 1 month, the heterogeneity of the analysis decreases from considerable (I2 = 96.1%, 95% CI: 91.7 to 98.2) to not important (0%). SRP + SLreutDA maintains the clinical relevance (MD > 0.5 mm) (MD = 1.16, 95% CI: 1.06 to 1.25, p = 0.0001).
Therapy > 1 m
The heterogeneity of this outcome remains considerable (> 70%), even with the sensitivity analysis, and no clinically relevant alterations were observed compared to the initial network estimations.
BOP (short-term studies)
The heterogeneity of this outcome remains considerable (> 70%), even with the sensitivity analysis, and no clinically relevant alterations were observed compared to the initial network estimations.
Ranking of the interventions
For all the outcomes measured, the best probiotic regimen in terms of PPD and BOP reduction and CAL gain is the Lactobacillus, specifically the specie reuteri (PPD ≤ 3 m: P-score = 1; PPD > 3 m: P-score = 0.9363; CAL > 3 m: P-score = 0.9650; BOP ≤ 3 m: P-score = 0.9671; 0.9417; BOP > 3 m: P-score = 0.9863). This probiotic, when used as an adjuvant to SRP, appears to be the most effective for both short and long-term follow-up periods, whether the therapy lasts for less or more than one month. The combination of Lactobacillus with Bifidobacterium and Saccharomyces seems to have better impact on CAL gain in studies with a follow up ≤ 3 m (P-score = 0.9922).
Discussion
This systematic review with network meta-analysis examined 33 RCTs to assess the efficacy of probiotics in enhancing clinical parameters (PPD, CAL, BOP). It represents the first comprehensive analysis of diverse probiotic proposals. Although most probiotics, in conjunction with SRP, showed improvements in PPD and CAL over Splac, certainty levels were very low at 92% and 71%, respectively (Appendix 6).
Our sensitivity analysis substantially reduced the heterogeneity of the outcomes measured, specifically for PPD. It concludes that the duration of probiotic therapy regimens can directly impact the success of the supplementary intervention. Our findings did not show sustained benefits beyond one month of probiotic therapy, suggesting that there is no difference providing probiotic therapy for more than one mouth. Quantitative analysis for secondary outcomes, PI and CFU, faced constraints due to network disconnection and high clinical data heterogeneity, respectively. However, the qualitative evaluation of the included studies measuring the effect of probiotics as an adjuvant to subgingival instrumentation in terms of reducing CFU counts follows the biological plausibility of the mechanism of action of probiotics in oral microflora. Almost all the included studies show that groups with subgingival instrumentation adjuvated with probiotic therapy experienced more CFU reduction compared to control/placebo. This is valid for the total load of bacteria [17, 23, 29, 33, 42, 44, 47] or specific periodontal bacteria (Aggregatibacter actinomycetemcomitans [22, 25, 42, 47], Porphyromonas gingivalis [17, 22, 25, 37, 42, 47], Prevotella intermedia [17, 22, 25, 42, 47], Fusobacterium nucleatum [42, 47], Tannerella forsythia [42, 47], and Treponema denticola [47]) demonstrating the action of probiotics in replacing dysbiotic microflora with symbiotic microflora.
Despite theoretically distinct systemic and local routes of probiotic administration, we refrained from conducting a subgroup analysis based on the type of administration. This decision was influenced by the local application of probiotics, such as dissolving tablets under the tongue, applying gels, sucking lozenges, and dissolving capsules in the mouth, where it’s challenging to ensure that the patient does not inadvertently swallow the content, making it difficult to control for a completely local application route.
In our statistical analysis, we differentiated between two control arms (SRP; SRP + plac) based on the well-established placebo response, even in cases where outcomes are objectively measurable. While PPD and CAL measurements are objective, we acknowledge that improvement and response to periodontal therapy result from a collaborative effort between the dentist and the patient’s commitment to oral healthcare. In periodontal diseases, the placebo effect is explained as a psychological response to the therapeutic context or treatment received, possibly associated with the patient’s motivation to improve [48]. However, in our results, we only observed significant and clinically relevant differences between SRP and SRP + plac for the BOP outcome (-21.51, 95% CI: -30.35 to -12.67, p = 0.001, 95% PdI: -89.1755; 46.1555), leading us to conclude that SRP alone is an inferior therapy compared to SRP + plac. Nevertheless, the measurement of this outcome is subjective compared to the objective measurements of PPD and CAL outcomes. For PPD, there were no differences between SRP and SRP + plac (-0.02, 95% CI: -0.21 to 0.17, p = 0.8402, 95% PdI: -1.2799; 1.2399). For the CAL outcome, the differences were statistically significant between SRP and SRP + plac but not clinically relevant (0.27, 95% CI: 0.01 to 0.053, p = 0.0430, 95% PdI: -1.4251; 1.9651). These differences were only proven for the short-term studies, as the network loses connection for the long-term studies (to apply the network algorithm, it was mandatory to remove the arms of SRP alone treatment).
