Abstract
Background
Malaria remains a significant public health concern, especially for the deadliest parasite, Plasmodium falciparum. During acute malaria, various cytokines, including osteopontin (OPN), regulate the immune response. OPN has been shown to be protective against malaria in mice. Nonetheless, its precise function and potential ability to control parasites during acute malaria in humans remain poorly understood.
Results
Blood samples were collected from Swedish adults with imported malaria, Ugandan children and adults with symptomatic malaria (including follow-up after 42 days), Ugandans with non-malarial fever and healthy individuals from both Uganda and Sweden. Parasitemia was determined by microscopy. Malaria-negative samples were verified by LAMP. OPN and interferon-γ (IFN- γ) levels were measured using ELISA. In children, OPN levels were significantly higher during acute infection compared to levels after 42 days, whereas Ugandan adults showed no difference. Swedish adults with imported malaria had elevated OPN levels compared to both Swedish controls and Ugandan adults with malaria. Parasitemia was significantly correlated with both OPN and IFN-γ levels across the entire cohort. While a significant correlation between OPN and IFN-γ was evident overall, it remained statistically significant only in Ugandan adults when analyzed by subgroups. This suggests that OPN is not just a general marker of inflammation but may be regulated differently during the development of malaria immunity.
Conclusions
In acute malaria, elevated OPN levels showed a stronger correlation with lack of immunity than age. These findings underscore the potential importance of OPN in malaria, particularly in non-immune individuals.
Keywords: Malaria, Osteopontin, Parasitemia, Imported malaria, Immunity, ELISA, LAMP, IFN- γ
Background
Despite advancements in the implementation of malaria intervention strategies, Plasmodium falciparum (P. falciparum) malaria remains a significant public health concern, causing notable morbidity and mortality, particularly among children under 5 years of age [1]. Malaria is endemic in Sub-Saharan Africa, and Uganda is one of the countries with the highest burden of the disease, estimated at 12.7 million cases annually [1].
An infection with P. falciparum can manifest itself through a range of symptoms, from mild to severe, including anemia and cerebral complications. Disease severity is typically related to the parasitemia levels, with high parasitemia associated with severe malaria. The World Health Organization (WHO) defines severe malaria as the presence of P. falciparum asexual parasitemia accompanied by organ dysfunction and/or hyperparasitemia [2]. Individuals residing in malaria-endemic regions typically acquire anti-parasite immunity and immunity to clinical disease over time, often termed semi-immunity, as it does not confer complete protection against infection [3, 4]. This immunity is acquired more rapidly against severe forms of malaria but takes longer against milder forms [3]. Furthermore, immunity wanes over time without regular exposure to P. falciparum. This is evidenced by the higher susceptibility to malaria among individuals born in endemic regions but later residing in non-endemic countries and then returning to visit friends and relatives (VFRs), a group that constitutes a significant proportion of imported malaria cases in Europe [5].
In the context of malaria, diverse inflammatory mediators intricately interact to modulate the immune response against the parasite, as supported by both experimental and clinical observations [6, 7]. However, the underlying mechanisms and determining factors involved in the development of naturally acquired immunity remain incompletely understood.
Full-length osteopontin (OPN), a multifunctional, highly phosphorylated, and glycosylated matricellular protein, assumes a role in diverse physiological contexts such as wound repair, cancer biology, angiogenesis, bone resorption and immune functions [8, 9]. Its extracellular form is found in multiple tissues and body fluids and have been shown to contribute as a cytokine in both pro-inflammatory and anti-inflammatory responses [10, 11]. The pleiotropic features of OPN are, in part, attributable to its ability to interact with multiple receptors, including integrin and CD44 variants [12]. OPN functions as an immunoregulatory mediator and is expressed by a range of immune cells, including B- and T cells, dendritic cells, macrophages, and neutrophils, contributing to various cellular processes [9, 12]. Both the innate and the adaptive immune response play a vital role in the defense against intracellular pathogens. OPN actively contributes to this response by stimulating the production of IL-12, IL-17 and TNF‐α from macrophages, T helper cells, and dendritic cells, thereby inducing the development of Th1 and Th17 cells, as well as promoting B cell proliferation and production of immunoglobulins [13–16]. The significance of OPN is underscored by observations in OPN knockout mice, which exhibit impaired type-1 immunity against both viral and bacterial infections [17]. Recent studies have further shown OPN to be highly expressed during various infections, including TB [18], COVID-19 [19], dengue [20], and trypanosomiasis [21].
However, little is known about the basic mechanisms of OPN in the context of malaria. OPN appeared to have a protective effect as shown in a mouse model of the murine malaria P. chabaudi chabaudi. In this study, OPN knockout mice succumbed to the infection, whereas wildtype mice survived, indicating that OPN might have an important role in resolving the infection [22]. Moreover, in individuals with P. falciparum malaria, those expressing OPN mRNA showed significantly lower parasitemia levels compared to those lacking OPN mRNA, indicating a potential suppressive role for OPN against P. falciparum [23]. Interestingly, our recent study conducting growth inhibition assays (GIAs) did not detect a direct effect in cultured parasites with the addition of OPN [24]. Nevertheless, we demonstrated correlations between OPN and P. falciparum-specific atypical memory B cells and complement-fixing antibodies in people living in malaria endemic areas [24, 25], suggesting a potential role for OPN in contributing to naturally acquired immunity against malaria.
Interferon-γ (IFN-γ) is a key pro-inflammatory cytokine in the Th1 response, playing an important role in defending against malaria and clearing the parasite [26]. Alongside TNF-α and IL-12, IFN-γ helps inhibit parasite replication and promotes the removal of infected red blood cells [27]. Elevated IFN-γ levels are often associated with disease severity [28, 29]. Evidence suggests that populations with lower acquired immunity to malaria may exhibit higher IFN-γ levels during acute infections compared to populations with more established immunity [30]. However, the relationship between IFN-γ and OPN remains poorly understood.
The aim of this study was to improve our understanding of the role of OPN in malaria. Specifically, we investigated three key aspects within a cohort that included both adults and children with P. falciparum malaria: (1) the plasma OPN levels during the acute phase of infection, (2) the correlation of OPN with parasite density and IFN- γ and (3) the assessment of OPN levels following convalescence from the infection. In addition, we examined plasma OPN levels in individuals with varying degrees of exposure and immunity to malaria by analyzing a cohort of imported malaria in Sweden. These findings contribute to increased knowledge of OPN’s involvement in the context of P. falciparum malaria. Understanding the dynamics of the immune response and the development of acquired immunity against malaria is crucial for developing effective strategies for vaccines or treatments to combat the disease.