In 2020, Nikolaos Donos et al. published a systematic review evaluating the efficacy of host modulators combined with subgingival instrumentation in reducing probing pocket depth in patients with periodontitis. The study concluded that based on five RCTs, treatment with probiotics resulted in a non-statistically significant benefit in PPD reduction of 0.38 mm [5]. On the other hand, a study published by J Li and his team, supports the use of probiotics as adjuvant to non-surgical periodontal treatment for PPD (MD=-0.60, 95% CI: -0.9 to -0.3, p < 0.001) and CAL (MD=-0.52, 95% CI: -0.75 to -0.28, p < 0.001) outcomes [4]. This study suggest that the administration of probiotics together with scaling and root planing can somewhat improve chronic periodontitis patient clinical outcomes and reduce levels of periodontal pathogens [4]. Our results, in addition to incorporating a network analysis, have included an additional number of randomized controlled trials (33) and increased the overall study population from 193 [5] and 647 [4] patients to 1290, respectively. This expansion provides innovative evidence for this topic and enhances statistical robustness. With SLreutDA, the reduction in PPD was statistically significant (MD = 1.16, 95% CI: 1.06 to 1.25, p = 0.001) and clinically relevant (difference > 0.5 mm), with the results being better than that reported by Donos et al. [5] and consistent with the results of Li et al. [4]. The confidence interval and I2 statistic (0%) in our results instilled confidence in this information. The purpose of conducting a network meta-analysis in this field is to compare all available probiotic therapy regimens head-to-head and understand which one is the most effective as an adjunct to periodontal therapy. Although our study supports the clinical benefits of probiotics, there are still studies that do not demonstrate these benefits, with some authors advocating against the use of probiotics in the treatment of periodontal diseases [5, 14].
To ensure a homogeneous sample and meet the transitivity assumption, we focused on untreated patients diagnosed with periodontitis. Additionally, we also adhered to the definition provided by Armitage (1999) to avoid excluding studies published before 2018 [1]. Given that chronic and aggressive periodontitis have different disease story, we included only studies on chronic periodontitis published before 2018. Individual diagnostic criteria for chronic periodontitis were analyzed for each included study. This led to the exclusion of three studies due to missing patient selection information or different criteria for diagnosing the disease. This situation highlights the importance of adhering to FAIR principles in biomedical research to ensure data is findable, accessible, interoperable (using standardized vocabularies), and reusable.
Smoking is a well-known risk factor for experiencing periodontitis, and it appears to have more impact on the CAL outcome, increasing the risk of periodontal attachment loss compared to non-smokers. Analyzing the subgroup of smokers with a network model created two subnetworks, thus preventing comparison. The findings of this study align with existing literature since the interventions associated with significantly greater CAL gain are primarily from studies that excluded smokers.
The included studies’ follow-up period ranged from 1 month to 1 year. For the presentation of results, we chose to divide the data by follow-up periods: ≤3 months (short-term) and > 3 months (long-term), since periodontal patients, contrary to the general population, require a more frequent recall system. The truth is that this follow-up period is not established for all periodontal patients, as it varies greatly depending on the case. However, the 3-month follow-up period seems to be the most acceptable time for periodontal patient recall in maintenance [49]. The most favorable results for the measured outcomes were observed at 1, 3, and 6 months, indicating probiotic therapies’ short- and long-term success. These results are novel, as the evidence of probiotics’ clinical efficacy at 6-month of follow-up was still to be proven, according to published papers [14].
Considerable heterogeneity was observed in clinical data, particularly for the PPD outcome. This variability can be attributed to differences in probiotic therapy duration (ranging from a single application to 1 year), various methods of administration, the use of single probiotic versus combinations, and variations in clinical data collection methods.