Methods
Study site and participant enrollment
This prospective longitudinal study was conducted at Iganga General Hospital, which is located about 120 km east of Kampala, the capital city of Uganda. It is a government-owned hospital that serves Iganga District and parts of the surrounding districts. It’s strategic location on the Jinja-Tororo Highway makes it accessible to a large number of people, including those living in remote areas. In this study area malaria transmission occurs throughout the year, with peaks following the rains during April-June and September-December.
Study participants were 41 adults and 40 children diagnosed with malaria at the Outpatient Department at Iganga General Hospital during workdays. Enrollment took place between March and December 2022, spanning two malaria peak seasons. Malaria diagnosis was based on clinical presentation and confirmed by a positive rapid diagnostic test (RDT) in combination with a positive microscopy for P. falciparum asexual parasites, or a positive test result in Plasmodium species LAMP. Inclusion criteria were a history of fever in the previous 24 h and/or an axillary temperature ≥ 37.5 °C, and informed written consent from participants or their parents/guardians for minors. Participants were required to agree to a 42-day follow-up. Exclusion criteria included infants below 6 months of age, anemia with hemoglobin < 5 g/dl, recent blood transfusion within the last 3 months, and individuals suffering from known chronic disease conditions such as sickle cell disease, severe malnutrition, cancer, or HIV. The study also included three control groups from Uganda. The first group included 46 individuals (23 children and 23 adults) who presented with a febrile non-malarial illness at Iganga General Hospital during the study period. The second group consisted of 81 adults who were accompanying their children at Kasangati Health Centre in Uganda. These adults were assessed by clinicians and reported feeling well. The third control group consisted of children participating in the follow-up visit, all of whom reported feeling well and appeared healthy.
In addition, a prospective study was conducted at Skåne University Hospital in Lund and Malmö, Sweden. 14 adult individuals admitted with imported malaria, confirmed by positive RDT and microscopy at the Department of Infectious Diseases between 2018 and 2022, were enrolled into the study. Details were collected on their history of previous malaria exposure, origin and length-of-time residing in Sweden. As controls, the study included 13 healthy volunteers who had no history of travel to malaria-endemic regions.
Sample collection and processing
In Uganda, venous blood samples of 5 mL were collected from adults and 2 mL from children into EDTA tubes at the time of enrollment and after 42 days of follow-up. All blood samples were obtained prior to the initiation of anti-malarial treatment. Blood samples were transported to the Makerere University, Biomedical Cross Cutting Laboratory for processing within 4 h after collection. Peripheral blood mononuclear cells (PBMCs) and plasma were separated, aliquoted and stored in a -80 freezer. Samples were transported to Sweden for downstream assays.
In Sweden, venous blood was collected in EDTA and serum tubes, and transported to the laboratory at Skåne University Hospital in Lund, Sweden for processing within 4 h. All blood samples were taken within 4 to 24 h after the administration of the first dose of anti-malarial treatment. PBMCs and plasma were separated, aliquoted and stored in a -80 freezer.
Malaria microscopy
For confirmation of malaria parasites in Uganda, thick blood smears were prepared by standard methods. Blood samples were collected before the initiation of anti-malarial treatment, and the smears were examined using standard microscopy. Plasmodium species blood stage parasites were then counted in microscope fields containing at least 200 white blood cells (WBCs). The parasite levels were determined according to WHO guidelines.
Similarly, in Sweden, blood samples for microscopy were collected before anti-malarial treatment. Parasites were detected and enumerated by light microscopy of Giemsa stained thick and thin blood smears at the Department of Infectious Diseases and at the Department of Clinical Microbiology at Skåne University Hospital in Lund and Malmö.
Loop mediated isothermal amplification (LAMP)
To verify that the group of 46 individuals categorized with malaria-like febrile illness truly tested negative for malaria, each plasma sample underwent testing with the LAMP-kit HumaTurb C + A Loopamp™ Malaria Pan Detection kit (Human Diagnostics Worldwide, Wiesbaden, Germany). The tests were conducted at the Clinical Microbiology Laboratory of Region Skåne, adhering to the manufacturer’s recommendations.
OPN ELISA
The concentration of OPN in plasma was measured using the Quantikine Human Osteopontin Immunoassay (R&D Systems, Abingdon, UK), following the manufacturer’s guidelines. In brief, plasma samples were diluted at a ratio of 1:25. 100 µL of assay diluent was added to each well, followed by the addition of 50 µL of either standard or plasma sample. The plates were incubated for two hours, washed × 4, and then resuspended with 200 µL of conjugate per well, followed by an additional 2-h incubation and 4 wash cycles. Subsequently, 200 µL of substrate solution was added and left to incubate for 30 min. The plate was read within 30 min after the addition of 50 µL of stop solution per well. Assays were performed in duplicate for both diluted samples and standards. To minimize batch/plate effects, baseline and follow-up samples from the same individuals were analyzed on the same plates. Plasma OPN concentrations were determined using the provided in-kit standard curve, ranging from 0.313 ng/mL to 20 ng/mL. Optical density readings were recorded at 450 nm using a Multiscan Sky microplate reader. OPN concentrations were calculated utilizing GraphPad Prism software, version 10 (GraphPad Software Inc., San Diego, CA, USA).
Interferon-γ ELISA
The concentration of human IFN-γ in plasma was measured using the Enzyme-linked Immunosorbent Assay (BMS228, Invitrogen, USA), following the manufacturer’s instructions. In brief, human IFN-γ standard was added in different dilutions to create a standard curve ranging from 1.6 to 100 pg/mL. The plasma samples were diluted 1:2, with 50 µL of sample diluent added into each well followed by 50 µL plasma. Then 50 µL Biotin-Conjugate was added, the plates were incubated for two hours, washed × 3, 100 µL Streptavidin-HRP was added, followed by one hour incubation. All incubations were performed at room temperature. Subsequently, 100 µL of substrate solution was added and left for 10 min. The reaction was stopped by adding 100 µL stop solution per well. Optical density was recorded within 30 min using a Multiscan Sky microplate reader at 450 nm. Assays were performed in duplicate for both diluted samples and standards. To minimize batch/plate effects, baseline and follow-up samples from the same individuals were analyzed on the same plates. IFN-γ concentrations were calculated utilizing Microsoft EXCEL, version 16.78.3, and GraphPad Prism software, version 10 (GraphPad Software Inc., San Diego, CA, USA).
Data analysis
Continuous data were expressed as mean and standard deviation (SD) or as median and interquartile range (IQR) in case of non-normal distributions. Qualitative variables were expressed as number and percentage. Comparisons of plasma OPN and IFN-γ levels between groups were done by using the non-parametric Mann–Whitney test or the Kruskal Wallis test with Dunn’s multiple comparison test. Differences between paired data were done using Wilcoxon matched-pairs signed-rank test. Correlations between plasma OPN levels and parasitemia were assessed using Spearman’s rank correlation coefficients. Two-sided P values were calculated for all test statistics and P < 0.05 was considered significant. All statistical analyses were performed using GraphPad Prism software, version 10 (GraphPad Software Inc., San Diego, CA, USA).
Results
Characteristics of study participants
The study in Uganda included a total of 81 participants, all confirmed to have malaria either through positive results from RDTs along with confirmatory microscopy, or via a positive LAMP test. Table 1 shows the characteristics of the participants at enrollment: The study group comprised 40 adults (aged ≥ 15 years) and 41 children (aged < 15 years), ranging in age from 1 to 61 years. Out of the recruited children, 11 (27%) were ≤ 5 years of age, with a mean age of 3.2 ± 1.3, while 30 (73%) fell within the 6–14 range, with a mean age of 9.5 ± 2.7. Five children presenting with severe malaria at admission received intravenous treatment with artesunate, while all other participants, both children and adults, were treated with an oral combination therapy of artemether/lumefantrine. A subgroup of 42 individuals (18 adults and 24 children) participated in a follow-up visit after 42 days.
Table 1.
General characteristics of the study cohorts
| Patient characteristics | Ugandan malaria cohort | Malaria-like febrile illness, Uganda | Swedish malaria cohort | Ugandan adults in good health | Ugandan children in good health | |||
|---|---|---|---|---|---|---|---|---|
| Total (n = 81) | Children (n = 41) | Adults (n = 40) | Children (n = 23) | Adults (n = 23) | Total (n = 14) | Total (n = 81) | Total (= 24) | |
| Age, years, median (IQR) | 14 (8 – 21.75) | 8 (4.8—10) | 22 (18 – 46) | 6 (3—10) | 32 (19—38) | 45.5 (38.25 – 58.25) | 23.0 (20.0 – 27.0) | 8 (6–11) |
| Female, n (%) | 41 (51) | 18 (44) | 29 (73) | 12 (52) | 5 (22) | 3 (21) | 81 (100) | 9 (37.5) |
| Previous malaria, n (%) | 81 (100) | 41 (100) | 40 (100) | 23 (100) | 23 (100) | 11 (76) | 81 (100) | 24(100) |
| Characteristics at admission | ||||||||
| Severe malaria, n (%) | 5 (6) | 5 (12) | 0 | - | - | 0 | - | - |
| Positive for Plasmodium spp in microscopy, n (%) | 81 (100) | 41 (100) | 40 (100) | 0 (0) | 0 (0) | 14 (100) | 2 (2.5) | 3 (12.5) |
| Parasitemia, parasites/µL, median (IQR) | 3791 (928 – 19 100) | 7920 (1360 – 36 420 | 1860 (750—8470) | - | - | 18 000 (4000–37 100) | 1160 (800 – 1520) | 1240 (96 – 3040) |
Forty-six individuals (23 adults and 23 children) sought healthcare at Iganga General Hospital for fever and malaise and were enrolled in the study. After testing negative for malaria by LAMP, they were categorized as having malaria-like febrile illness. Like the malaria cohort, all participants had a history of prior malaria infection.
Out of the 14 Swedish participants diagnosed with malaria, 10 were confirmed to have P. falciparum malaria, two P. malariae, one P. vivax, and one P. ovale, as determined by examination with light microscopy. Treatment varied among participants: one received intravenous artesunate, two were treated with oral chloroquine phosphate, and the remaining 10 were given oral combination therapy with artemether/lumefantrine. Two individuals were born in Sweden, while the remaining 12 originated from Sub-Saharan Africa, all currently residing in Sweden with varying lengths of stay, ranging from several years to recent arrivals. Twelve participants had contracted malaria in Sub-Saharan Africa, and the remaining two in South-East Asia. The majority of these individuals had contracted malaria while visiting friends and relatives, with only three people having taken malaria prophylaxis during their visits.
Parasite levels
As presented in Table 1, in the Ugandan cohort, the median parasite density was 3791 parasites/µL (IQR: 928 – 19 100), with levels ranging from 16 to 189 304 parasites/µL.
Among the participants, 17% (n = 14) individuals had a parasite density below 500 parasites/µL, while 75% (n = 61) surpassed 1000 parasites/µL. 66% (n = 19) of individuals with a parasitemia > 10 000 were children under the age of 15. Median parasitemia levels in children under 15 were significantly higher than in adults (p = 0.0046). No difference was observed in median parasitemia levels between children ≤ 5 years of age and those > 5 years.
In the Swedish cohort, the median parasitemia was 18 000 parasites/µL (IQR: 4000 -37 000), with levels ranging from 3200 to 48 000 parasites/µL.
Elevated plasma OPN levels in children during acute malaria infection in Uganda
The median plasma OPN level in the entire study group from Uganda was 71 ng/µL (IQR: 41–191 ng/µL). Upon categorizing the group into two subgroups based on age; adults (≥ 15 years) and children (< 15 years), the median plasma OPN levels were 49 ng/µL (IQR: 26 – 103 ng/µL) and 159 ng/µL (IQR: 66 – 304 ng/µL), respectively. As seen in Fig. 1, the median OPN level in children with malaria was significantly higher compared to that in adults (P = 0.0001). No difference was observed in median OPN levels between children ≤ 5 years of age and those > 5 years.
Fig. 1.