The primary limitation in probiotic research stems from the fact that these agents were initially developed for treating gastrointestinal disorders. As a result, there are currently no approved probiotics for use in dental practice, necessitating extensive clinical research to comprehend the specificities of these agents in the oral environment. It is precisely the duration and route of administration of the probiotics that pose the greatest challenge, as it is necessary to understand how long and for how long probiotics need to be taken to prevent the pathogenic microflora from becoming dominant again [13]. In our statistical analysis, we combined data from different administration protocols and a wide range of microorganisms. While we acknowledge this approach as a significant limitation of the study, it reflects the available evidence on probiotics that we were able to work with. For this reason, the results of this study should be interpreted conscientiously and with caution. Nevertheless, it is apparent that incorporating probiotics as an adjunctive therapy in periodontal treatment is safe, as evidenced by the absence of reported adverse effects in patients [50].
Our results suggest that Lactobacillus, particularly the specie reuteri, appear to be an effective adjuvant to subgingival instrumentation in improving clinical parameters, as they performed significantly and clinically better across all considered outcomes. This finding is consistent with the most recent published literature in this field [7, 13, 51]. Although the results do not align with the recommendations outlined in the clinical guideline published by the EFP [7], the authors believe that it could be due to the additional available evidence since guidelines publication in 2020. Since then, twenty-nine additional RCTs have been published, with a substantial increase in the overall study population from 193 patients to 1290 patients. Nevertheless, it is important to mention that the perceived effectiveness of Lactobacillus reuteri as the most effective probiotic may be influenced by its strong representation in RCTs, which is likely due to funding from pharmaceutical companies. This represents a limitation and underscores, once again, the need for caution when interpreting and extrapolating the results.
The network diagrams for the three outcomes were categorized as ‘star networks’ due to numerous proposed protocols in the literature, resulting in a low percentage of direct evidence. This reliance on indirect evidence is a limitation, cautioning against definitive conclusions. While Lactobacillus emerged as the most effective protocol for all outcomes in both short and long-term studies, the findings are based on low-quality indirect evidence. Thus, further clinical validation in oral healthcare settings is crucial. Additionally, a decline in probiotic effectiveness between 3 and 6 months underlines the need for extended-duration research to evaluate sustained efficacy and inform more robust clinical practices.
The analyzed evidence suggests that combining SRP with probiotics regimens as adjuvants to subgingival instrumentation is effective in improving clinical parameters (PPD and CAL). Lactobacillus reuteri seems to be the most comprehensive and effective of the studied probiotic. Although SRP + Lactobacillus reuteri ranked higher than most other genera, these results must be cautiously interpreted due to the network’s weak connection for the considered outcomes.
This systematic review underscores the need for future research, advocating for long-term RCTs (minimum one year) to evaluate sustained probiotic effects. Standardizing administration routes, comparing single vs. combined probiotic regimens, and enhancing clinical data collection methods in RCTs are crucial for improved comparability and reliability of results. Additionally, it would be interesting to combine all possible therapies used as adjuncts to non-surgical periodontal treatment, such as antibiotics [52–58], ozonized gels [59], and hyaluronic acid [60], to determine which interventions work best.
Conclusions
This systematic review with network meta-analysis highlights the potential role of probiotics, particularly Lactobacillus reuteri, as an effective adjuvant to professional mechanical plaque removal in improving clinical parameters in periodontal therapy. The findings underscore the possibility of integrating probiotics into periodontal treatment protocols, especially in light of the growing issue of antimicrobial resistance, as probiotics do not seem to cause adverse effects. Furthermore, this review calls for further long-term RCTs to validate these results. Standardizing probiotic administration and addressing clinical data heterogeneity are essential for advancing the use of probiotics.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not Applicable.
Abbreviations
- BOP
Bleeding on probing.
- CAL
Clinical attachment level.
- CFU
Colony forming units.
- CI
Confidence interval.
- MD
Mean difference.
- NMA
Network meta-analysis.
- PdI
Prediction interval.
- PI
Plaque index.
- Plac
Placebo.
- PMPR
Professional mechanical plaque removal.
- PPD
Probing pocket depth.
- RCT
Randomized controlled trial.
- SBlactDN
SRP + Bifidobacterium lactis DN.
- SLacidLrhamBlongSboul
SRP + Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium longum and Saccharomyces boulardii.
- SLreut
SRP + Lactobacillus reuteri.
- SLreutAA
SRP + Lactobacillus reuteri AA.
- SLreutD
SRP + Lactobacillus reuteri D.
- SLreutDA
SRP + Lactobacillus reuteri DA.
- Slreutincrem
SRP + Lactobacillus reuteri incremental.
- SLreutsingle
SRP + Lactobacillus reuteri single.
- Splac
SRP + placebo.
- SR
Systematic review.
- SRP
Scaling and root planing.
Author contributions
C.D.M., A.M., L.A., J.F.M., J.S., and D.M. contributed to the conception and design. C.D.M. contributed to acquisition, analysis, and interpretation. C.D.M., A.M., L.A., and D.M. contributed to interpretation and analysis. C.D.M. drafted the manuscript. A.M., L.A., J.F.M., J.S., and D.M. critically revised the manuscript.All authors gave final approval and agreed to be accountable for all aspects of the work ensuring integrity and accuracy.
Funding
Not Applicable. No funding was obtained for this study. This work was self-funded by the CEMDBE (Center for Evidence-Based Dental Medicine), Faculty of Dental Medicine of the University of Lisbon.
Data availability
This systematic review and network meta-analysis was registered in the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; trial No. CRD42021250678).All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Caton JG, et al. A new classification scheme for periodontal and peri-implant diseases and conditions - introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45(20):S1–8. 10.1111/jcpe.12935. [DOI] [PubMed] [Google Scholar]
- 2.Nazir M, et al. Global prevalence of Periodontal Disease and Lack of its Surveillance. ScientificWorldJournal. 2020;2020(2146160). 10.1155/2020/2146160. [DOI] [PMC free article] [PubMed]
- 3.IHME I. f. H. M. a. E.-. Global Burden of Disease Collaborative Network | Global Burden of Disease Study (2021).
- 4.Li J, Zhao G, Zhang HM, Zhu FF. Probiotic adjuvant treatment in combination with scaling and root planing in chronic periodontitis: a systematic review and meta-analysis. Benef Microbes. 2023;14:95–107. 10.3920/BM2022.0056. [DOI] [PubMed] [Google Scholar]
- 5.Donos N, et al. The adjunctive use of host modulators in non-surgical periodontal therapy. A systematic review of randomized, placebo-controlled clinical studies. J Clin Periodontol. 2020;47(22):199–238. 10.1111/jcpe.13232. [DOI] [PubMed] [Google Scholar]
- 6.Tomasi C, Leyland AH, Wennstrom JL. Factors influencing the outcome of non-surgical periodontal treatment: a multilevel approach. J Clin Periodontol. 2007;34:682–90. 10.1111/j.1600-051X.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 7.Sanz M, et al. Treatment of stage I-III periodontitis-the EFP S3 level clinical practice guideline. J Clin Periodontol. 2020;47:4–60. 10.1111/jcpe.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng E, et al. Antibiotic resistance in the microbiota of periodontitis patients: an update of current findings. Crit Rev Microbiol. 2024;50:329–40. 10.1080/1040841X.2023.2197481. [DOI] [PubMed] [Google Scholar]
- 9.Saha S, Tomaro-Duchesneau C, Tabrizian M, Prakash S. Probiotics as oral health biotherapeutics. Expert Opin Biol Ther. 2012;12:1207–20. 10.1517/14712598.2012.693474. [DOI] [PubMed] [Google Scholar]
- 10.Zidar A, Kristl J, Kocbek P, Zupančič Š. Treatment challenges and delivery systems in immunomodulation and probiotic therapies for periodontitis. Expert Opin Drug Deliv. 2021;18:1229–44. 10.1080/17425247.2021.1908260. [DOI] [PubMed] [Google Scholar]
- 11.Anusha RL, Umar D, Basheer B, Baroudi K. The magic of magic bugs in oral cavity: Probiotics. J Adv Pharm Technol Res. 2015;6:43–7. 10.4103/2231-4040.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng E, et al. Probiotic therapy for periodontal and peri-implant health - silver bullet or sham? Benef Microbes. 2021;12:215–30. 10.3920/BM2020.0182. [DOI] [PubMed] [Google Scholar]
- 13.Homayouni Rad A, Pourjafar H, Mirzakhani E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front Cell Infect Microbiol. 2023;13:1120995. 10.3389/fcimb.2023.1120995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng E, et al. Adjunctive probiotics after periodontal debridement versus placebo: a systematic review and meta-analysis. Acta Odontol Scand. 2022;80:81–90. 10.1080/00016357.2021.1942193. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of interventions Version 5.1.0 [updated March 2011]. Cochrane Collab (2011).