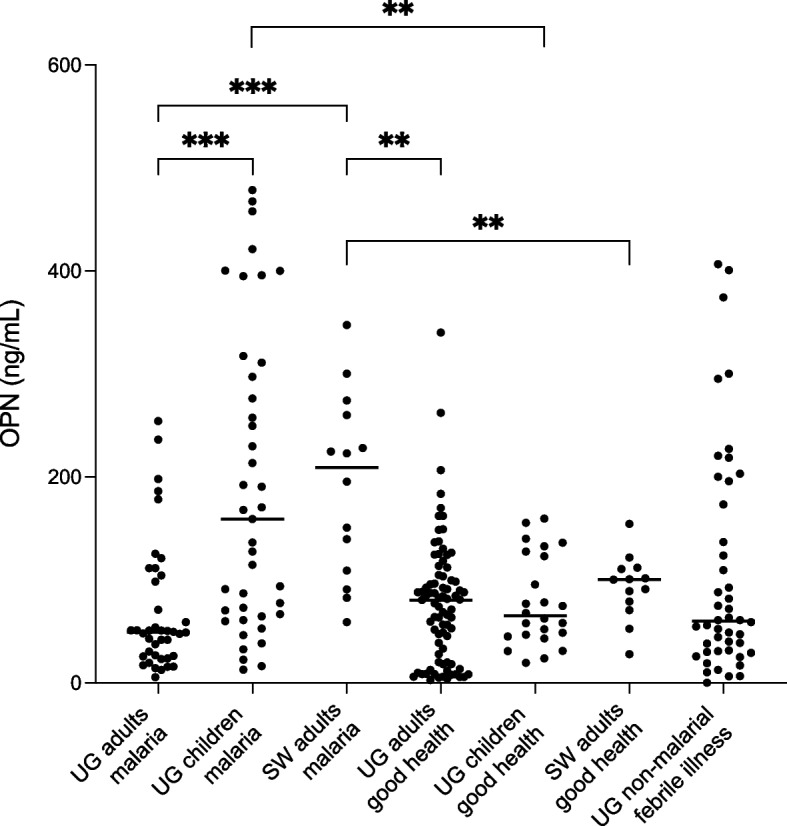
Plasma levels of OPN in various study groups. Scatterplots depict individual values, with lines representing the medians. *** Indicates significance at P < 0.001, ** indicates significance at P < 0.01, as determined by Kruskal–Wallis test with Dunn’s correction for multiple comparisons. UG = Ugandan, SW = Swedish
Plasma OPN levels show no difference in Ugandan adults with and without P. falciparum malaria
In the control group of healthy Ugandan adults, the median plasma OPN level was 80 ng/µL (IQR:20 – 104 ng/µL), with no significant difference in median plasma OPN level compared to the group of adults with acute malaria (Fig. 1).
Elevated plasma OPN levels in imported malaria in Sweden compared to Ugandan adults, but similar to Ugandan children
The median plasma OPN level in the Swedish study cohort with malaria was 209 ng/mL (IQR: 105 – 264 ng/mL), which was significantly higher compared to the median plasma OPN level of the Swedish control group consisting of healthy volunteers (100 ng/mL, IQR: 75 – 111; p = 0.0047) (Fig. 1). Moreover, the median plasma OPN level in the Swedish malaria cohort was also markedly elevated when compared to the adult Ugandan groups, with or without an acute malaria infection (p = 0.0007 and p = 0.004, respectively). When comparing individuals with P. falciparum malaria to those with non-falciparum malaria, no significant difference in plasma OPN levels was found. No significant difference was observed between the plasma OPN levels of the Swedish malaria cohort and those of the children in Uganda with malaria.
Plasma levels of OPN correlate with parasite density
To investigate the potential correlation between plasma OPN levels and parasitemia during acute malaria infections, we employed Spearman’s rank correlation coefficient. In the overall analysis of all participants in the Ugandan and Swedish malaria cohorts, plasma OPN levels showed a correlation with parasitemia (rho = 0.41, P < 0.0001) (Fig. 2A). Moreover, upon subgroup analysis, when stratifying participants into adults (≥ 15 years old) and children (< 15 years old), a significant correlation between OPN and parasitemia levels was observed both within the group of adults (rho = 0.33, P = 0.01) (Fig. 2B) and in the children subgroup (rho = 0.34, P = 0.03) (Fig. 2C). However, when Ugandan adults and Swedish adults with imported malaria were analyzed separately, no significant correlations were found between parasite density and OPN levels in either cohort.
Fig. 2.
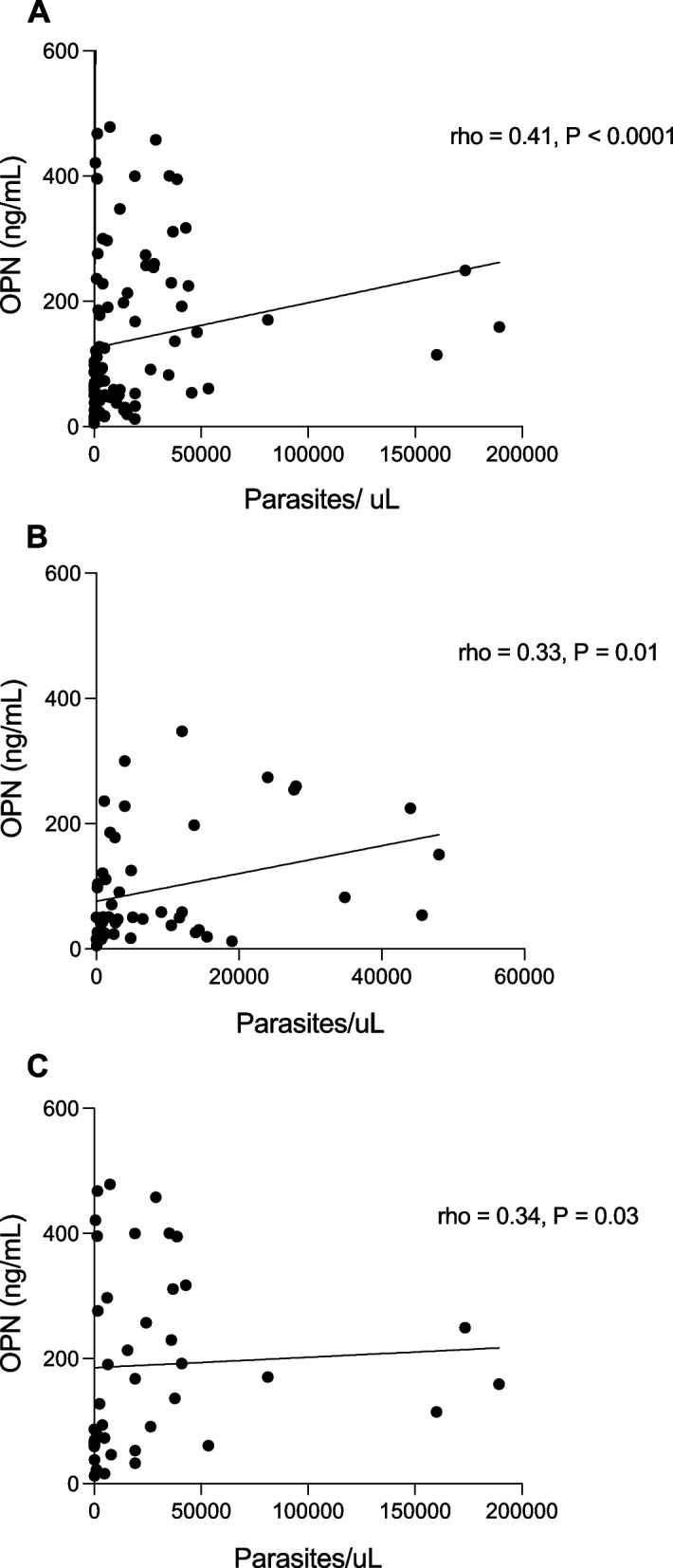
Correlation between plasma OPN levels and parasite density. Figures representing (A) Ugandan and Swedish adults and children with malaria, B Ugandan and Swedish adults with malaria, and (C) Ugandan children with malaria. Spearman’s rank coefficient was used to detect correlations between plasma OPN and parasite density
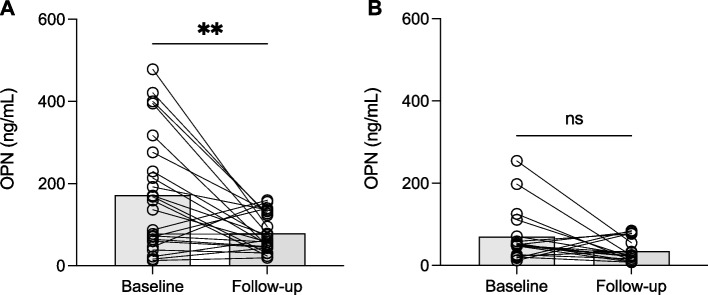
Reduced plasma OPN levels in follow-up after 42 days in Ugandan study participants
At the 42 days follow-up, a significant decrease in plasma OPN levels was observed in the overall group (P = 0.0004). Upon stratification by age, a significant decrease persisted in the < 15 years age group (P = 0.0039) (Fig. 3A). However, no significant difference between baseline and follow-up was detected in the adult group (Fig. 3B). All participants, except three asymptomatic children, tested negative for malaria by microscopy at follow-up. There were no significant differences in plasma OPN levels between these children and those who tested negative.