- 16.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Abuazab DR, El-Shinnawi UM, El-Daker MA. Efficacy of locally delivered Bifidobacterium Probiotic Gel as an adjunctive therapy in Periodontitis patients (clinical and microbiological study). Mansoura J Dentistry. 2021;8:1–11. 10.21608/mjd.2021.200160. [Google Scholar]
- 18.Alshareef A, et al. Effectiveness of Probiotic lozenges in Periodontal Management of Chronic Periodontitis patients: clinical and immunological study. Eur J Dent. 2020;14:281–7. 10.1055/s-0040-1709924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandra RV, et al. Effect of a locally delivered ProbioticPrebiotic mixture as an Adjunct to Scaling and Root Planing in the management of chronic Periodontitis. J Int Acad Periodontol. 2016;18:67–75. [PubMed] [Google Scholar]
- 20.Costacurta M, Sicuro L, Margiotta S, Ingrasciotta I, Docimo R. Clinical effects of lactobacillus reuteri probiotic in treatment of chronic periodontitis. A randomized, controlled trial. ORAL Implantology. 2018;4:191–8. [Google Scholar]
- 21.de Oliveira AM, Lourenco TGB, Colombo APV. Impact of systemic probiotics as adjuncts to subgingival instrumentation on the oral-gut microbiota associated with periodontitis: a randomized controlled clinical trial. J Periodontol. 2022;93:31–44. 10.1002/JPER.21-0078. [DOI] [PubMed] [Google Scholar]
- 22.Dhaliwal PK, Grover V, Malhotra R, Kapoor A. Clinical and microbiological investigation of the effects of Probiotics Combined with Scaling and Root Planing in the management of chronic periodontitis: a Randomized, controlled study. J Int Acad Periodontol. 2017;19:101–8. [PubMed] [Google Scholar]
- 23.El-Bagoory GKM, El-Guindy HM, Shoukheba MYM, El-Zamarany EA. The adjunctive effect of probiotics to nonsurgical treatment of chronic periodontitis: a randomized controlled clinical trial. J Indian Soc Periodontol. 2021;25:525–31. 10.4103/jisp.jisp_114_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghasemi S, Babaloo AR, Mohammadi B, Esmailzadeh M. Evaluating the effect of probiotic supplementation in the form of mouthwash along with scaling and root planing on periodontal indices in patients with stage III and grade a generalized periodontitis: a randomized clinical trial. J Adv Periodontology Implant Dentistry. 2020;12:73–8. 10.34172/japid.2020.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grover V. Thesis - handsearch.
- 26.Ikram S, et al. Clinical efficacy of Probiotics as an Adjunct to Scaling and Root Planning in the treatment of chronic Periodontitis. Annals Abbasi Shaheed Hosp Karachi Med Dent Coll. 2019;24:31–7. [Google Scholar]
- 27.Ince G, et al. Clinical and biochemical evaluation of lozenges containing Lactobacillus reuteri as an Adjunct to Non-surgical Periodontal Therapy in Chronic Periodontitis. J Periodontol. 2015;86:746–54. 10.1902/jop.2015.140612. [DOI] [PubMed] [Google Scholar]
- 28.Invernici MM, et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: a randomized clinical trial. J Clin Periodontol. 2018;45:1198–210. 10.1111/jcpe.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jebin AA, Nisha KJ, Padmanabhan S. Oral Microbial Shift following 1-Month supplementation of Probiotic Chewable tablets containing Lactobacillus reuteri UBLRu-87 as an Adjunct to Phase 1 Periodontal Therapy in Chronic Periodontitis patients: a Randomized Controlled Clinical Trial. Contemp Clin Dent. 2021;12:121–7. 10.4103/ccd.ccd_135_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanagaraj SS, Elavarasu S, Thangavelu A, Subaramoniam MK, Dutta T. The evaluation of probiotic as an adjunct to scaling and root planing in chronic periodontitis patients - a clinical, microbiological and biochemical study. Int J Oral Health Dentistry. 2019;5:32–6. 10.18231/j.ijohd.2019.008. [Google Scholar]