Fig. 3.
Distribution of plasma OPN levels at baseline and 42 days follow-up in children (A) and adults (B). Bars representing the median and interconnecting lines paired subjects; ** significant at P < 0.005 and ns = not significant as tested by Wilcoxon matched-pairs signed-rank test
No significant difference in plasma OPN between P. falciparum malaria and non-malarial febrile illness
Plasma levels of OPN were compared in 81 Ugandan individuals with verified malaria infection, and 46 participants experiencing non-malarial febrile illness. The median OPN level was significantly elevated in the non-malarial group aged < 15 years (123, IQR:63 – 221) when compared to the adult group (30, IQR:17 – 49) (P < 0.0001). As demonstrated in Fig. 1, no significant differences were observed between the malaria-infected group and the non-malaria febrile illness group, even upon further subgroup analysis based on age (adults and children).
Plasma IFN- γ levels in acute malaria
To compare OPN plasma levels with another marker of inflammation, we measured IFN- γ levels in 152 samples, including all individuals with acute malaria, several follow-up samples and control samples. In the Ugandan cohort with acute malaria the median IFN- γ level in children (< 15 years) was 3.7 pg/mL (IQR 2.1–6.1 pg/mL) and 3.7 ng/mL (IQR 2.0–5.6 pg/mL) in adults (≥ 15 years) (Fig. 4). No significant differences were observed in the respective control groups. In the Swedish malaria cohort, the median IFN- γ level was 4.3 pg/mL (IQR 3.7–9.5 pg/mL), which was significantly elevated compared to the Swedish individuals in good health (P = 0.0007) (Fig. 4).
Fig. 4.
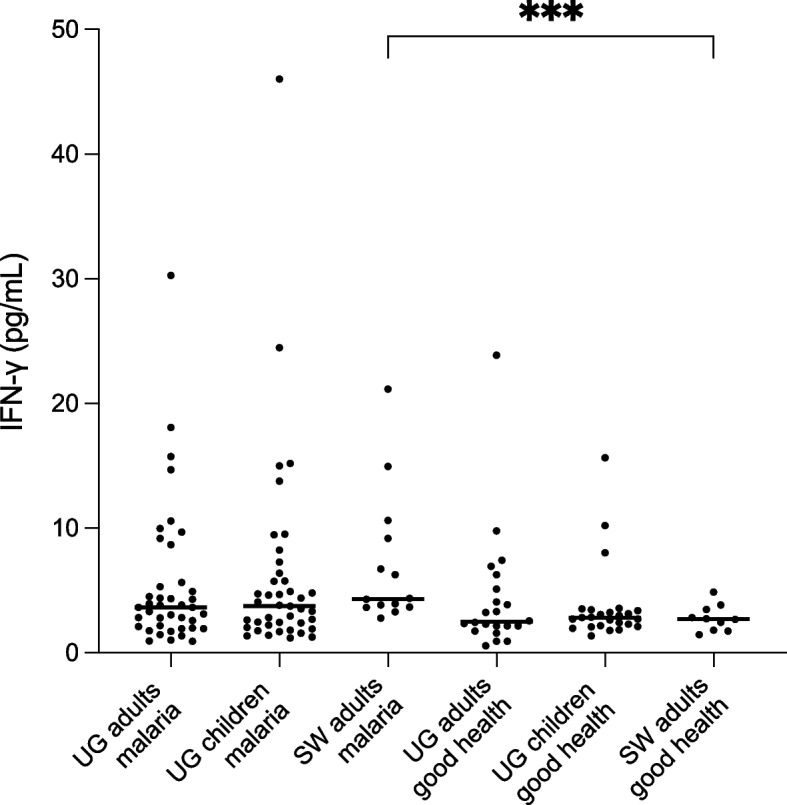
IFN- γ plasma levels in Ugandan and Swedish individuals with and without acute malaria. Scatterplots depict individual values, with lines representing the medians. *** Indicates significance at P < 0.0005, as determined by non-parametric Mann–Whitney-test. UG = Ugandan, SW = Swedish
Plasma IFN-γ levels correlate with parasitemia
Spearman’s correlation analyses were conducted to examine the relationship between parasitemia and IFN-γ levels. The analysis revealed a significant positive correlation between IFN-γ and parasitemia across the entire malaria cohort, which included participants from both Uganda and Sweden (rho = 0.24, P = 0.012). In subgroup analyses, a significant correlation was found in Ugandan children (rho = 0.33, P = 0.036). No significant correlations were observed in other groups (data not shown).
Correlations between plasma OPN and IFN- γ levels
To examine the relationship between OPN and IFN-γ, correlation analyses were performed. In the combined malaria cohorts and control groups, Spearman's correlation revealed a significant positive relationship (rho = 0.35, P < 0.0001). However, when analyzed by subgroup, a significant correlation was observed only in Ugandan adults, (rho = 0.59, P < 0.0001). No significant correlations were detected in the other groups (data not shown).