- 31.Kumar V, et al. Localized probiotic-guided pocket recolonization in the treatment of chronic periodontitis: a randomized controlled clinical trial. J Periodontal Implant Sci. 2021;51:199–212. 10.5051/jpis.2004140207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laleman I, et al. The effect of a streptococci containing probiotic in periodontal therapy: a randomized controlled trial. J Clin Periodontol. 2015;42:1032–41. 10.1111/jcpe.12464. [DOI] [PubMed] [Google Scholar]
- 33.Meenakshi SS, Varghese S. Adjunctive effect of probiotic (Lactobacillus casei Shirota) to scaling and root planing in the management of chronic periodontitis. Drug Invention Today. 2018;10:1381–6. [Google Scholar]
- 34.Minic I, Pejcic A, Bradic-Vasic M. Effect of the local probiotics in the therapy of periodontitis A randomized prospective study. Int J Dent Hyg. 2022;20:401–7. 10.1111/idh.12509. [DOI] [PubMed] [Google Scholar]
- 35.Morales A, et al. Clinical effects of Lactobacillus rhamnosus in non-surgical treatment of chronic periodontitis: a randomized placebo-controlled trial with 1-Year Follow-Up. J Periodontol. 2016;87:944–52. 10.1902/jop.2016.150665. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira AM, d., Lourenço TGB, Colombo APV. Impact of systemic probiotics as adjuncts to subgingival instrumentation on the oral-gut microbiota associated to periodontitis: a randomized controlled clinical trial. peer Rev. 2021. 10.1111/jper.10797. [DOI] [PubMed] [Google Scholar]
- 37.Paul GT, Gandhimadhi D, Babu SP. K. K. A Double-blind, Placebo Controlled Study to assess the Clinical and Microbiological effects of a probiotic lozenge as an adjunctive therapy in the management of chronic Periodontitis. J Health Res. 2019;6:57–63. 10.4103/cjhr.cjhr_71_18. [Google Scholar]
- 38.Pelekos G, et al. Effects of adjunctive probiotic L. reuteri lozenges on S/RSD outcomes at molar sites with deep pockets. J Clin Periodontol. 2020;47:1098–107. 10.1111/jcpe.13329. [DOI] [PubMed] [Google Scholar]
- 39.Pudgar P, et al. Probiotic strains of Lactobacillus brevis and Lactobacillus plantarum as adjunct to non-surgical periodontal therapy: 3-month results of a randomized controlled clinical trial. Clin Oral Investig. 2021;25:1411–22. 10.1007/s00784-020-03449-4. [DOI] [PubMed] [Google Scholar]
- 40.Ranjith A, Nazimudeen NB, Baiju KV. Probiotic mouthwash as an adjunct to mechanical therapy in the treatment of stage II periodontitis: a randomized controlled clinical trial. Int J Dent Hyg. 2022;20:415–21. 10.1111/idh.12589. [DOI] [PubMed] [Google Scholar]
- 41.Tekce M, et al. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: a 1-year follow-up study. J Clin Periodontol. 2015;42:363–72. 10.1111/jcpe.12387. [DOI] [PubMed] [Google Scholar]
- 42.Teughels W, et al. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol. 2013;40:1025–35. 10.1111/jcpe.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Theodoro LH, et al. Effects of Lactobacillus reuteri as an adjunct to the treatment of periodontitis in smokers: randomised clinical trial. Benef Microbes. 2019;10:375–84. 10.3920/BM2018.0150. [DOI] [PubMed] [Google Scholar]
- 44.Ozener HO, Kuru L, Kadir T, Kuru B. Bifidobacterium animalis subsp. lactis as adjunct to non-surgical periodontal treatment in periodontitis: a randomized controlled clinical trial. Clin Oral Investig. 2023;27:1965–72. 10.1007/s00784-023-04870-1. [DOI] [PubMed] [Google Scholar]
- 45.Invernici MM, et al. Bifidobacterium animalis subsp lactis HN019 presents antimicrobial potential against periodontopathogens and modulates the immunological response of oral mucosa in periodontitis patients. PLoS ONE. 2020;15:e0238425. 10.1371/journal.pone.0238425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vicario M, Santos A, Violant D, Nart J, Giner L. Clinical changes in periodontal subjects with the probiotic Lactobacillus reuteri Prodentis: a preliminary randomized clinical trial. Acta Odontol Scand. 2013;71:813–9. 10.3109/00016357.2012.734404. [DOI] [PubMed] [Google Scholar]
- 47.Butera A, et al. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: evaluation of clinical and microbiological parameters. Microorganisms. 2020;9. 10.3390/microorganisms9010069. [DOI] [PMC free article] [PubMed]
- 48.Vollert J, et al. Assessment of Placebo Response in Objective and subjective outcome measures in rheumatoid arthritis clinical trials. JAMA Netw Open. 2020;3:e2013196. 10.1001/jamanetworkopen.2020.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farooqi OA, Wehler CJ, Gibson G, Jurasic MM, Jones JA. Appropriate recall interval for Periodontal maintenance: a systematic review. J Evid Based Dent Pract. 2015;15:171–81. 10.1016/j.jebdp.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bessa LJ, Botelho J, Machado V, Alves R, Mendes JJ. Managing oral health in the context of Antimicrobial Resistance. Int J Environ Res Public Health. 2022;19. 10.3390/ijerph192416448. [DOI] [PMC free article] [PubMed]
- 51.Arbildo-Vega HI, et al. Clinical effectiveness of Lactobacillus reuteri in the treatment of peri-implant diseases: a systematic review and meta-analysis. J Biol Regul Homeost Agents. 2021;35:79–88. 10.23812/21-2supp1-7. [DOI] [PubMed] [Google Scholar]
- 52.Afrashtehfar KI, Assery MK. From dental science to clinical practice: knowledge translation and evidence-based dentistry principles. Saudi Dent J. 2017;29:83–92. 10.1016/j.sdentj.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyeena L, Koduganti RR, Panthula VR, Jammula SP. Comparison of efficacy of probiotics versus tetracycline fibers as adjuvants to scaling and root planing. J Indian Soc Periodontol. 2019;23:539–44. 10.4103/jisp.jisp_590_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghazal M, et al. A placebo-controlled randomized clinical trial of antibiotics versus probiotics as an adjuvant to nonsurgical periodontal treatment among smokers with stage III, Grade C generalized periodontitis. Clin Adv Periodontics. 2023;13:197–204. 10.1002/cap.10253. [DOI] [PubMed] [Google Scholar]
- 55.Ikram S, et al. Effect of local probiotic (Lactobacillus reuteri) vs systemic antibiotic therapy as an adjunct to non-surgical periodontal treatment in chronic periodontitis. J Investig Clin Dent. 2019;10:e12393. 10.1111/jicd.12393. [DOI] [PubMed] [Google Scholar]
- 56.Morales A, et al. Clinical effects of probiotic or azithromycin as an adjunct to scaling and root planning in the treatment of stage III periodontitis: a pilot randomized controlled clinical trial. BMC Oral Health. 2021;21:12. 10.1186/s12903-020-01276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morales A, et al. Microbiological and clinical effects of probiotics and antibiotics on nonsurgical treatment of chronic periodontitis: a randomized placebo- controlled trial with 9-month follow-up. J Appl Oral Sci. 2018;26:e20170075. 10.1590/1678-7757-2017-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramos TCS, et al. Effect of systemic antibiotic and probiotic therapies as adjuvant treatments of subgingival instrumentation for periodontitis: a randomized controlled clinical study. J Appl Oral Sci. 2022;30:e20210583. 10.1590/1678-7757-2021-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scribante A, Gallo S, Pascadopoli M, Frani M, Butera A. Ozonized gels vs chlorhexidine in non-surgical periodontal treatment: a randomized clinical trial. Oral Dis. 2023. 10.1111/odi.14829. [DOI] [PubMed] [Google Scholar]
- 60.Ramanauskaite E, et al. Microbiological effects of Sodium Hypochlorite/-Amino acids and cross-linked hyaluronic acid adjunctive to non-surgical Periodontal treatment. Oral Health Prev Dent. 2024;22:171–80. 10.3290/j.ohpd.b5281925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This systematic review and network meta-analysis was registered in the International Prospective Register of Ongoing Systematic Reviews (PROSPERO; trial No. CRD42021250678).All data generated or analysed during this study are included in this published article [and its supplementary information files].