Discussion
This study is the first to explore the plasma OPN dynamics and their correlation with parasitemia in both acute P. falciparum malaria and during convalescence in children and adults in an endemic setting, as well as in individuals with imported malaria in Sweden. We found that OPN plasma levels were elevated during acute P. falciparum infection in Ugandan children and adults with imported malaria in Sweden. Additionally, plasma OPN levels correlated with parasite density, suggesting a role for OPN in immunity development and the natural immune response to malaria.
In this study, plasma OPN levels increased significantly among children during acute P. falciparum infection, while levels in Ugandan adults remained unchanged. The increase in OPN levels among children aligns with studies showing elevated OPN in other acute infections such as schistosomiasis, dengue, leptospirosis, and COVID-19 [19, 20, 31, 32]. However, these studies focused solely on adult participants. Interestingly, in cases of schistosomiasis, OPN levels were notably higher during acute infection compared to the chronic hepatosplenic form of the disease [26]. Given that plasma OPN levels in Ugandan adults with acute P. falciparum infection did not increase as compared to the control group, our results suggest that OPN regulation may depend on factors such as age and the development of semi-immunity. An acute P. falciparum infection induces significant immunological changes, marked by elevated inflammatory signals such as IFN‐γ, TNF‐α, and IL‐6 [33], which are known to upregulate or be induced by OPN expression [14, 34]. Children have been shown to have a higher pro-inflammatory response of TNF-α and IL-10 compared to adults during acute P. falciparum infection [35], and younger children had increased levels of pro-inflammatory cytokines TNF‐α, IL-2, IL-6 and Th1-biased cytokines compared to older children at the same stage of infection [36]. A high pro-inflammatory response can lead to a more rapid control of parasite growth but also lead to severe clinical disease, as evidenced by both experimental and clinical studies [28, 37, 38], and has been suggested to be an important factor in the development of severe malaria [39]. These signals may directly or indirectly contribute to elevated OPN levels. Soluble OPN is known to promote dendritic cell migration and TNF-α secretion, which in turn supports a Th1-polarized immune response and enhances T-cell production of IFN-γ [14]. In our study, we compared the plasma levels of OPN and IFN-γ to better understand the relationship between these two inflammatory cytokines during malaria. Interestingly, we found significant correlations between OPN and IFN-γ only in Ugandan adults. In contrast, this correlation was absent in other groups, which presumably have less developed immunity compared to the adults. This indicates that OPN plays an intricate role in immune regulation beyond merely reflecting inflammation.
Furthermore, an interesting finding in the Swedish cohort was the elevated plasma levels of OPN in individuals with acute malaria. Despite the majority of the individuals being born in malaria-endemic regions, most resided in Sweden and thus lacked regular exposure to P. falciparum, likely resulting in the loss of or reduced semi-immunity [5]. The discrepancy in OPN levels between the two Swedish cohorts suggests a similar inflammatory response during acute malaria for both children in Uganda, who have not yet developed semi-immunity, and for the adults with imported malaria who either have no or limited immunity. Together, this suggests that OPN elevation may be related more to immune status rather than age, indicating OPN’s potential role in the initial immune response to malaria, especially in non-immune individuals. However, given the limited understanding of the mechanisms governing OPN regulation and its expression in various cell types, the exact role of OPN in acute malaria remains to be fully understood. OPN could potentially contribute to the acute inflammatory response against the parasite or play a role in the adaptive immune response by regulating B- and T cell responses and immunoglobulin production. Recent studies reporting a decrease in P. falciparum invasion in erythrocytes lacking CD44, a key receptor for OPN-signaling, present an intriguing connection between P. falciparum malaria and OPN [40, 41]. Further research is needed to elucidate the precise functions of OPN in the context of malaria infection.
Parasitemia is an important marker of illness severity. We found interesting correlations between OPN and parasite levels in the Ugandan cohort, particularly pronounced among younger children. This suggest that as parasitemia levels increases, there is a corresponding rise in OPN secretion. This response may be generated either by the presence of the parasites, or by the immunoinflammatory activity resulting from the infection. Interestingly, the correlation between OPN and parasitemia levels disappeared when analyzing the adult groups separately. This implies that OPN’s significance may be greater in non-immune individuals, possibly serving as a marker for severe infection or contributing to the host response in the absence of acquired immunity. Our findings differ from prior research that reported an inverse relationship between OPN mRNA and parasitemia, suggesting a potential suppressive effect of OPN on P. falciparum [23]. However, the study relied on mRNA analysis from dried blood spots, a method that may pose challenges in obtaining precise results, as evidenced by the inability to detect OPN in many samples [23]. The results of our study are in accordance with a previous study, where an OPN growth inhibition assay demonstrated no discernable impact on parasite growth [24], suggesting that OPN does not directly impact parasite levels. Moreover, our current results are in line with existing literature that has documented associations between pro-inflammatory cytokines such as IL-6, IL-10 and IFN-γ and parasitemia [33, 42, 43].
The majority of adults and children had experienced malaria previously and were categorized as having mild malaria, indicating that most participants had developed some level of semi-immunity against severe disease. Nevertheless, children had higher median parasite levels than adults, likely due to less advanced immune responses. The degree of parasitemia likely depends on several factors, including immune status and the infection stage at the time of blood sampling. At follow-up, all participants were in good health, with all but three children testing negative for P. falciparum via microscopy. OPN levels had decreased in children but remained unchanged in adults. The differences between children and adults support the theory that OPN is upregulated in response to an infection with P. falciparum in non-immune individuals.
The conditions under which OPN is upregulated in acute infections, whether it depends on the etiology or as a general febrile response, remains unclear. To address this, we compared OPN levels between individuals with non-malarial febrile illnesses and those diagnosed with malaria. Interestingly, we found no significant difference in OPN plasma levels between the two groups, despite distinct etiologies. As in the cohorts with malaria, we observed higher OPN levels in children compared to adults in the non-malaria febrile cohort. Nonetheless, interpreting these findings is challenging due to the lack of information regarding the etiology of the febrile illnesses – whether viral, bacterial, or parasitic. A larger, age-matched cohort, with a known infectious agent as a control group, would be necessary for more comprehensive comparisons.
This study has some limitations. Firstly, follow-up samples were analyzed from only a small subgroup of patients. Additionally, variability in reported physiological OPN levels may arise from differences in study methodologies, such as the choice of ELISA-kits, anti-coagulants, and whether the analysis measured full-length or cleaved OPN [20, 44]. Although previous studies indicate that younger children have higher OPN levels than adults [24, 45], the age at which this difference diminishes remains uncertain. One study suggested around 14 years of age, however, the comparison between OPN levels in ill, hospitalized children with healthy adults, make the results difficult to interpret, as OPN tend to be higher during infections [45]. Another limitation is the exclusion of HIV-positive individuals from the study cohort. Including these participants could have provided insights into how co-infection affects OPN levels and made the study more representative of the local population, where HIV prevalence were reported at 4.5% in 2021 [46]. Additionally, comparing OPN levels with PfHRP2 could have enhanced the study's value, as plasma PfHRP2 could more accurately reflect the total P. falciparum burden compared to parasite density in peripheral blood [47].
Conclusions
In summary, we have demonstrated that OPN plasma levels were increased during acute P. falciparum infection in non-immune children and adults, and that OPN plasma levels correlated with parasitemia levels, indicating a potential involvement in the immune response of non-immune individuals. While these findings suggest a possible role for OPN in malaria, further investigation is needed to fully understand its mechanisms and specific role in immune development.
Acknowledgements
We are grateful to all study participants for kindly donating blood samples. We also thank the clinical and administrative staff at Iganga General Hospital and the Department of Infectious Diseases at Skåne University Hospital for assistance in the study.
Clinical trial number
Not applicable.
Abbreviations
- P. falciparum
Plasmodium falciparum
- OPN
Osteopontin
- LAMP
Loop-mediated isothermal amplification
- IFN-γ
Interferon-γ
- WHO
World Health Organization
- RDT
Rapid diagnostic test
- PBMCs
Peripheral blood mononuclear cells
- WBCs
White blood cells
- SD
Standard deviation
- IQR
Interquartile range
Authors’ contributions
Conception and study design SM, AL, HN, and KP, sample collection SM, AL, and LD, laboratory work and analysis SM, LD, BW and KP, drafting and revision of manuscript SM, AL, LD, BW, HN, and KP. All authors read and approved the final manuscript.
Funding
Open access funding provided by Lund University. Funding was provided from ALF (Region Skåne/Lund University) and SUS Fonder. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
All results are visible in the figures, and exact datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
A written informed consent was obtained from all the participants or their guardian prior to the study enrollment. The study was approved by the School of Biomedical Sciences Research and Ethics Committee of Makerere University, the Uganda National Council of Science and Technology (approval SBS-REC- 765 and HS1267ES) and by Regionala Etikprövningsnämnden, Lund (Dnr 2015/7) and Etikprövningsmyndigheten in Stockholm, Sweden (204/478–32 and 2022–03160-01).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. World Malaria Report 2023. Geneva: World Health Organization; 2023. Available from: https://www.who.int/publications/i/item/9789240086173.
- 2.World Health Organization. Guidelines for the treatment of malaria. Third edition. Geneva: World Health Organization; 2015. Available from: https://www.afro.who.int/sites/default/files/2017-06/9789241549127_eng.pdf
- 3.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22(1):13–36 Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tran TM, Li S, Doumbo S, Doumtabe D, Huang CY, Dia S, et al. An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis. 2013;57(1):40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mischlinger J, Rönnberg C, Álvarez-Martínez MJ, Bühler S, Paul M, Schlagenhauf P, et al. Imported Malaria in Countries where Malaria Is Not Endemic: a Comparison of Semi-immune and Nonimmune Travelers. Clin Microbiol Rev. 2020;33(2). [DOI] [PMC free article] [PubMed]
- 6.Dobaño C, Nhabomba AJ, Manaca MN, Berthoud T, Aguilar R, Quintó L, et al. A Balanced Proinflammatory and Regulatory Cytokine Signature in Young African Children Is Associated With Lower Risk of Clinical Malaria. Clin Infect Dis. 2019;69(5):820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezema CA, Okagu IU, Ezeorba TPC. Escaping the enemy’s bullets: an update on how malaria parasites evade host immune response. Parasitol Res. 2023;122(8):1715–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan Y, Zhao L, Yang YG, Liu W. The Role of Osteopontin in Tumor Progression Through Tumor-Associated Macrophages. Front Oncol. 2022;12:953283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. 2018;59:17–24. [DOI] [PubMed] [Google Scholar]
- 10.Rittling SR, Singh R. Osteopontin in Immune-mediated Diseases. J Dent Res. 2015;94(12):1638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lund SA, Giachelli CM, Scatena M. The role of osteopontin in inflammatory processes. J Cell Commun Signal. 2009;3(3–4):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin EY, Xi W, Aggarwal N, Shinohara ML. Osteopontin (OPN)/SPP1: from its biochemistry to biological functions in the innate immune system and the central nervous system (CNS). Int Immunol. 2023;35(4):171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19(5–6):333–45. [DOI] [PubMed] [Google Scholar]
- 14.Renkl AC, Wussler J, Ahrens T, Thoma K, Kon S, Uede T, et al. Osteopontin functionally activates dendritic cells and induces their differentiation toward a Th1-polarizing phenotype. Blood. 2005;106(3):946–55. [DOI] [PubMed] [Google Scholar]
- 15.Murugaiyan G, Mittal A, Weiner HL. Increased osteopontin expression in dendritic cells amplifies IL-17 production by CD4+ T cells in experimental autoimmune encephalomyelitis and in multiple sclerosis. J Immunol. 2008;181(11):7480–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan H, Nagarkatti PS, Nagarkatti M. Role of CD44 in the differentiation of Th1 and Th2 cells: CD44-deficiency enhances the development of Th2 effectors in response to sheep RBC and chicken ovalbumin. J Immunol. 2009;183(1):172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860–4. [DOI] [PubMed] [Google Scholar]
- 18.Hasibuan FM, Shiratori B, Senoputra MA, Chagan-Yasutan H, Koesoemadinata RC, Apriani L, et al. Evaluation of matricellular proteins in systemic and local immune response to Mycobacterium tuberculosis infection. Microbiol Immunol. 2015;59(10):623–32. [DOI] [PubMed] [Google Scholar]
- 19.Bai G, Furushima D, Niki T, Matsuba T, Maeda Y, Takahashi A, et al. High Levels of the Cleaved Form of Galectin-9 and Osteopontin in the Plasma Are Associated with Inflammatory Markers That Reflect the Severity of COVID-19 Pneumonia. Int J Mol Sci. 2021;22(9). [DOI] [PMC free article] [PubMed]
- 20.Chagan-Yasutan H, Lacuesta TL, Ndhlovu LC, Oguma S, Leano PS, Telan EF, et al. Elevated levels of full-length and thrombin-cleaved osteopontin during acute dengue virus infection are associated with coagulation abnormalities. Thromb Res. 2014;134(2):449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiberti N, Hainard A, Lejon V, Robin X, Ngoyi DM, Turck N, et al. Discovery and verification of osteopontin and Beta-2-microglobulin as promising markers for staging human African trypanosomiasis. Mol Cell Proteomics. 2010;9(12):2783–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeno Y, Nakazawa S, Yamamoto N, Shinzato M, Nagashima S, Tanaka K, et al. Osteopontin participates in Th1-mediated host resistance against nonlethal malaria parasite Plasmodium chabaudi chabaudi infection in mice. Infect Immun. 2006;74(4):2423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maeno Y, Nakazawa S, le Dao D, Van Tuan N, Giang ND, Van Hanh T, et al. Osteopontin is involved in Th1-mediated immunity against Plasmodium falciparum infection in a holoendemic malaria region in Vietnam. Acta Trop. 2006;98(3):305–10. [DOI] [PubMed] [Google Scholar]
- 24.Mortazavi SE, Lugaajju A, Kaddumukasa M, Tijani MK, Kironde F, Persson KEM. Osteopontin and malaria: no direct effect on parasite growth, but correlation with P. falciparum-specific B cells and BAFF in a malaria endemic area. BMC Microbiol. 2021;21(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortazavi SE, Lugaajju A, Nylander M, Danielsson L, Tijani MK, Beeson JG, et al. Acquisition of complement fixing antibodies targeting Plasmodium falciparum merozoites in infants and their mothers in Uganda. Front Immunol. 2023;14:1295543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King T, Lamb T. Interferon-γ: The Jekyll and Hyde of Malaria. PLoS Pathog. 2015;11(10):e1005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gun SY, Claser C, Tan KS, Rénia L. Interferons and interferon regulatory factors in malaria. Mediators Inflamm. 2014;2014:243713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mandala WL, Msefula CL, Gondwe EN, Drayson MT, Molyneux ME, MacLennan CA. Cytokine Profiles in Malawian Children Presenting with Uncomplicated Malaria, Severe Malarial Anemia, and Cerebral Malaria. Clin Vaccine Immunol. 2017;24(4). [DOI] [PMC free article] [PubMed]
- 29.Oyegue-Liabagui SL, Bouopda-Tuedom AG, Kouna LC, Maghendji-Nzondo S, Nzoughe H, Tchitoula-Makaya N, et al. Pro- and anti-inflammatory cytokines in children with malaria in Franceville. Gabon Am J Clin Exp Immunol. 2017;6(2):9–20. [PMC free article] [PubMed] [Google Scholar]
- 30.Mahittikorn A, Mala W, Masangkay FR, Kotepui KU, Wilairatana P, Kotepui M. Increased interferon-γ levels and risk of severe malaria: a meta-analysis. Sci Rep. 2022;12(1):18917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira TA, Syn WK, Amâncio FF, Cunha PH, Caporali JF, Trindade GV, et al. Osteopontin Is Upregulated in Human and Murine Acute Schistosomiasis Mansoni. PLoS Negl Trop Dis. 2016;10(10):e0005057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hattori T, Iwasaki-Hozumi H, Bai G, Chagan-Yasutan H, Shete A, Telan EF, et al. Both Full-Length and Protease-Cleaved Products of Osteopontin Are Elevated in Infectious Diseases. Biomedicines. 2021;9(8). [DOI] [PMC free article] [PubMed]
- 33.Oyegue-Liabagui SL, Mbani Mpega Ntigui CN, Ada Mengome MF, Kouna LC, Tsafack Tegomo NP, Longo Pendy NM, et al. Cytokine response in asymptomatic and symptomatic Plasmodium falciparum infections in children in a rural area of south-eastern Gabon. PLoS One. 2023;18(2):e0280818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uchibori T, Matsuda K, Shimodaira T, Sugano M, Uehara T, Honda T. IL-6 trans-signaling is another pathway to upregulate Osteopontin. Cytokine. 2017;90:88–95. [DOI] [PubMed] [Google Scholar]
- 35.Maurizio PL, Fuseini H, Tegha G, Hosseinipour M, De Paris K. Signatures of divergent anti-malarial treatment responses in peripheral blood from adults and young children in Malawi. Malar J. 2019;18(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrington L, Vance H, Rek J, Prahl M, Jagannathan P, Katureebe A, et al. Both inflammatory and regulatory cytokine responses to malaria are blunted with increasing age in highly exposed children. Malar J. 2017;16(1):499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walther M, Woodruff J, Edele F, Jeffries D, Tongren JE, King E, et al. Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol. 2006;177(8):5736–45. [DOI] [PubMed] [Google Scholar]
- 38.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72(10):5630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumar R, Ng S, Engwerda C. The Role of IL-10 in Malaria: A Double Edged Sword. Front Immunol. 2019;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baro B, Kim CY, Lin C, Kongsomboonvech AK, Tetard M, Peterson NA, et al. Plasmodium falciparum exploits CD44 as a coreceptor for erythrocyte invasion. Blood. 2023;142(23):2016–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanjee U, Grüring C, Chaand M, Lin KM, Egan E, Manzo J, et al. CRISPR/Cas9 knockouts reveal genetic interaction between strain-transcendent erythrocyte determinants of Plasmodium falciparum invasion. Proc Natl Acad Sci U S A. 2017;114(44):E9356–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, et al. The prognostic and pathophysiologic role of pro- and antiinflammatory cytokines in severe malaria. J Infect Dis. 1999;180(4):1288–97. [DOI] [PubMed] [Google Scholar]
- 43.Hugosson E, Montgomery SM, Premji Z, Troye-Blomberg M, Björkman A. Relationship between antipyretic effects and cytokine levels in uncomplicated falciparum malaria during different treatment regimes. Acta Trop. 2006;99(1):75–82. [DOI] [PubMed] [Google Scholar]
- 44.Vordermark D, Said HM, Katzer A, Kuhnt T, Hänsgen G, Dunst J, et al. Plasma osteopontin levels in patients with head and neck cancer and cervix cancer are critically dependent on the choice of ELISA system. BMC Cancer. 2006;6:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nourkami-Tutdibi N, Graf N, Beier R, Zemlin M, Tutdibi E. Plasma levels of osteopontin from birth to adulthood. Pediatr Blood Cancer. 2020;67(7):e28272. [DOI] [PubMed] [Google Scholar]
- 46.Uganda AIDS Commission. Annual Joint AIDS Review Report FY 2021/22. Kampala: Uganda AIDS Commision: 2022. Available from: https://uac.go.ug/images/2024/jar-2024/jar-report-2021-22_nov-21-2022_final.pdf.
- 47.Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, et al. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2005;2(8):e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All results are visible in the figures, and exact datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.